Nonenzymatic Lactic Acid Detection Using Cobalt Polyphthalocyanine/Carboxylated Multiwalled Carbon Nanotube Nanocomposites Modified Sensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
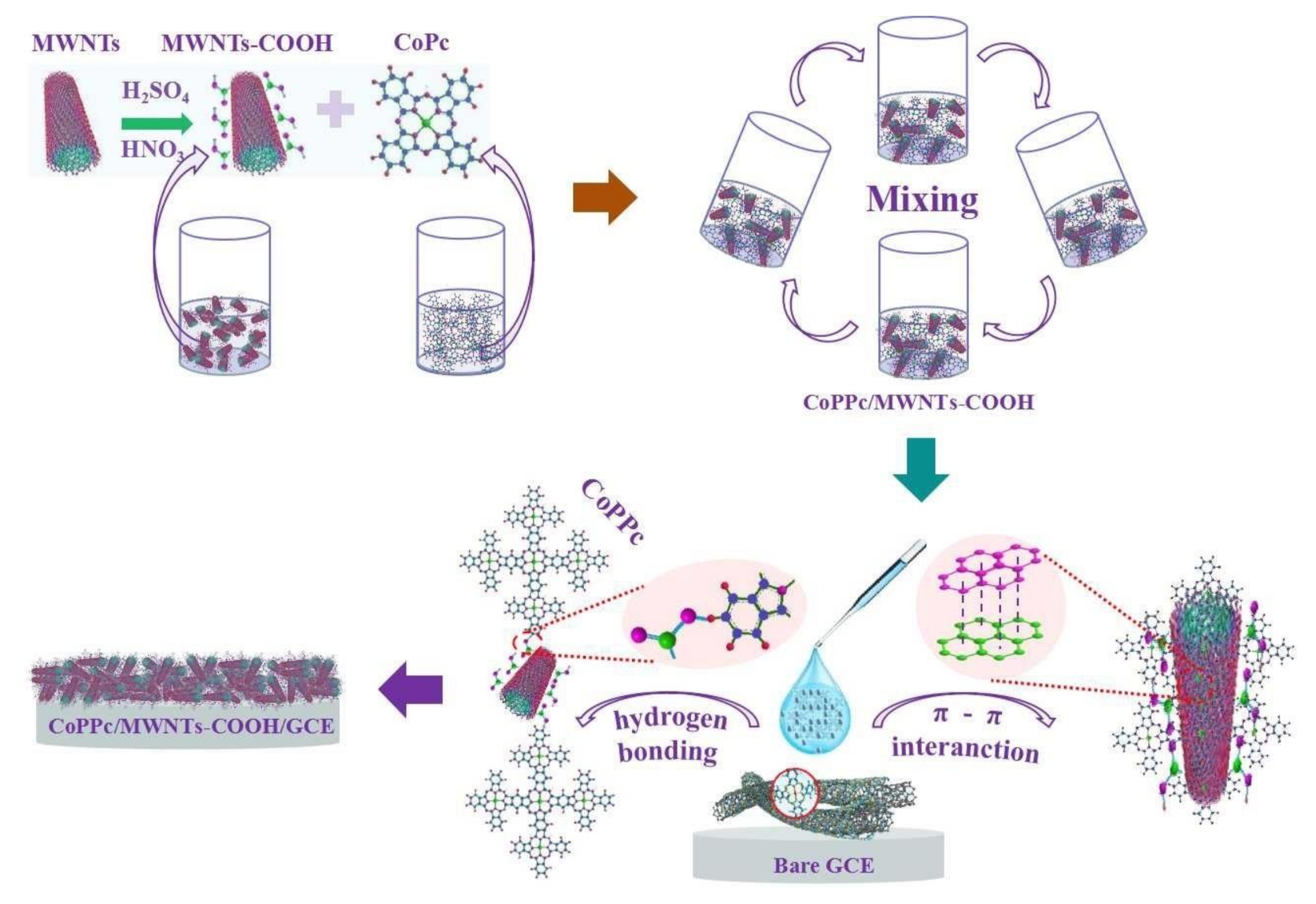
2.2. Fabrication of CoPPc/MWCNTs-COOH Nanocomposites Modified Sensor
2.2.1. Functionalization of MWCNTs
- Purification of MWCNTs. Initially, 5 mg MWCNTs was ultrasonically dispersed in 75 mL of H2SO4 at 25 °C for 30 min. Secondly, the suspension liquid was poured into a 250-mL conical flask and refluxed at 90 °C for 3 h with constant stirring. Then, the mixture was centrifuged at 6000 rpm and washed with deionized water several times until the pH was nearly neutral. Finally, the purified MWCNTs were obtained after drying in a vacuum oven at 70 °C. Impurities were removed by O2, gas or soluble nitrate. The reaction equations were as follows:
- 2.
- Carboxylation of MWCNTs. The purified MWCNTs were treated with 60 mL mixture of H2SO4 and HNO3 (3/1, v/v) under ultrasonication at 30 °C for 3 h. After that, the mixture was added into a beaker with 200 mL ultrapure water and then cooled to room temperature. Then, the obtained suspension was centrifuged at 6000 rpm for 10 min, 8000 rpm for 5 min, and 10,000 rpm for 5 min successively. Finally, the precipitation was washed with deionized water (until the pH of the filtrate tested neutral) and dried under vacuum at 80 °C for 8 h to get MWCNTs-COOH.
- 3.
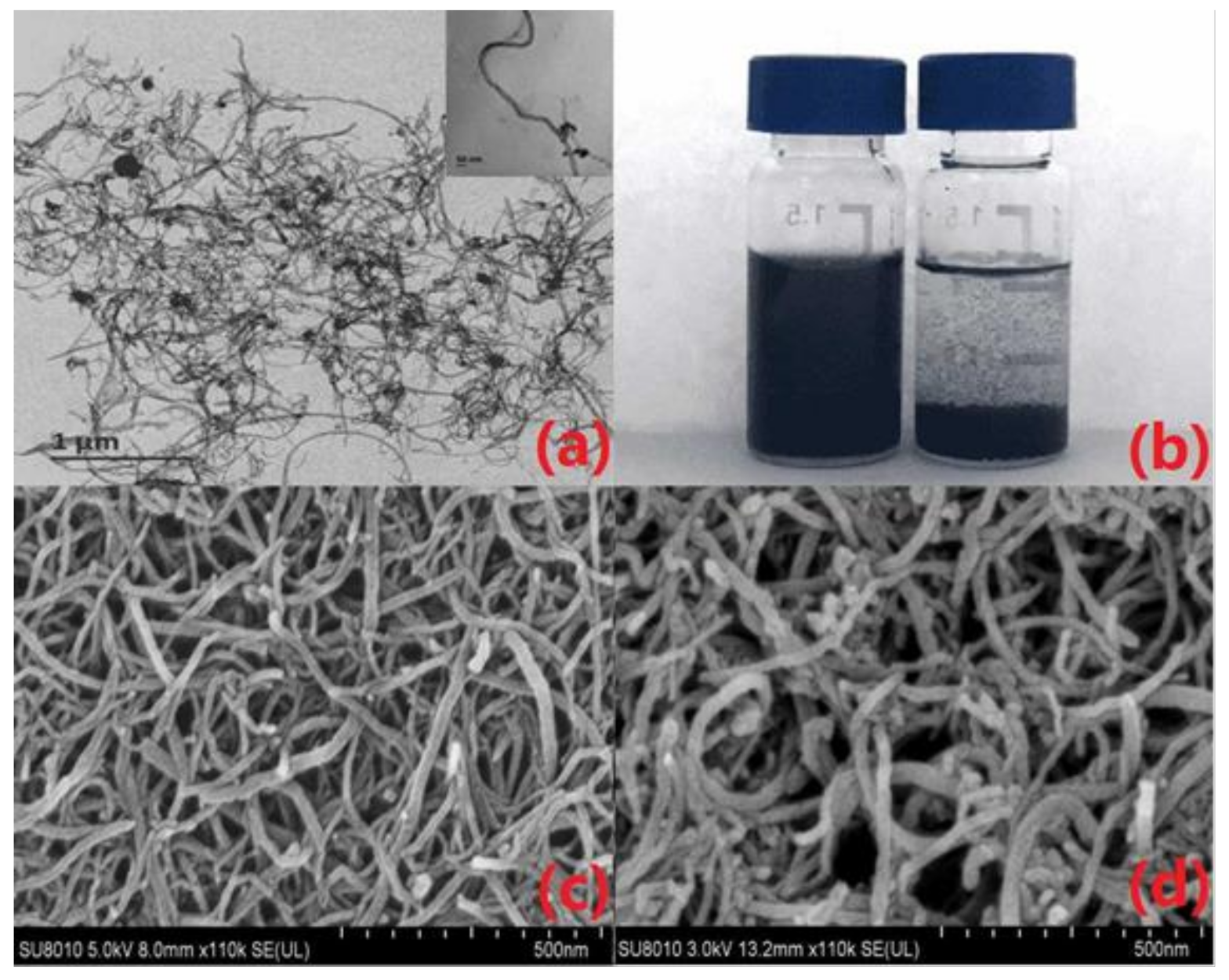
- The dispersity of raw and functional MWCNTs in aqueous solution are shown in Figure 1b. MWCNTs-COOH presented a well dispersed suspension, and the homogeneous dispersion maintained stability for a long time (i.e., over one week). The raw MWCNTs were poorly dispersed, and the suspension stratified in a short time (less than 12 h). This might be explained by the fact that the MWCNTs were chemically shortened after purification and functionalization, and the modified hydrophilic group (-COOH) enhanced the solubility of the composite. Good distribution of MWCNTs-COOH in aqueous solution was beneficial for further modifications by improving the uniformity and stability of drop-casting on the surface of GCE.
2.2.2. Fabrication of the Nonenzymatic Lactic Acid Sensor
2.3. Material Characterizations and Electrochemical Tests
2.4. Detection of Lactic Acid in Rice Wine Samples
3. Results
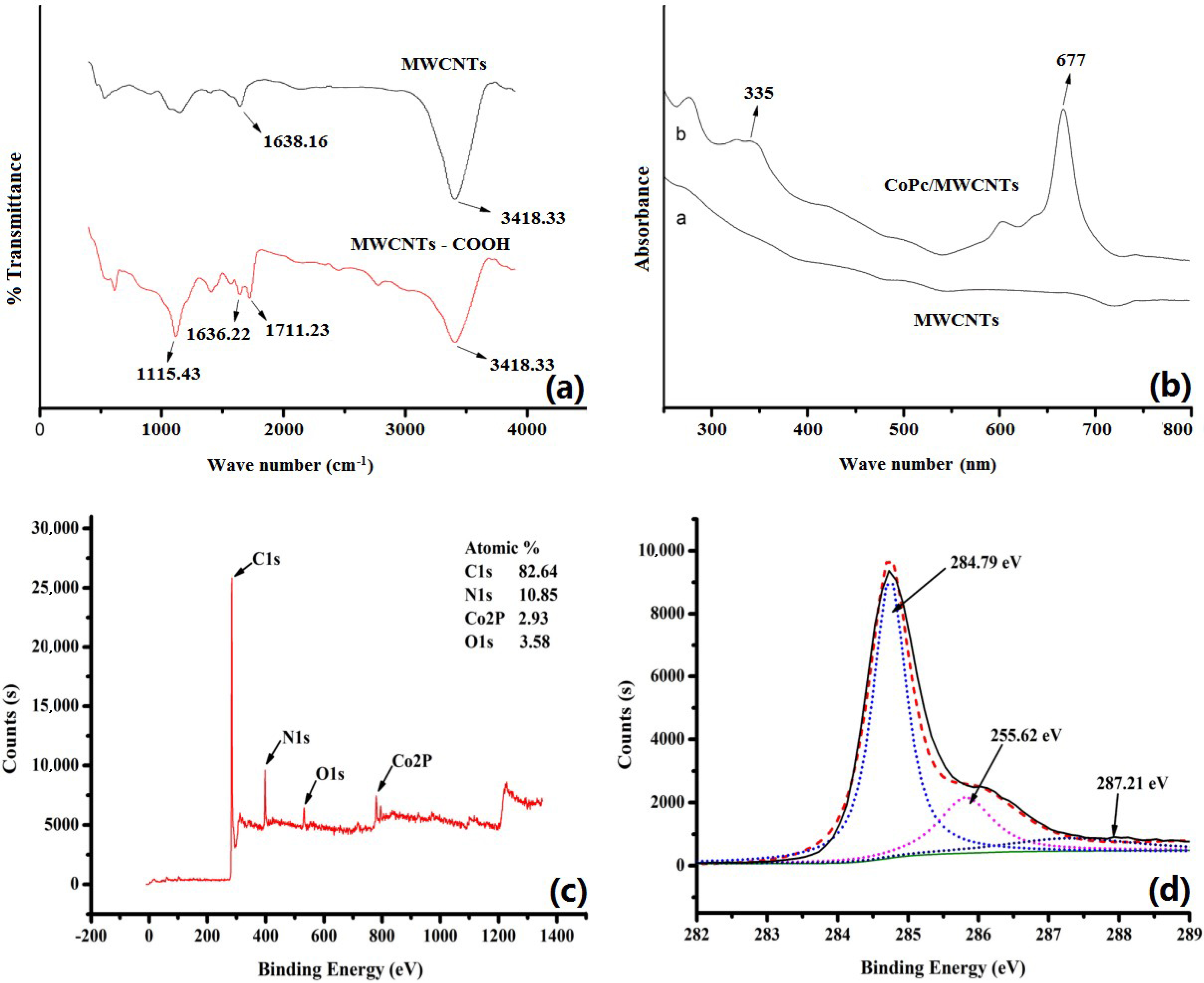
3.1. Characterization of CoPPc/MWCNTs-COOH Nanocomposites
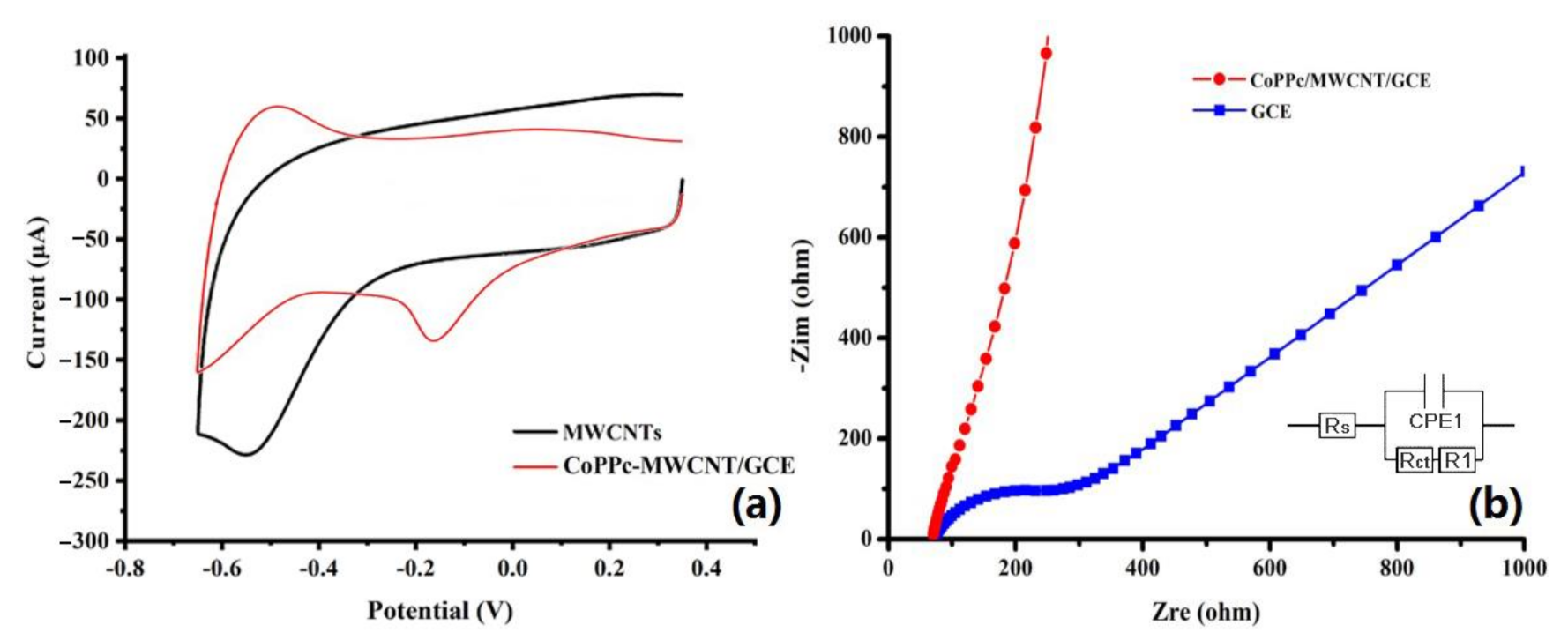
3.2. Electrochemical Properties of the Modified CoPPc/MWCNTs-COOH/GCE
3.3. Optimization of Experimental Parameters
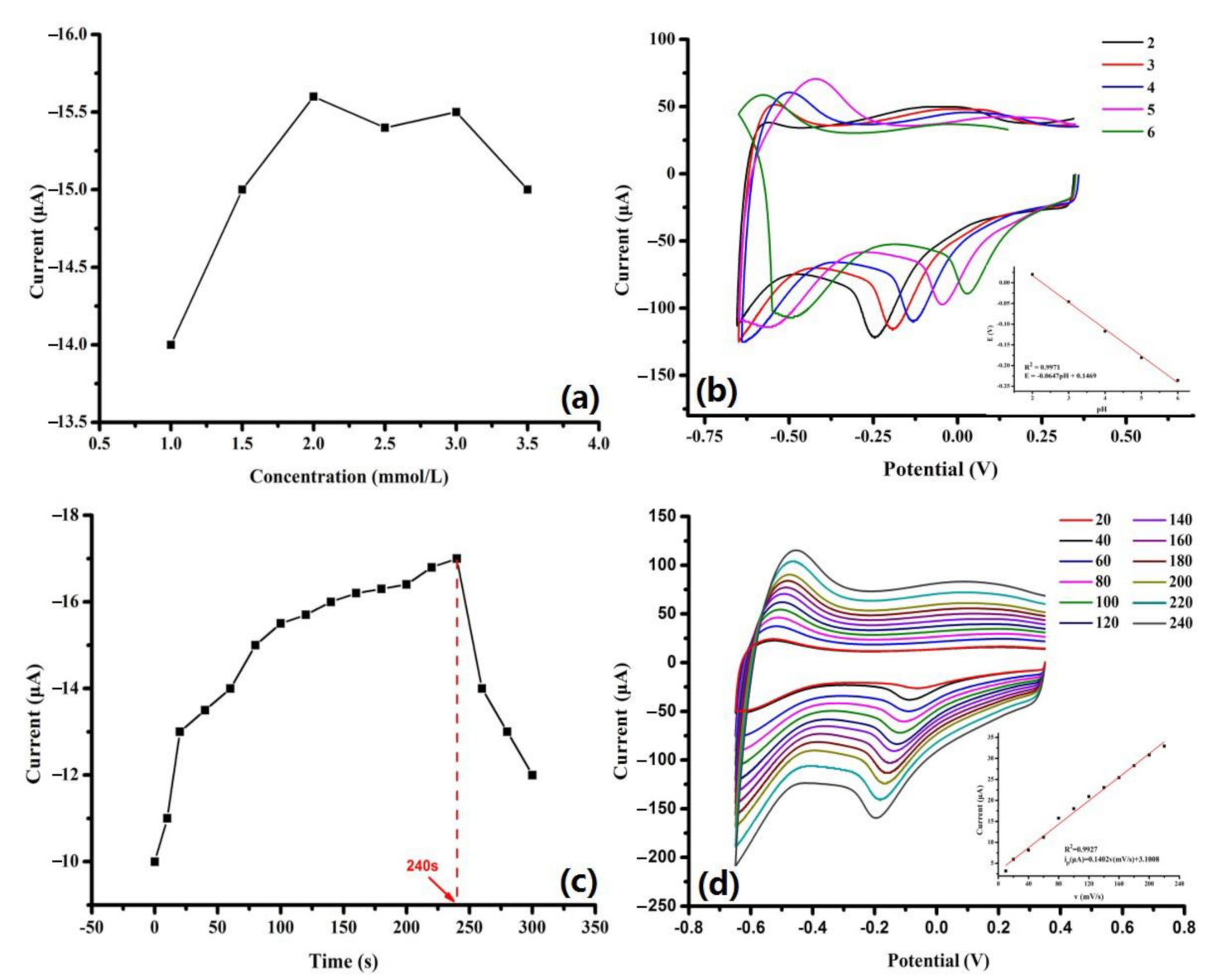
3.3.1. Influence of Modifier Dosage
3.3.2. Influence of pH
3.3.3. Effect of Accumulation Time
3.3.4. Effect of Scan Rate
3.4. Kinetic Analysis of Lactic Acid on Modified Electrode
3.4.1. The Number of Electrons and Protons Participated in the Electrocatalysis Process
3.4.2. The Effective Surface Area of the Modified Electrode
3.4.3. The Analysis of Diffusion Coefficient
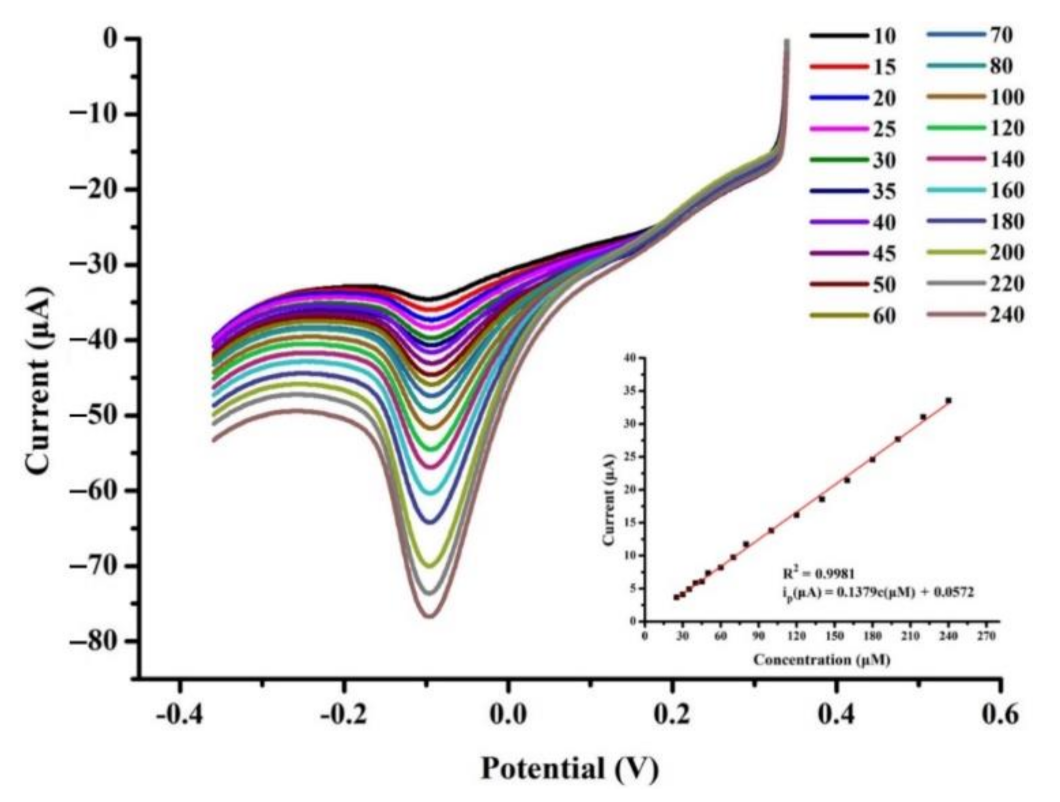
3.5. Determination of Lactic Acid Concentration
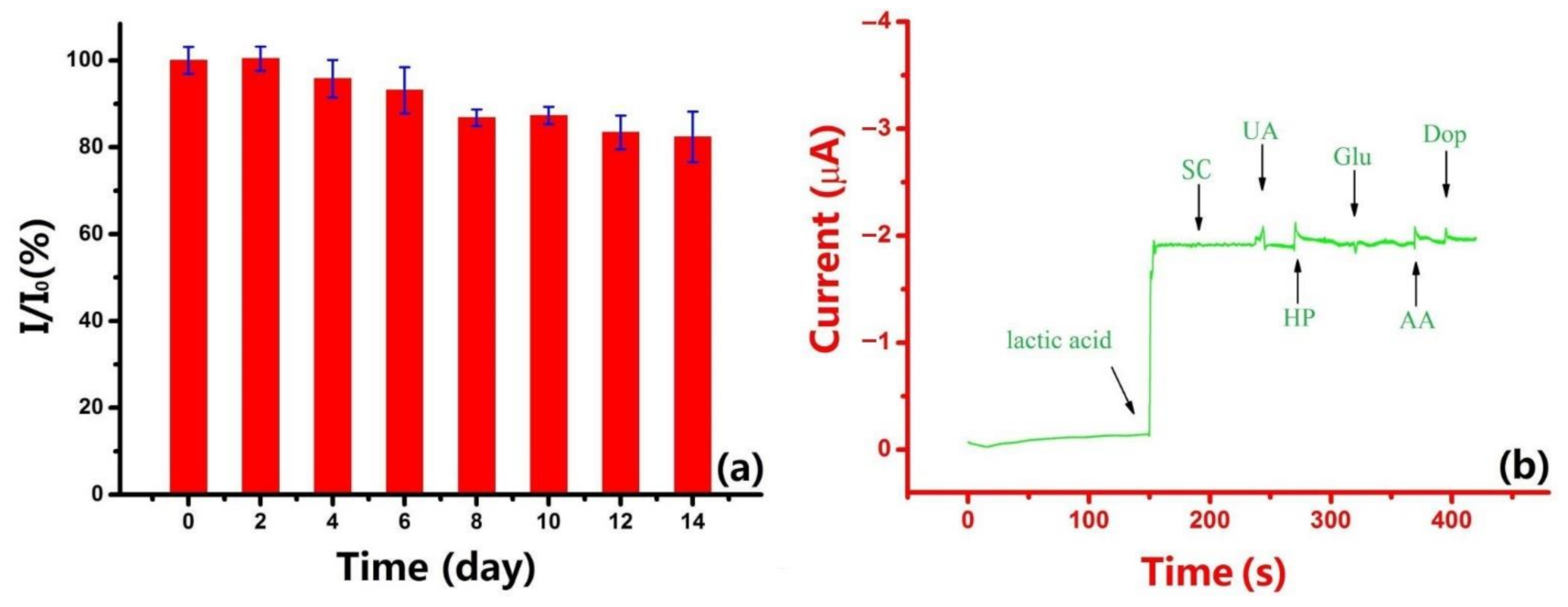
3.6. Reproducibility, Stability, and Interference Analysis
3.7. Real Samples Analysis
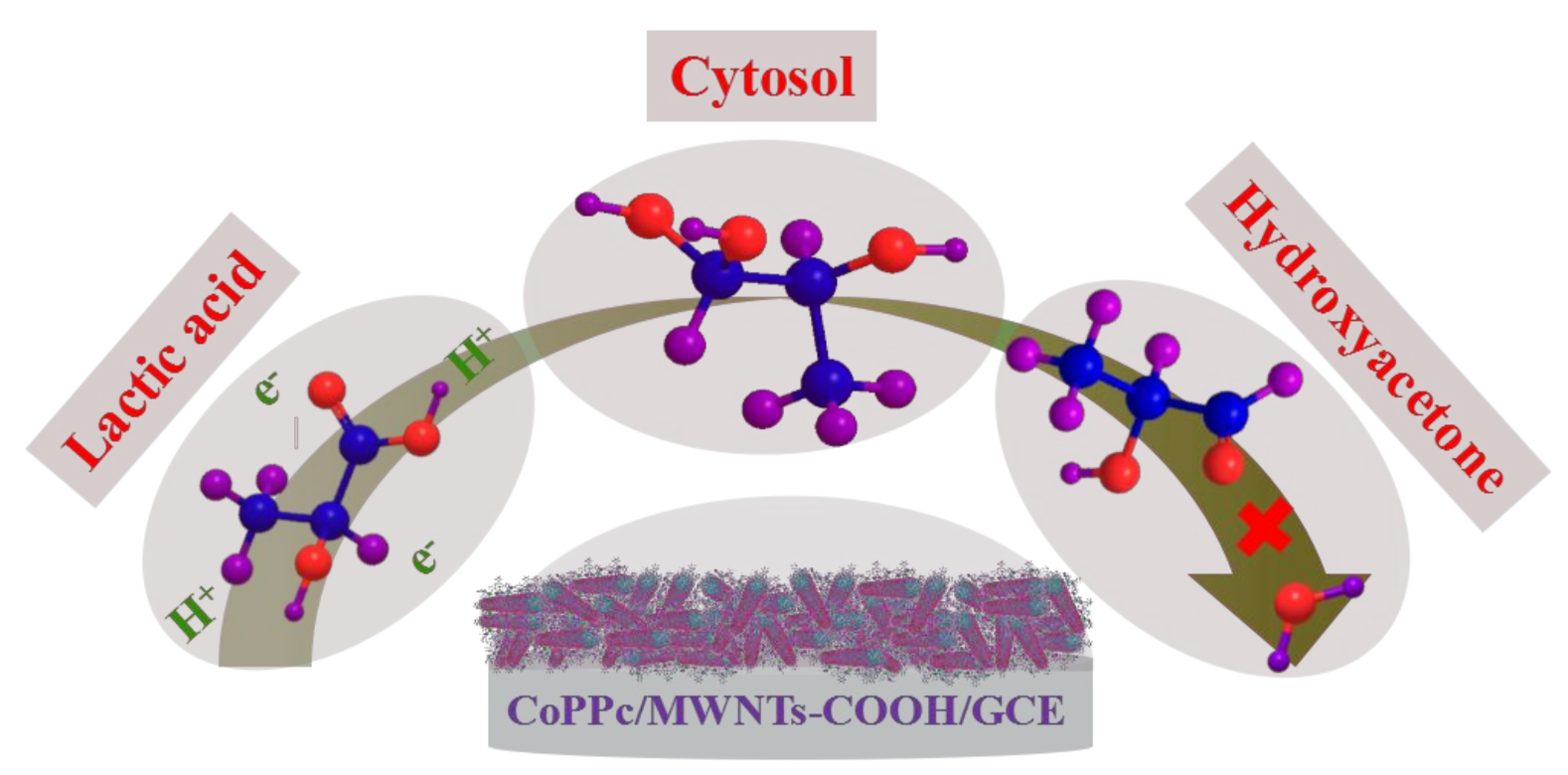
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liaud, N.; Navarro, D.; Vidal, N.; Sigoillot, J.C.; Raouche, S. High throughput automated colorimetric method for the screening of l-lactic acid producing microorganisms. Methods X 2014, 1, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Borshchevskaya, L.N.; Gordeeva, T.L.; Kalinina, A.N.; Sineokii, S.P. Spectrophotometric determination of lactic acid. J. Anal. Chem. 2019, 71, 755–758. [Google Scholar] [CrossRef]
- Wijayasinghe, R.; Bogahawaththa, D.; Huppertz, T.; Chandrapala, J.; Vasiljevic, T. Influence of lactic, citric and phosphoric acids on the properties of concentrated lactose solutions. Food Chem. 2019, 293, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Zuriarrain, A.; Zuriarrain, J.; Puerias, A.I.; Duenas, M.T.; Berregi, I. Quantitative determination of lactic and acetic acids in cider by H-1 NMR spectrometry. Food Control 2015, 52, 49–53. [Google Scholar] [CrossRef]
- Czarnecka, M.; Czarnecki, Z.; Roszyk, H. Determination of lactic acid in plant material. Zywnosc 2000, 7, 92–101. [Google Scholar]
- Sokullu, E.; Palabiyik, I.M.; Onur, F.; Boyaci, I.H. Chemometric methods for simultaneous quantification of lactic, malic and fumaric acids. Eng. Life Sci. 2010, 10, 297–303. [Google Scholar] [CrossRef]
- Alam, F.; RoyChoudhury, S.; Jalal, A.H.; Umasankar, Y.; Forouzanfar, S.; Akter, N.; Bhansali, S.; Pala, N. Lactate biosensing: The emerging point-of-care and personal health monitoring. Biosens. Bioelectron. 2018, 117, 818–829. [Google Scholar] [CrossRef]
- Pundir, C.S.; Narwal, V.; Batra, B. Determination of lactic acid with special emphasis on biosensing methods: A review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef]
- Calio, A.; Dardano, P.; Di Palma, V.; Bevilacqua, M.F.; Di Matteo, A.; Iuele, H.; De Stefano, L. Polymeric microneedles based enzymatic electrodes for electrochemical biosensing of glucose and lactic acid. Sens. Actuators B Chem. 2016, 236, 343–349. [Google Scholar] [CrossRef]
- Vargas, E.; Ruiz, M.A.; Campuzano, S.; de Rivera, G.G.; Lopez-Colino, F.; Reviejo, A.J.; Pingarron, J.M. Implementation of a new integrated lactic acid biosensor in a semiautomatic FIA system for the simultaneous determination of lactic acid enantiomers, Application to the analysis of beer samples. Talanta 2016, 152, 147–154. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Annalakshmi, M.; Chen, S.M.; Chen, T.W. Ultrasensitive non-enzymatic electrochemical sensing of glucose in noninvasive samples using interconnected nanosheets-like NiMnO3 as a promising electrocatalyst. Sens. Actuators B Chem. 2019, 299, 126974. [Google Scholar] [CrossRef]
- Hu, Q.Y.; Yang, J.Y.; Zheng, Z.Y.; Ding, Y.P.; Chen, Y.W.; Gao, W.H. In situ H2O2 generation with gold nanoflowers as the coreactant accelerator for enzyme-free electrochemiluminescent immunosensing. Biosens. Bioelectron. 2019, 143, 111627. [Google Scholar] [CrossRef]
- Hao, X.K.; Jia, J.C.; Chang, Y.; Jia, M.L.; Wen, Z.H. Monodisperse copper selenide nanoparticles for ultrasensitive and selective non-enzymatic glucose biosensor. Electrochim. Acta 2019, 327, 135020. [Google Scholar] [CrossRef]
- Vasuki, K.; Siva, G.; Balasubramani, A.; Pannipara, M.; Al-Sehemi, A.G.; Xia, Y.; Fang, R.Y.; Yoo, D.J.; Kumar, T.R.; Ramachandran, R. Surfactant and binder free hierarchical NCNPs@CuO nanostructures on ITO for the cost effective enzyme-free glucose sensor applications. Appl. Phys. A-Mater. Sci. Process. 2019, 125, 384. [Google Scholar] [CrossRef]
- Rabti, A.; Raouafi, N.; Merkoci, A. Bio(Sensing) devices based on ferrocene-functionalized graphene and carbon nanotubes. Carbon 2016, 108, 481–514. [Google Scholar] [CrossRef]
- Hu, Z.F.; Peng, W.C.; Tian, W.; Wang, F.F.; Kang, X.; Zhang, Y.X.; Yue, H.R.; Zhang, L.L.; Ji, J.Y.; Wang, S.B. A general strategy for in-situ fabrication of uniform carbon nanotubes on three-dimensional carbon architectures for electrochemical application. Appl. Surf. Sci. 2019, 496, 143704. [Google Scholar] [CrossRef]
- Kanninen, P.; Eriksson, B.; Davodi, F.; Buan, M.E.M.; Sorsa, O.; Kallio, T.; Lindström, R.W. Carbon corrosion properties and performance of multi-walled carbon nanotube support with and without nitrogen-functionalization in fuel cell electrodes. Electrochim. Acta 2020, 332, 135384. [Google Scholar] [CrossRef]
- Anojčić, J.; Guzsvány, V.; Kónya, Z.; Mikov, M. Rapid, trace-level direct cathodic voltammetric determination of dopamine by oxidized multiwalled carbon nanotube modified carbon paste electrode in selected samples of pharmaceutical importance. Ionics 2019, 25, 6093–6106. [Google Scholar] [CrossRef]
- Nagata, M.; Shukla, S.; Nakanishi, Y.; Liu, Z.; Lin, Y.C.; Shiga, T.; Nakamura, Y.; Koyama, T.; Kishida, H.; Inoue, T. Isolation of single-wired transition-metal monochalcogenides by carbon nanotubes. Nano Lett. 2019, 19, 4845–4851. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Gao, E.L.; Xu, Z.P. Interfacial failure boosts mechanical energy dissipation in carbon nanotube films under ballistic impact. Carbon 2019, 146, 139–146. [Google Scholar] [CrossRef]
- Zamani, F.G.; Moulahoum, H.; Ak, M.; Demirkol, D.O.; Timur, S. Current trends in the development of conducting polymers-based biosensors. TrAC Trend. Anal. Chem. 2019, 118, 264–276. [Google Scholar] [CrossRef]
- El Rhazi, M.; Majid, S.; Elbasri, M.; Salih, F.E.; Oularbi, L.; Lafdi, K. Recent progress in nanocomposites based on conducting polymer: Application as electrochemical sensors. Int. Nano Lett. 2018, 8, 79–99. [Google Scholar] [CrossRef]
- Sterby, M.; Emanuelsson, R.; Mamedov, F.; Strømme, M.; Sjödin, M. Investigating electron transport in a PEDOT/Quinone conducting redox polymer with in situ methods. Electrochim. Acta 2019, 308, 277–284. [Google Scholar] [CrossRef]
- Wang, J.F.; Wang, J.R.; Kong, Z.; Lv, K.L.; Teng, C.; Zhu, Y. Conducting-polymer-based materials for electrochemical energy conversion and storage. Adv. Mater. 2017, 29, 703044. [Google Scholar] [CrossRef]
- Li, M.R.; Wang, W.; Chen, Z.; Song, Z.L.; Luo, X.L. Electrochemical determination of paracetamol based on Au@graphene core-shell nanoparticles doped conducting polymer PEDOT nanocomposite. Sens. Actuators B Chem. 2018, 260, 778–785. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, W.; Lin, G.Y.; Wu, B.; Xu, M.Y.; Huang, X.W.; Luo, J.; Zhu, Y.; Liu, X.Y. Long conducting and water-compatible polymer/carbon nanotubes nanocomposite with “beads-on-a-string” structure as a highly effective electrochemical sensing material. ACS Sustain. Chem. Eng. 2019, 7, 3556–3566. [Google Scholar] [CrossRef]
- Heredia, D.A.; Lopez, E.J.G.; Durantini, E.N.; Durantini, J.; Dittrich, T.; Rappich, J.; Macor, L.; Solis, C.; Morales, G.M.; Gervaldo, M.; et al. Electrochemical, spectroelectrochemical and surface photovoltage study of ambipolar C60-EDOT and C60-Carbazole based conducting polymers. Electrochim. Acta 2019, 311, 178–191. [Google Scholar] [CrossRef]
- Zhou, H.H.; Han, G.Y. One-step fabrication of heterogeneous conducting polymers-coated graphene oxide/carbon nanotubes composite films for high-performance supercapacitors. Electrochim. Acta 2016, 192, 448–455. [Google Scholar] [CrossRef]
- Yen, S.C.; Liu, Z.W.; Juang, R.S.; Sahoo, S.; Huang, C.H.; Chen, P.L.; Hsiao, Y.S.; Fang, J.T. Carbon nanotube/conducting polymer hybrid nanofibers as novel organic bioelectronic interfaces for efficient removal of protein-bound uremic toxins. ACS Appl. Mater. Interfaces 2019, 11, 43843–43856. [Google Scholar] [CrossRef]
- Liu, J.H.; Xu, X.Y.; Yu, J.L.; Hong, J.L.; Liu, C.; Ouyang, X.; Lei, S.; Meng, X.; Tang, J.N.; Chen, D.Z. Facile construction of 3D porous carbon nanotubes/polypyrrole and reduced graphene oxide on carbon nanotube fiber for high-performance asymmetric supercapacitors. Electrochim. Acta 2019, 314, 9–19. [Google Scholar] [CrossRef]
- Fan, P.; Wang, S.; Liu, H.; Liao, L.B.; Lv, G.C.; Mei, L.F. Polyaniline nanotube synthesized from natural tubular halloysite template as high performance pseudocapacitive electrode. Electrochim. Acta 2020, 331, 135259. [Google Scholar] [CrossRef]
- Nxele, S.R.; Oluwole, D.O.; Nyokong, T. Electrocatalytic activity of a push pull Co(II) phthalocyanine in the presence of graphitic carbon nitride quantum dots. Electrochim. Acta 2019, 326, 134978. [Google Scholar] [CrossRef]
- Xia, Y.J.; Kashtanov, S.; Yu, P.F.; Chang, L.Y.; Feng, K.; Zhong, J.; Guo, J.H.; Sun, X.H. Identification of dual-active sites in cobalt phthalocyanine for electrochemical carbon dioxide reduction. Nano Energy 2020, 67, 104163. [Google Scholar] [CrossRef]
- Lin, L.; Li, H.B.; Yan, C.C.; Li, H.F.; Si, R.; Li, M.R.; Xiao, J.P.; Wang, G.X.; Bao, X.H. Synergistic catalysis over iron-nitrogen sites anchored with cobalt phthalocyanine for efficient CO2 electroreduction. Adv. Mater. 2019, 31, 1903470. [Google Scholar] [CrossRef]
- Wang, M.; Torbensen, K.; Salvatore, D.; Ren, S.X.; Joulie, D.; Dumoulin, F.; Mendoza, D.; Lassalle-Kaiser, B.; Isci, U.; Berlinguette, C.P. CO2 electrochemical catalytic reduction with a highly active cobalt phthalocyanine. Nat. Commun. 2019, 10, 3602. [Google Scholar] [CrossRef]
- Nemakal, M.; Aralekallu, S.; Mohammed, I.; Pari, M.; Sannegowda, L.K. Nanomolar detection of 4-aminophenol using amperometric sensor based on a novel phthalocyanine. Electrochim. Acta 2019, 318, 342–353. [Google Scholar] [CrossRef]
- Gulati, P.; Kaur, P.; Rajam, M.V.; Srivastava, T.; Mishra, P.; Islam, S.S. Vertically aligned multi-walled carbon nanotubes based flexible immunosensor for extreme low level detection of multidrug resistant leukemia cells. Sens. Actuators B Chem. 2019, 301, 127047. [Google Scholar] [CrossRef]
- Palomaki, T.; Peltola, E.; Sainio, S.; Wester, N.; Pitkanen, O.; Kordas, K.; Koskinen, J.; Laurila, T. Unmodified and multi-walled carbon nanotube modified tetrahedral amorphous carbon (ta-C) films as in vivo sensor materials for sensitive and selective detection of dopamine. Biosens. Bioelectron. 2019, 123, 281–284. [Google Scholar] [CrossRef]
- Shinde, P.A.; Seo, Y.; Ray, C.; Jun, S.C. Direct growth of WO3 nanostructures on multi-walled carbon nanotubes for high-performance flexible all-solid-state asymmetric supercapacitor. Electrochim. Acta 2019, 308, 231–242. [Google Scholar] [CrossRef]
- Ulusoy, H.İ.; Gülle, S.; Yilmaz, E.; Soylak, M. Trace determination of vitamin B12 in food samples by using Fe3O4 magnetic particles including multiwalled carbon nanotubes and nanodiamonds. Anal. Methods 2019, 11, 5108. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Xiao, H.L.; Cao, L.L.; Qin, H.S.; Wei, S.S.; Gu, M.; Zhao, F.J.; Chen, Z.C. Non-enzymatic lactic acid sensor based on AuPtNPs functionalized MoS2 nanosheet as electrode modified materials. J. Electroanal. Chem. 2021, 903, 115806. [Google Scholar] [CrossRef]
- Istrate, O.; Rotariu, L.; Bala, C. Amperometric L-Lactate Biosensor Based upon a Gold Nanoparticles/Reduced Graphene Oxide/Polyallylamine Hydrochloride Modified Screen-Printed Graphite Electrode. Chemosensors 2021, 9, 74. [Google Scholar] [CrossRef]
- Zhao, Z.; Kong, Y.; Liu, C.; Huang, G.S.; Xiao, Z.J.; Zhu, H.Q.; Bao, Z.H.; Mei, Y.F. Atomic layer deposition-assisted fabrication of 3D Co-doped carbon framework for sensitive enzyme-free lactic acid sensor. Chem. Eng. J. 2021, 417, 129285. [Google Scholar] [CrossRef]
- Nien, Y.H.; Kang, Z.X.; Chou, J.C.; Ho, C.S.; Lai, C.H.; Kuo, P.Y.; Chen, Y.Y.; Huang, Y.H. Study of the Nonenzymatic CZO Lactic Acid Sensor Modified by Graphitic Carbon Nitride and Iron-Platinum Nanoparticles. IEEE Trans. Electron Devices 2021, 68, 5142–5148. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Chen, A.C. Design of an enzyme-mimicking NiO@Au nanocomposite for the sensitive electrochemical detection of lactic acid in human serum and urine. Electrochim. Acta 2021, 368, 137612. [Google Scholar] [CrossRef]
- Zheng, H.J.; Zhang, S.; Liu, X.Q.; Zhou, Y.M.; Alwarappan, S. Synthesis of a PEDOT-TiO2 heterostructure as a dual biosensing platform operating via photoelectrochemical and electrochemical transduction mode. Biosens. Bioelectron. 2020, 162, 112234. [Google Scholar] [CrossRef]
- Narayanan, J.S.; Slaughter, G. Lactic Acid Biosensor Based on Lactate Dehydrogenase Immobilized on Au Nanoparticle Modified Microwire Electrode. IEEE Sens. J. 2020, 8, 4034–4040. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Tsao, P.-K.; Rinawati, M.; Chen, K.-J.; Chen, K.-Y.; Chang, C.-Y.; Yeh, M.-H. Designing ZIF-67 derived NiCo layered double hydroxides with 3D hierarchical structure for Enzyme-free electrochemical lactate monitoring in human sweat. Chem. Eng. J. 2022, 427, 131687. [Google Scholar] [CrossRef]
- GB 1886.173-2016; Food Additive—Lactic Acid. National Standards of People’s Republic of China: Beijing, China, 2016.
| Electrode | Sensor Type | Sensitivity (µA/mM·cm2) | LOD (μM) | Linear Range (mM) | Ref. |
|---|---|---|---|---|---|
| MoS2-AuPt@SPE | Nonenzymatic | - | 0.33 | 0.005–3 | [42] |
| FePt NPs-g-C3N4/CZO | Nonenzymatic | - | 110 | 0.2–10 | [43] |
| Co-NCF composite | Nonenzymatic | 1068 | 13.7 | 0.1–1 | [44] |
| AuNPs-ERGO-PAH | Enzymatic | 0.28 | 1 | 0.5–3 | [45] |
| GC/NiO@Au | Nonenzymatic | 8 | 11.6 | 0.1–500 | [46] |
| NAD+|LDH|PEDOT-TiONWs|FTO | Enzymatic | 0.1386 | 0.08 | 0.0005–0.3 | [47] |
| AuNP-cysteamine-LDH | Enzymatic | 73.16 | 411 | 0.5–7 | [48] |
| ZIF-67 derived NiCo LDH | Nonenzymatic | 83.98 | 399 | 2.0–26.1 | [49] |
| CoPPc/MWCNTs-COOH/GCE | Nonenzymatic | - | 2 | 0.01–0.24 | This work |
| Rice Wine Samples | Lactic Acid Concentration (mM) | Error (%) | Recovery (%) | |
|---|---|---|---|---|
| Reference Method | Sensor | |||
| 1 | 0.89 | 0.93 | 4.49% | 101.9% |
| 2 | 1.67 | 1.60 | −4.19% | 100.1% |
| 3 | 1.24 | 1.31 | 5.65% | 99.6% |
| 4 | 0.68 | 0.72 | 5.88% | 103.6% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, W.; Mai, J.; Wei, Z. Nonenzymatic Lactic Acid Detection Using Cobalt Polyphthalocyanine/Carboxylated Multiwalled Carbon Nanotube Nanocomposites Modified Sensor. Chemosensors 2022, 10, 83. https://doi.org/10.3390/chemosensors10020083
Shao W, Mai J, Wei Z. Nonenzymatic Lactic Acid Detection Using Cobalt Polyphthalocyanine/Carboxylated Multiwalled Carbon Nanotube Nanocomposites Modified Sensor. Chemosensors. 2022; 10(2):83. https://doi.org/10.3390/chemosensors10020083
Chicago/Turabian StyleShao, Wenqing, Jiayu Mai, and Zhenbo Wei. 2022. "Nonenzymatic Lactic Acid Detection Using Cobalt Polyphthalocyanine/Carboxylated Multiwalled Carbon Nanotube Nanocomposites Modified Sensor" Chemosensors 10, no. 2: 83. https://doi.org/10.3390/chemosensors10020083
APA StyleShao, W., Mai, J., & Wei, Z. (2022). Nonenzymatic Lactic Acid Detection Using Cobalt Polyphthalocyanine/Carboxylated Multiwalled Carbon Nanotube Nanocomposites Modified Sensor. Chemosensors, 10(2), 83. https://doi.org/10.3390/chemosensors10020083







