Porphyrin-Based Metal–Organic Frameworks for Efficient Electrochemiluminescent Chiral Recognition of Tyrosine Enantiomers
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Measurement Characterization
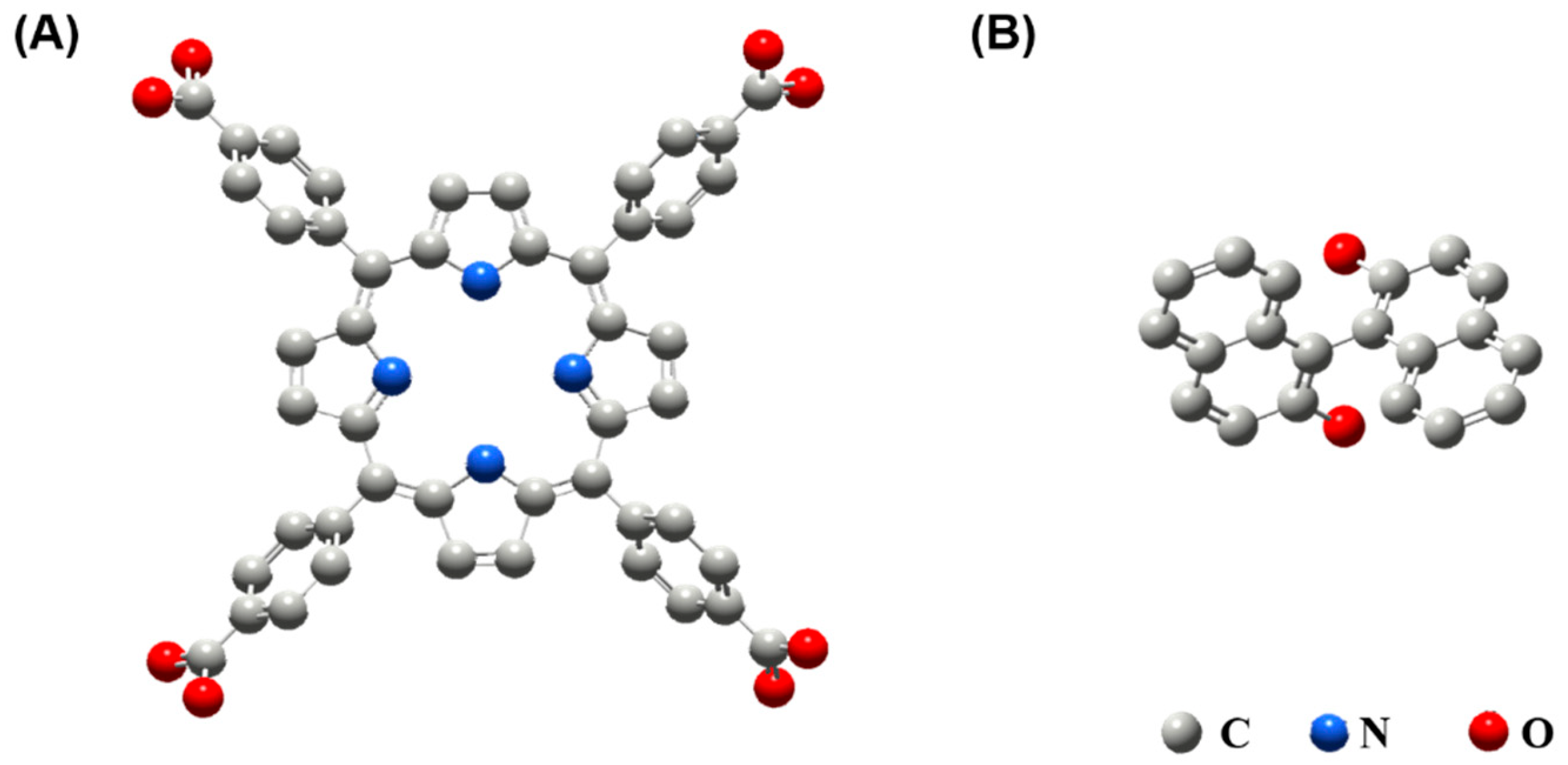
2.3. Synthesis of TCPP-Zn-(S)-BINOL
2.4. Electrode Preparation and Modification
2.5. ECL Chiral Recognition of Tyrosine (Tyr) Enantiomers
3. Results and Discussion
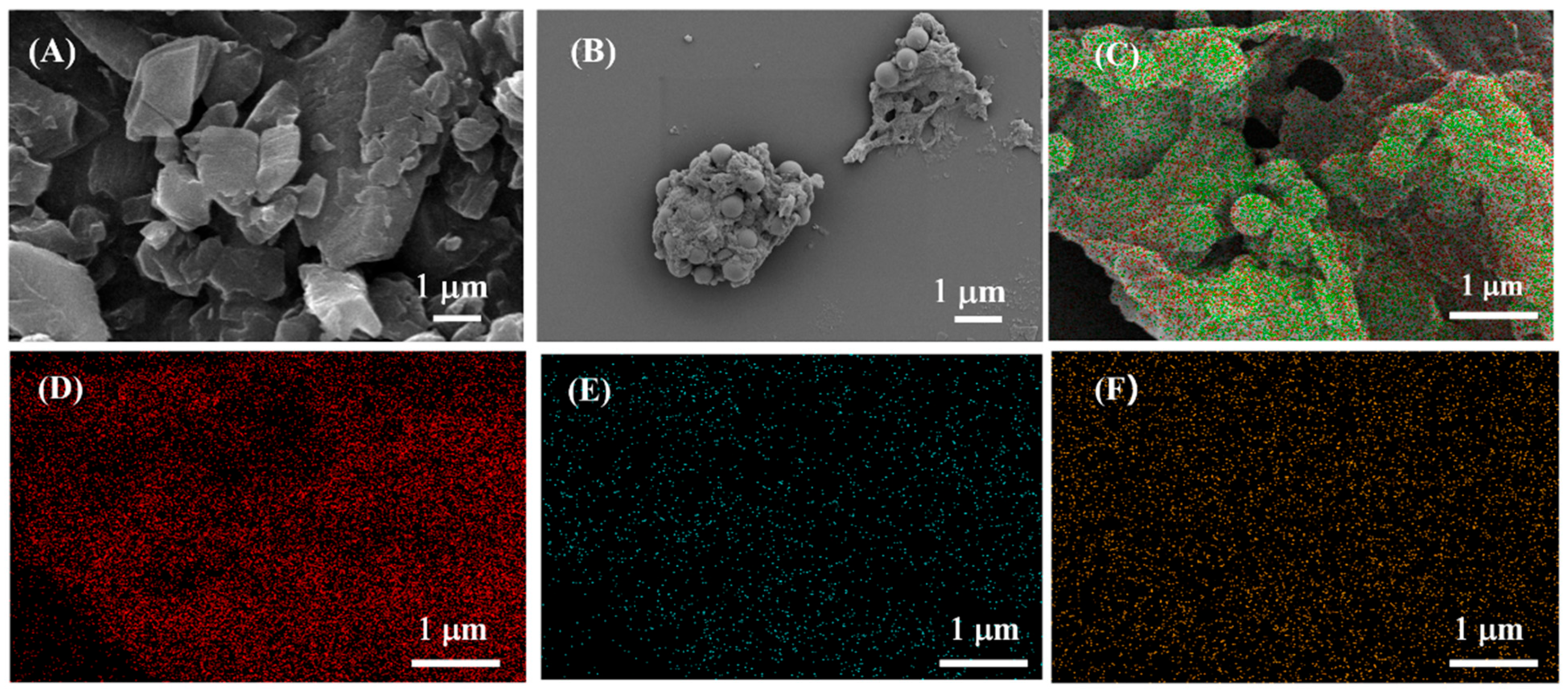
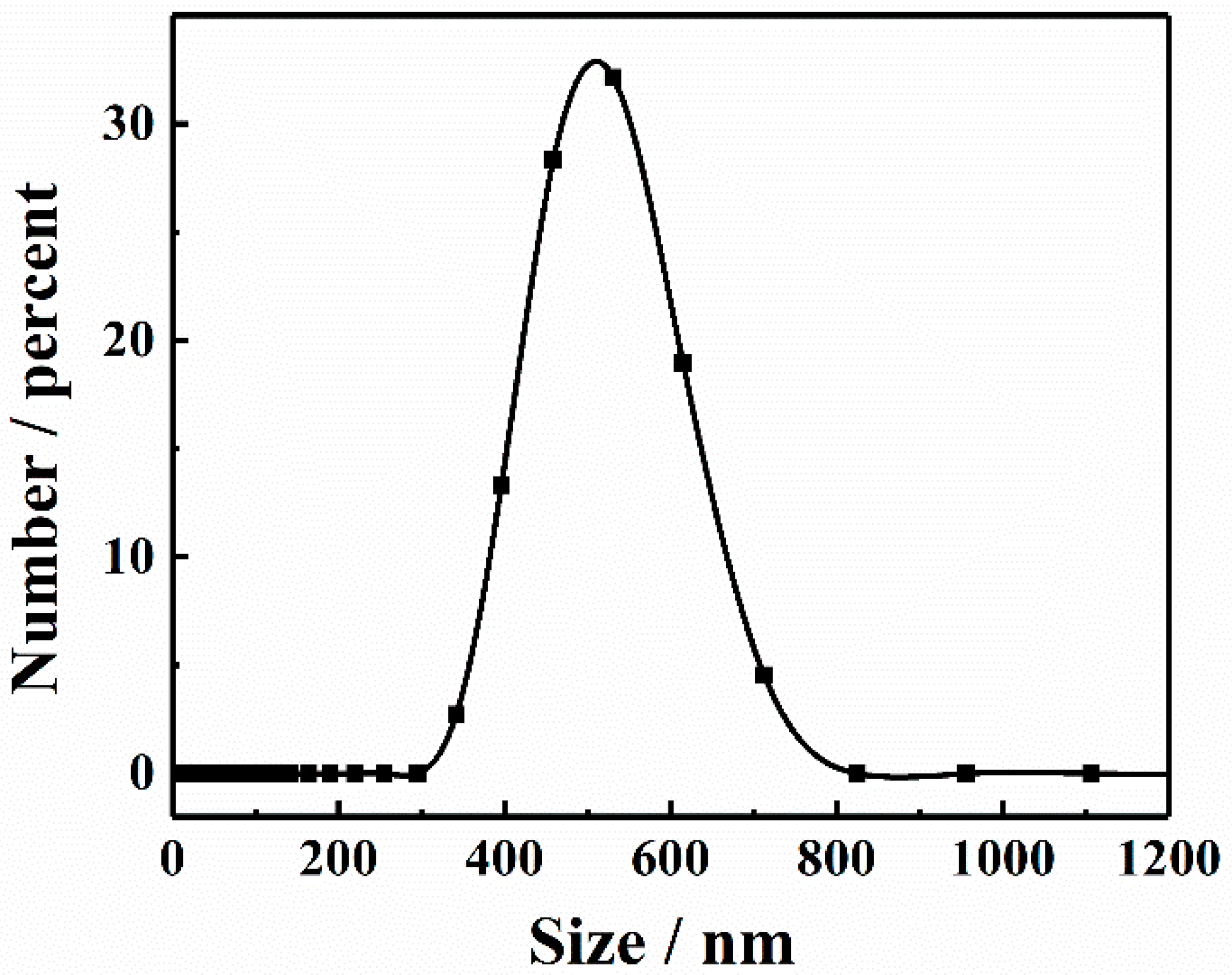
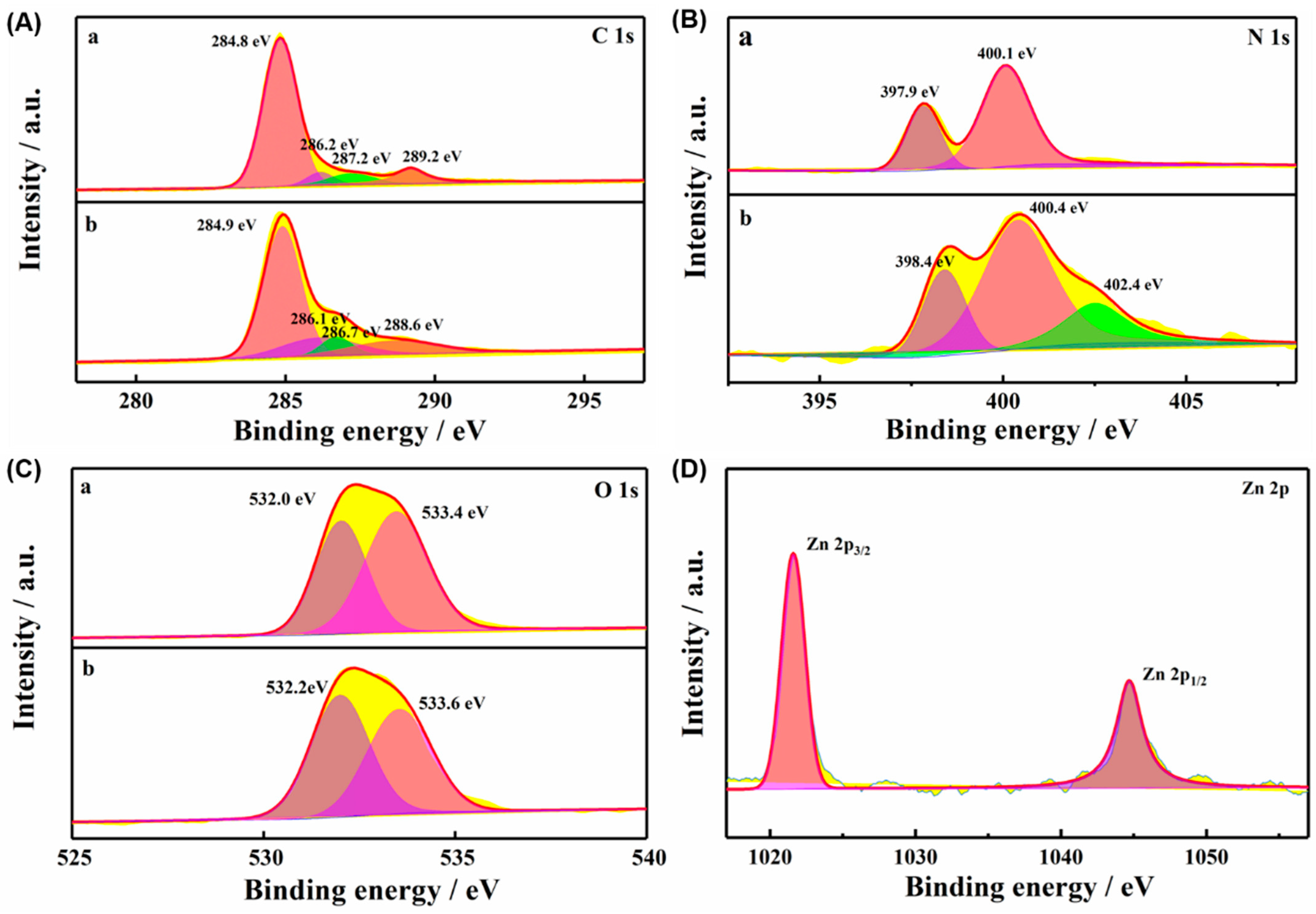
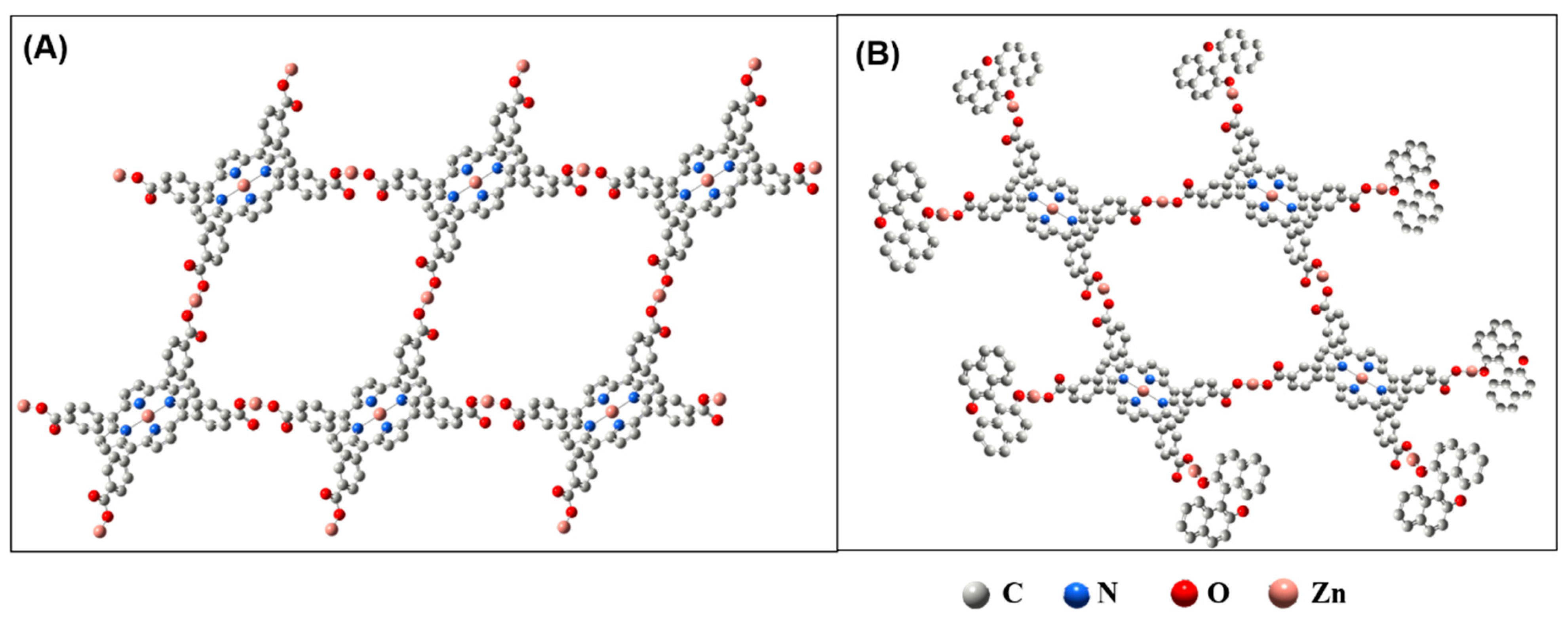
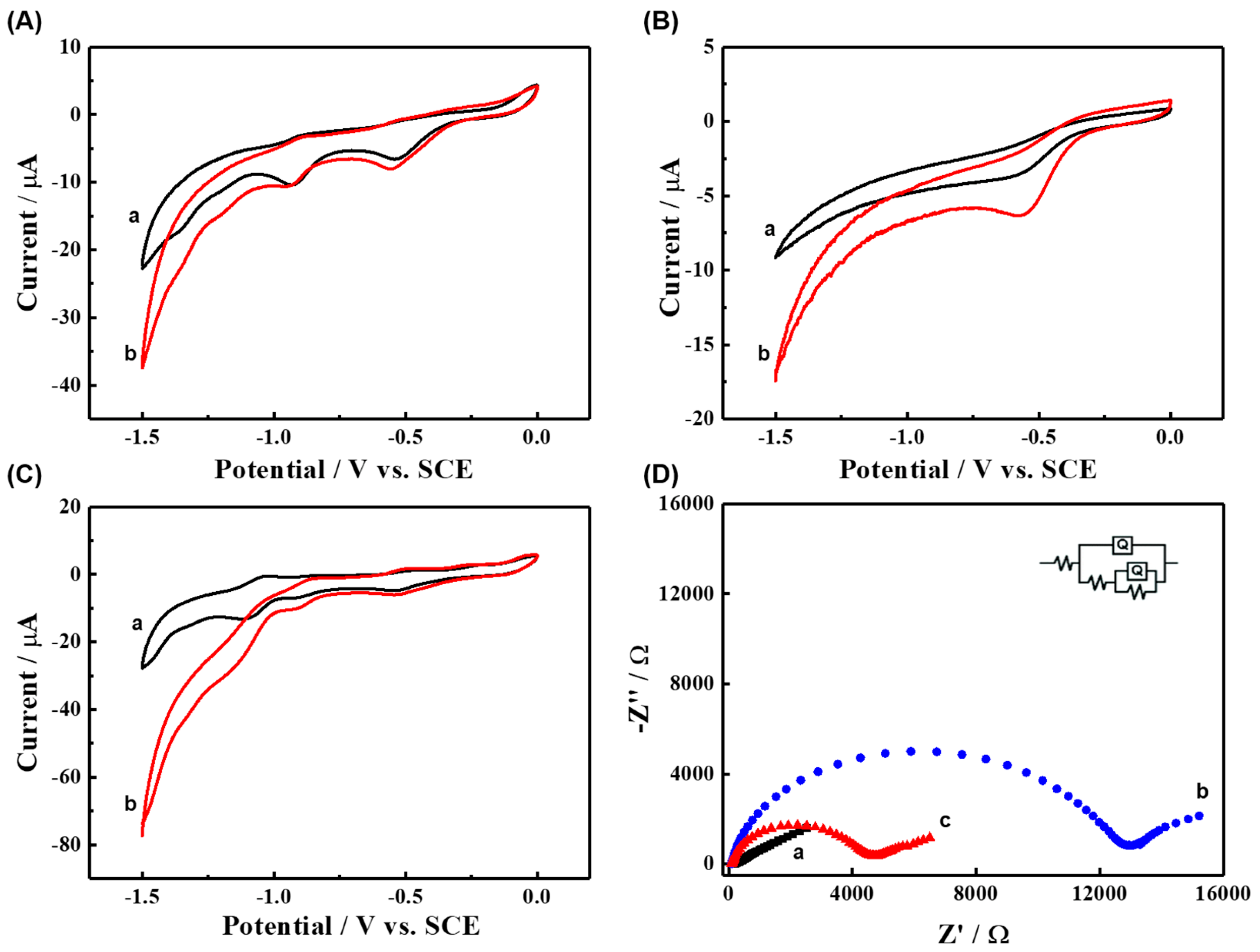
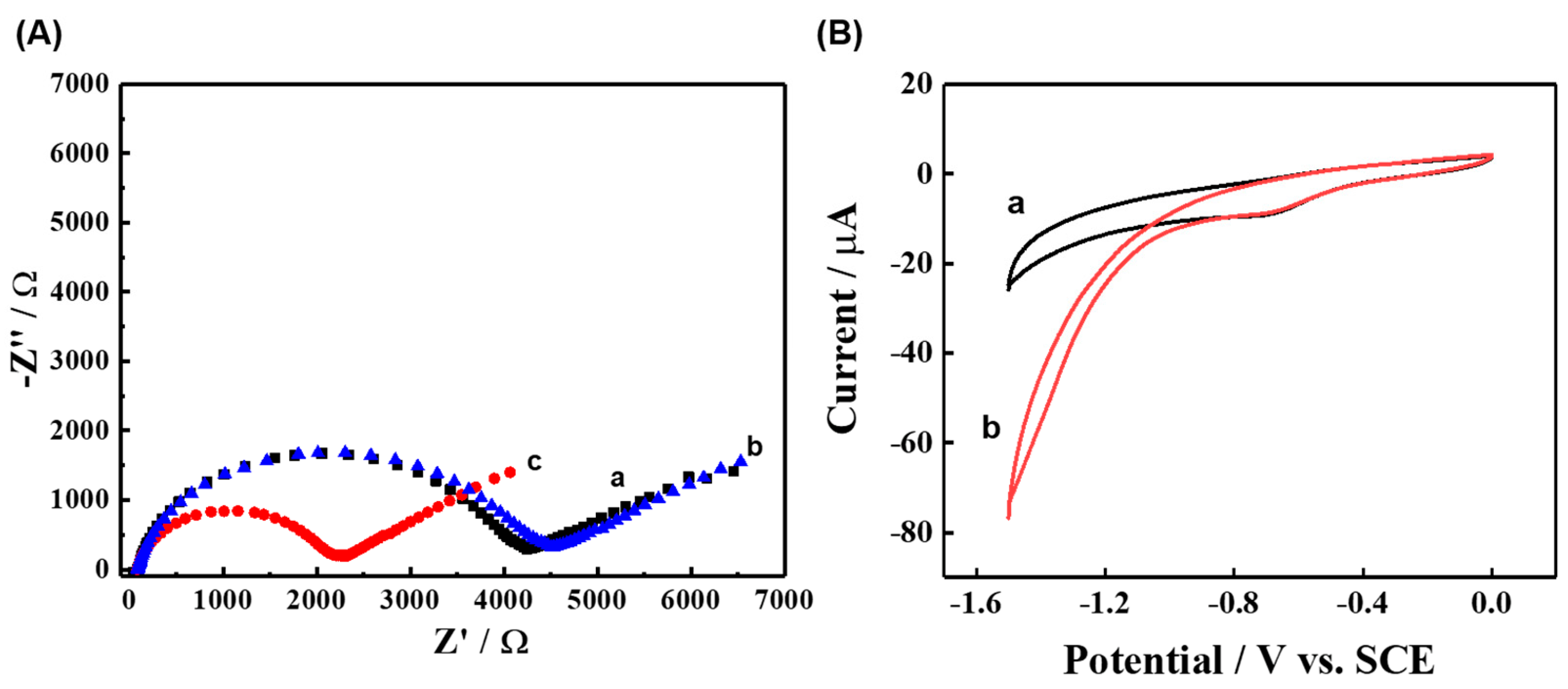
3.1. Characterization of TCPP-Zn-(S)-BINOL
3.2. Optimization of Experimental Conditions
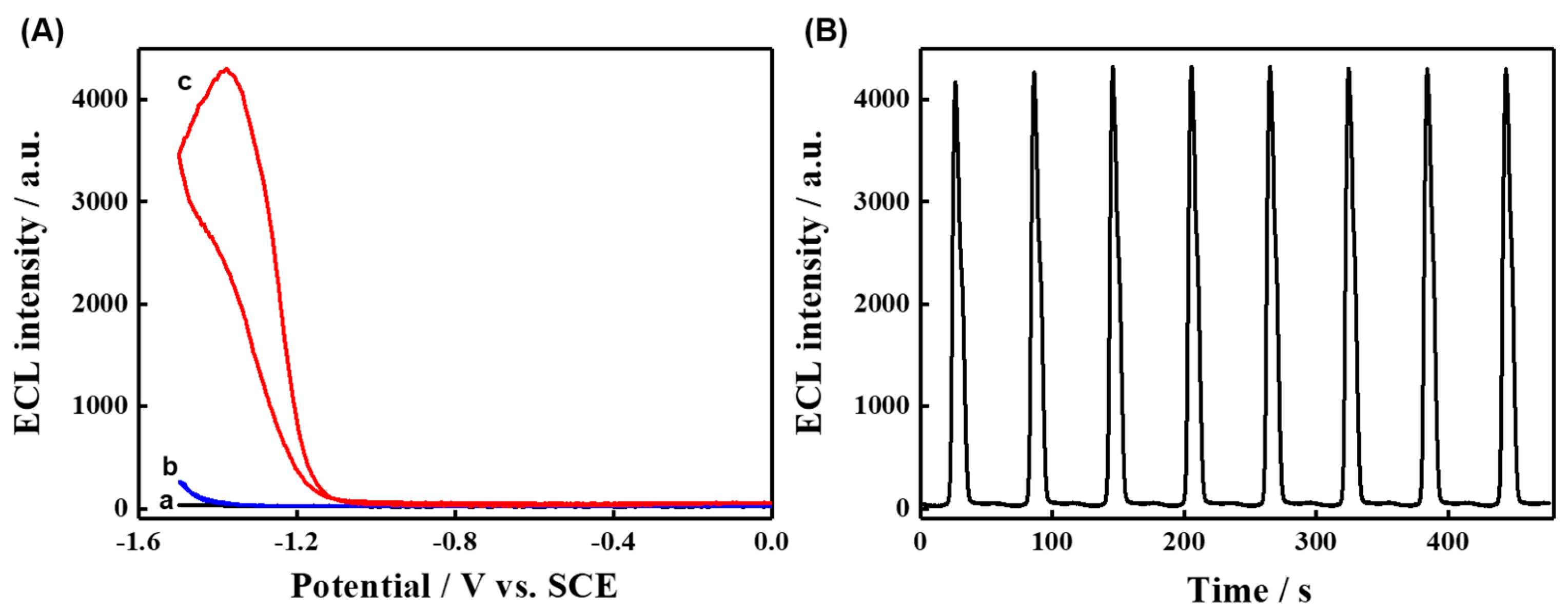
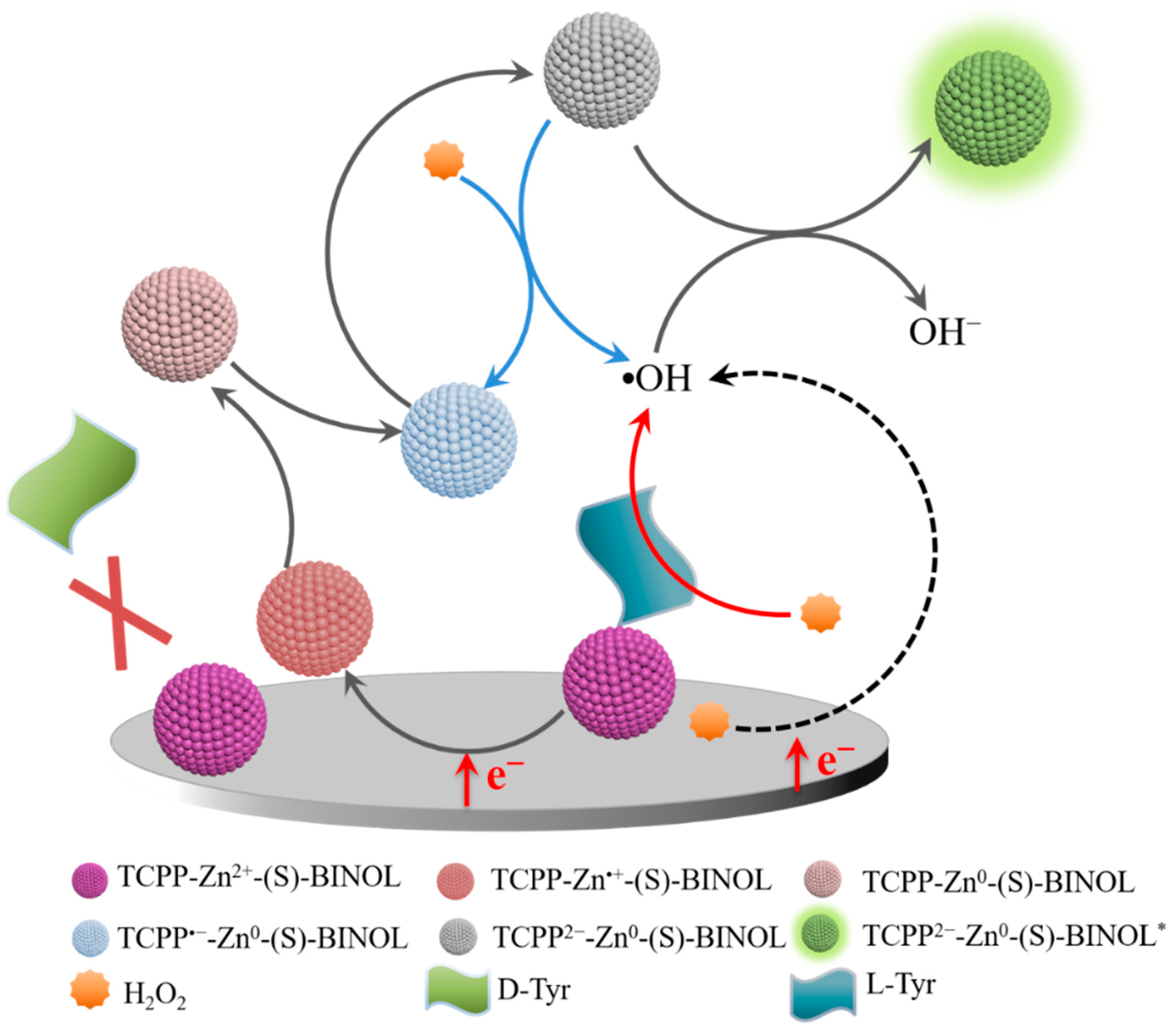
3.3. ECL Behavior and Mechanism of TCPP-Zn-(S)-BINOL
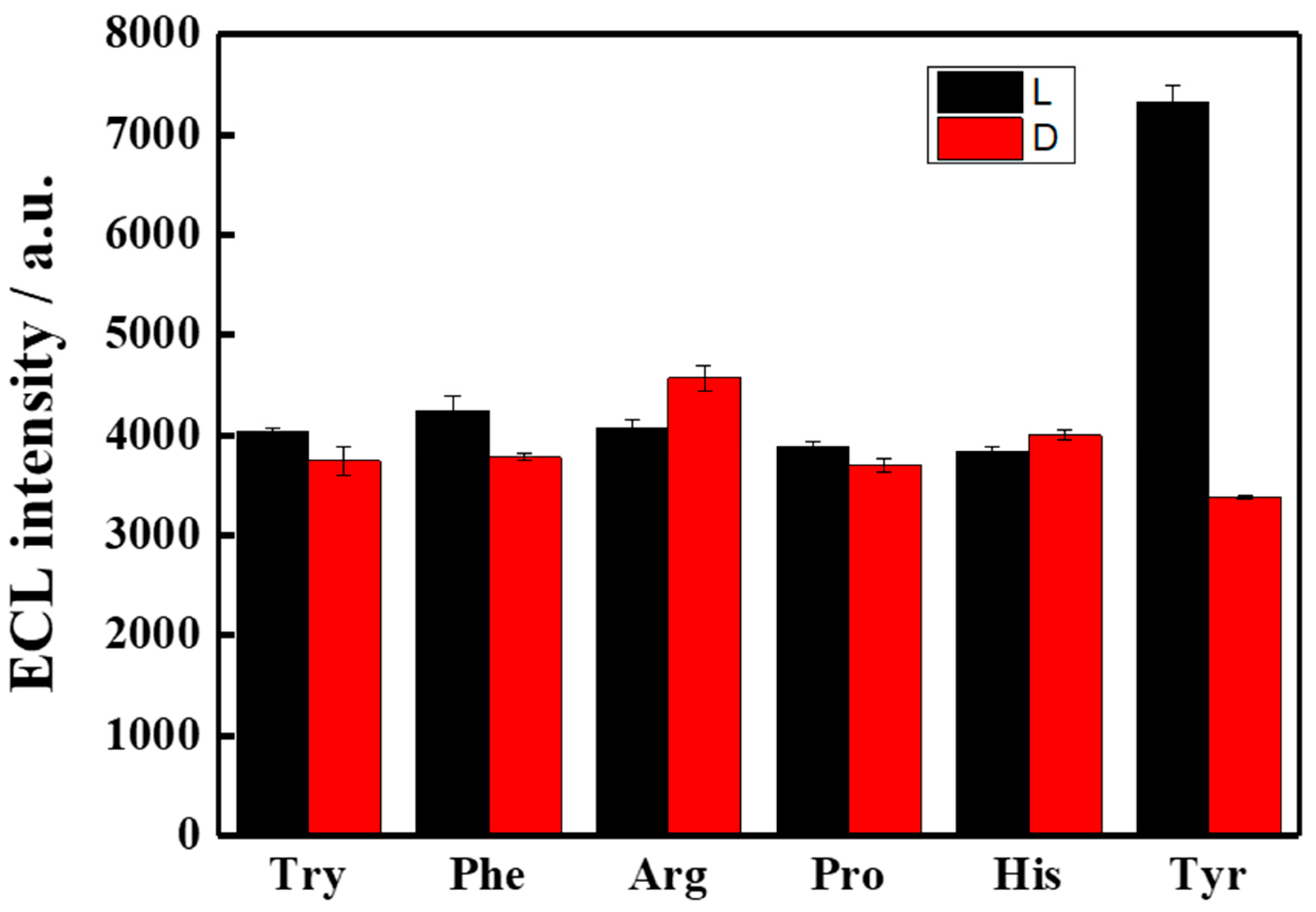
3.4. ECL Enantioselective Recognition of Tyr Enantiomers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanganyado, E.; Lu, Z.; Fu, Q.; Schlenk, D.; Gan, J. Chiral pharmaceuticals: A review on their environmental occurrence and fate processes. Water Res. 2017, 124, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.P.; Liu, C.M.; Meng, X.Y.; Wang, J.W.; Qiu, J.D. A novel open-tubular capillary electrochromatography using β-cyclodextrin functionalized graphene oxide-magnetic nanocomposites as tunable stationary phase. J. Chromatogr. A 2012, 1266, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Kingsford, O.J.; Yi, Y.; Wong, K.Y. Recent advances in electrochemical chiral recognition. J. Electrochem. Soc. 2019, 166, H205. [Google Scholar] [CrossRef]
- Zhao, Y.; Cui, L.; Ke, W.; Zheng, F.; Li, X. Electroactive Au@ Ag nanoparticle assembly driven signal amplification for ultrasensitive chiral recognition of d-/l-Trp. ACS Sustain. Chem. Eng. 2019, 7, 5157–5166. [Google Scholar] [CrossRef]
- Liu, H.; Shao, J.; Shi, L.; Ke, W.; Zheng, F.; Zhao, Y. Electroactive NPs and D-amino acids oxidase engineered electrochemical chiral sensor for D-alanine detection. Sens. Actuators B 2020, 304, 127333. [Google Scholar] [CrossRef]
- Wu, S.; Ye, Q.; Wu, D.; Tao, Y.; Kong, Y. Enantioselective recognition of chiral tryptophan with achiral glycine through the strategy of chirality transfer. Anal. Chem. 2020, 92, 11927–11934. [Google Scholar] [CrossRef]
- Myrgorodska, I.; Meinert, C.; Martins, Z.; le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Quantitative enantioseparation of amino acids by comprehensive two-dimensional gas chromatography applied to non-terrestrial samples. J. Chromatogr. A 2016, 1433, 131–136. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, W.X.; Chen, Z.; Rustum, A.M. Separation of chiral primary amino compounds by forming a sandwiched complex in reversed-phase high performance liquid chromatography. J. Chromatogr. A 2010, 1217, 4965–4970. [Google Scholar] [CrossRef]
- Feizi, F.; Shamsipur, M.; Barati, A.; Gholivand, M.B.; Mousavi, F. Chiral recognition and quantitative analysis of tyrosine enantiomers using L-cysteine capped CdTe quantum dots: Circular dichroism, fluorescence, and theoretical calculation studies. Microchem. J. 2020, 158, 105168. [Google Scholar] [CrossRef]
- Zhao, Q.; Wu, D.; Yin, Z.Z.; Cai, W.; Zhou, H.; Kong, Y. Fluorometric discrimination of tyrosine isomers based on the inner filter effect of chiral Au nanoparticles on MoS2 quantum dots. Anal. Methods 2021, 13, 2290–2296. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Z.; Wang, X.; Zeng, S.; Sun, C.; Pan, Y. Pair of stereodynamic chiral benzylicaldehyde probes for determination of absolute configuration of amino acid residues in peptides by mass spectrometry. Anal. Chem. 2017, 89, 11902–11907. [Google Scholar] [CrossRef]
- Wang, L.; Chai, Y.; Ni, Z.; Wang, L.; Hu, R.; Pan, Y.; Sun, C. Qualitative and quantitative analysis of enantiomers by mass spectrometry: Application of a simple chiral chloride probe via rapid in-situ reaction. Anal. Chim. Acta 2014, 809, 104–108. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Liu, C.; Li, B. Visual chiral recognition of tryptophan enantiomers using unmodified gold nanoparticles as colorimetric probes. Anal. Chim. Acta 2014, 809, 123–127. [Google Scholar] [CrossRef]
- Liu, C.; Lian, J.; Liu, Q.; Xu, C.; Li, B. β-Cyclodextrin-modified silver nanoparticles as colorimetric probes for the direct visual enantioselective recognition of aromatic α-amino acids. Anal. Methods 2016, 8, 5794–5800. [Google Scholar] [CrossRef]
- Contino, A.; Maccarrone, G.; Remelli, M. Exploiting thermodynamic data to optimize the enantioseparation of underivatized amino acids in ligand exchange capillary electrophoresis. Anal. Bioanal. Chem. 2013, 405, 951–959. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Z.; Ji, W.; Tanaka, Y.; Sui, H.; Zhao, B.; Ozaki, Y. Enantioselective discrimination of alcohols by hydrogen bonding: A SERS study. Angew. Chem. 2014, 126, 14086–14090. [Google Scholar] [CrossRef]
- Sun, X.; Kong, H.; Zhou, Q.; Tsunega, S.; Liu, X.; Yang, H.; Jin, R.H. Chiral plasmonic nanoparticle assisted raman enantioselective recognition. Anal. Chem. 2020, 92, 8015–8020. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; He, Y.; Kong, H.; Yang, H.; Liu, X. Molecule-specific vibration-based chiral differentiation of Raman spectra using cysteine modified gold nanoparticles: The cases of tyrosine and phenylalanine. J. Mater. Chem. B 2021, 9, 7167–7171. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, J.; Zhang, J.; Wu, D.; Tao, Y.; Kong, Y. Single-template molecularly imprinted chiral sensor for simultaneous recognition of alanine and tyrosine enantiomers. Anal. Chem. 2019, 91, 12546–12552. [Google Scholar] [CrossRef]
- Hesari, M.; Ding, Z. A grand avenue to Au nanocluster electrochemiluminescence. Acc. Chem. Res. 2017, 50, 218–230. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, Y.; Li, X.; Yue, Q.; Dong, X.; Du, B.; Cao, W.; Wei, Q. Dual-quenching electrochemiluminescence strategy based on three-dimensional metal–organic frameworks for ultrasensitive detection of amyloid-β. Anal. Chem. 2019, 91, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Farinone, M.; Urbańska, K.; Pawlicki, M. BODIPY-and porphyrin-based sensors for recognition of amino acids and their derivatives. Molecules 2020, 25, 4523. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Morisue, M.; Satake, A.; Kobuke, Y. Highly Fluorescent Self-Coordinated Phthalocyanine Dimers. Angew. Chem. Int. Ed. 2005, 44, 4763–4766. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Tanaka, S.; Shinkai, S. On the influence of porphyrin π–π stacking on supramolecular chirality created in the porphyrin-based twisted tape structure. Chem. Commun. 2005, 44, 5539–5541. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; De Rossi, M.; Sorrenti, A.; Laguzzi, G.; Gatto, E.; Stefanelli, M.; Venanzi, M.; Luvidi, L.; Mancini, G.; Paolesse, R. Supramolecular Chirality in Solvent-Promoted Aggregation of Amphiphilic Porphyrin Derivatives: Kinetic Studies and Comparison between Solution Behavior and Solid-State Morphology by AFM Topography. Chem. Eur. J. 2020, 16, 860–870. [Google Scholar] [CrossRef]
- Weyandt, E.; Filot, I.A.; Vantomme, G.; Meijer, E. Consequences of amide connectivity in the supramolecular polymerization of porphyrins: Spectroscopic observations rationalized by theoretical modelling. Chem. Eur. J. 2021, 27, 9700–9707. [Google Scholar] [CrossRef]
- Kari, N.; Zannotti, M.; Giovannetti, R.; Řeha, D.; Minofar, B.; Abliz, S.; Yimit, A. Metallic Effects on p-Hydroxyphenyl Porphyrin Thin-Film-Based Planar Optical Waveguide Gas Sensor: Experimental and Computational Studies. Nanomaterials 2022, 12, 944. [Google Scholar] [CrossRef]
- Qiao, X.; Arsalan, M.; Ma, X.; Wang, Y.; Yang, S.; Wang, Y.; Sheng, Q.; Yue, T. A hybrid of ultrathin metal-organic framework sheet and ultrasmall copper nanoparticles for detection of hydrogen peroxide with enhanced activity. Anal. Bioanal. Chem. 2021, 413, 839–851. [Google Scholar] [CrossRef]
- Zhang, C.; Han, M.; Yu, L.; Qu, L.; Li, Z. Fabrication an electrochemical sensor based on composite of Cu-TCPP nanosheets and PSS functionalized graphene for simultaneous and sensitive determination of dihydroxybenzene isomers. J. Electroanal. Chem. 2021, 890, 115232. [Google Scholar] [CrossRef]
- Hang, L.; Zhang, T.; Wen, H.; Liang, L.; Li, W.; Ma, X.; Jiang, G. Controllable photodynamic performance via an acidic microenvironment based on two-dimensional metal-organic frameworks for photodynamic therapy. Nano Res. 2021, 14, 660–666. [Google Scholar] [CrossRef]
- Tu, W.; Lei, J.; Wang, P.; Ju, H. Photoelectrochemistry of Free-Base-Porphyrin-Functionalized Zinc Oxide Nanoparticles and Their Applications in Biosensing. Chem. Eur. J. 2011, 17, 9440–9447. [Google Scholar] [CrossRef]
- Ibrahim, M.N.M.; Zakaria, N.; Sipaut, C.S.; Sulaiman, O.; Hashim, R. Chemical and thermal properties of lignins from oil palm biomass as a substitute for phenol in a phenol formaldehyde resin production. Carbohydr. Polym. 2011, 86, 112–119. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Ma, Q.; Huang, Y.; Zhang, X.; Ping, J.; Zhang, Z.; Lu, Q.; Yu, Y.; Xu, H.; et al. Ultrathin 2D metal–organic framework nanosheets. Adv. Mater. 2015, 27, 7372–7378. [Google Scholar] [CrossRef]
- Xiao, Y.; Guo, W.; Chen, H.; Li, H.; Xu, X.; Wu, P.; Shen, Y.; Zheng, B.; Huo, F.; Wei, W.D. Ultrathin 2D Cu-porphyrin MOF nanosheets as a heterogeneous catalyst for styrene oxidation. Mater. Chem. Front. 2019, 3, 1580–1585. [Google Scholar] [CrossRef]
- Cai, W.R.; Zeng, H.B.; Xue, H.G.; Marks, S.R.; Cosnier, S.; Zhang, X.J.; Shan, D. Enhanced Electrochemiluminescence of Porphyrin-Based Metal–Organic Frameworks Controlled via Coordination Modulation. Anal. Chem. 2019, 92, 1916–1924. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Deng, S.Y.; Zhang, X.J.; Shan, D. Cathodic electrochemiluminescence of singlet oxygen induced by the electroactive zinc porphyrin in aqueous media. Electrochim. Acta 2016, 190, 64–68. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, X.; Wang, K.; Zou, Y.; Gyimah, E.; Yakubu, S.; Zhang, Z. A novel signal amplification strategy based on the competitive reaction between 2D Cu-TCPP (Fe) and polyethyleneimine (PEI) in the application of an enzyme-free and ultrasensitive electrochemical immunosensor for sulfonamide detection. Biosens. Bioelectron. 2020, 150, 111883. [Google Scholar] [CrossRef]
- Ye, Q.; Guo, L.; Wu, D.; Yang, B.; Tao, Y.; Deng, L.; Kong, Y. Covalent functionalization of bovine serum albumin with graphene quantum dots for stereospecific molecular recognition. Anal. Chem. 2019, 91, 11864–11871. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, W.-R.; Zhu, W.-K.; Yang, B.-Z.; Wu, D.-T.; Li, J.-Y.; Yin, Z.-Z.; Kong, Y. Porphyrin-Based Metal–Organic Frameworks for Efficient Electrochemiluminescent Chiral Recognition of Tyrosine Enantiomers. Chemosensors 2022, 10, 519. https://doi.org/10.3390/chemosensors10120519
Cai W-R, Zhu W-K, Yang B-Z, Wu D-T, Li J-Y, Yin Z-Z, Kong Y. Porphyrin-Based Metal–Organic Frameworks for Efficient Electrochemiluminescent Chiral Recognition of Tyrosine Enantiomers. Chemosensors. 2022; 10(12):519. https://doi.org/10.3390/chemosensors10120519
Chicago/Turabian StyleCai, Wen-Rong, Wen-Kai Zhu, Bao-Zhu Yang, Da-Tong Wu, Jun-Yao Li, Zheng-Zhi Yin, and Yong Kong. 2022. "Porphyrin-Based Metal–Organic Frameworks for Efficient Electrochemiluminescent Chiral Recognition of Tyrosine Enantiomers" Chemosensors 10, no. 12: 519. https://doi.org/10.3390/chemosensors10120519
APA StyleCai, W.-R., Zhu, W.-K., Yang, B.-Z., Wu, D.-T., Li, J.-Y., Yin, Z.-Z., & Kong, Y. (2022). Porphyrin-Based Metal–Organic Frameworks for Efficient Electrochemiluminescent Chiral Recognition of Tyrosine Enantiomers. Chemosensors, 10(12), 519. https://doi.org/10.3390/chemosensors10120519





