Hydrogen-Sensing Properties of Ultrathin Pt-Co Alloy Films
Abstract
1. Introduction
2. Materials and Methods
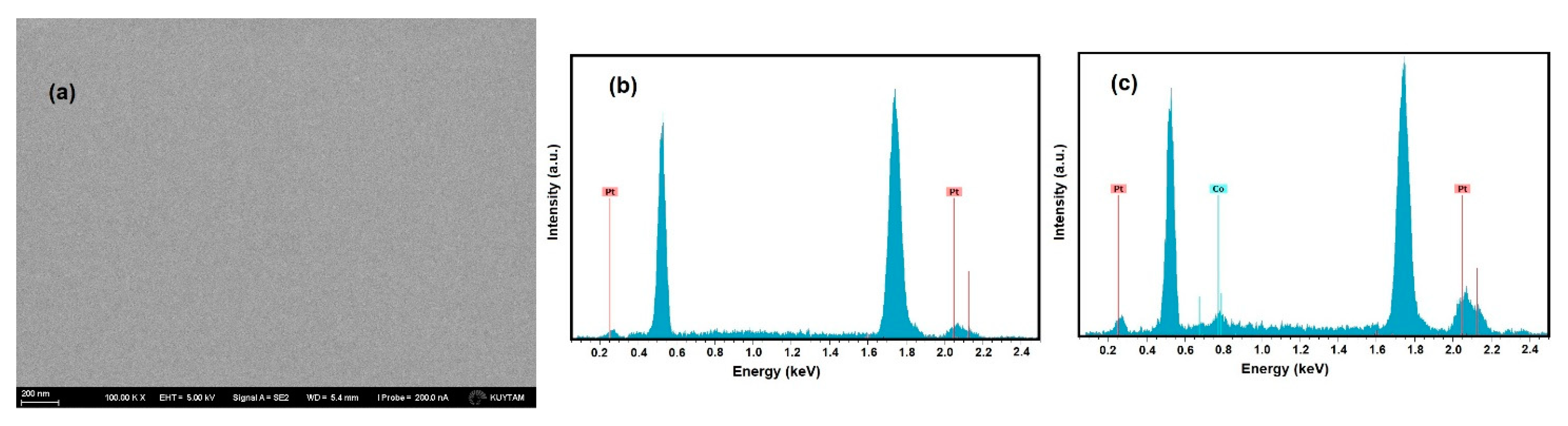
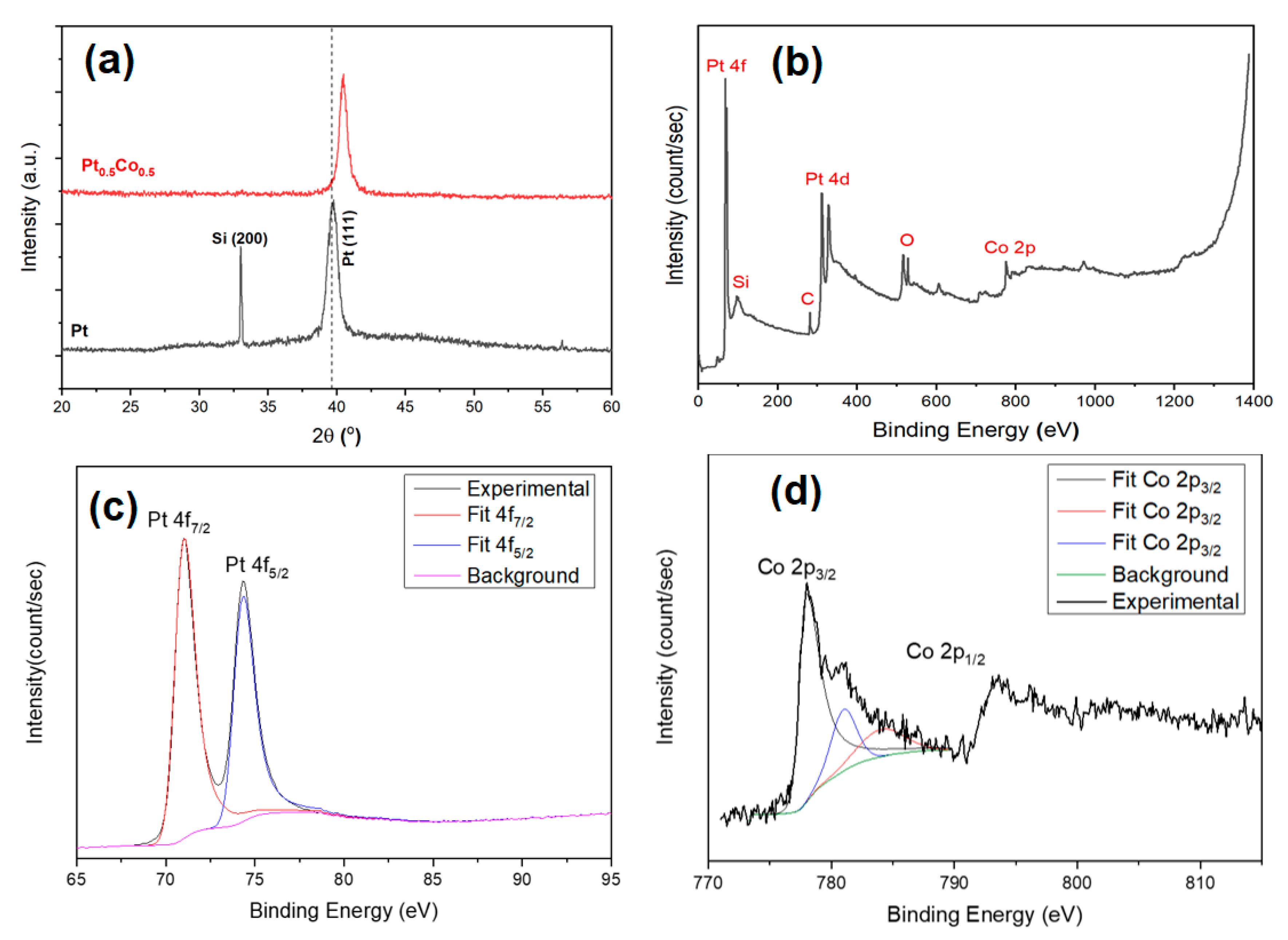
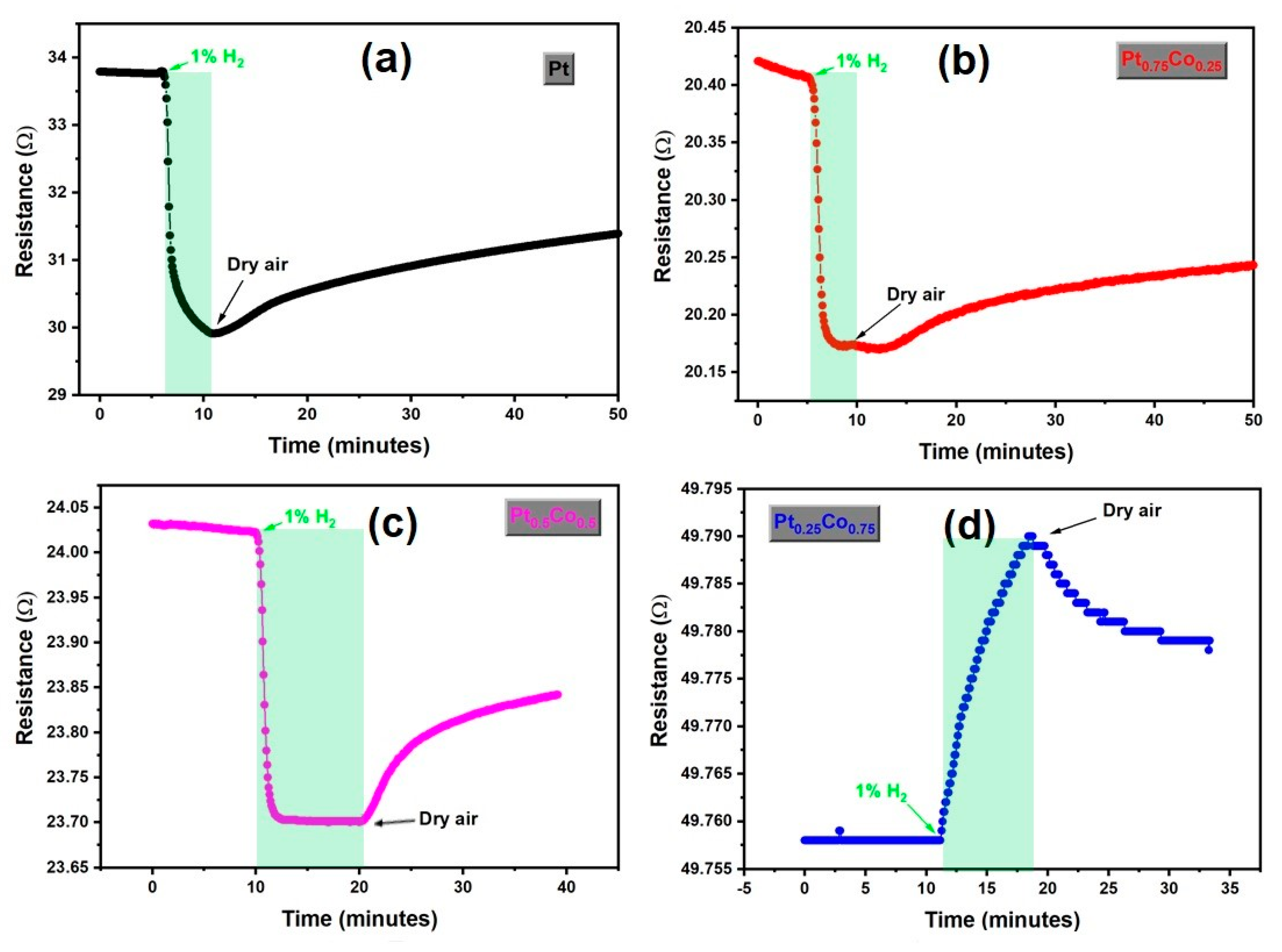
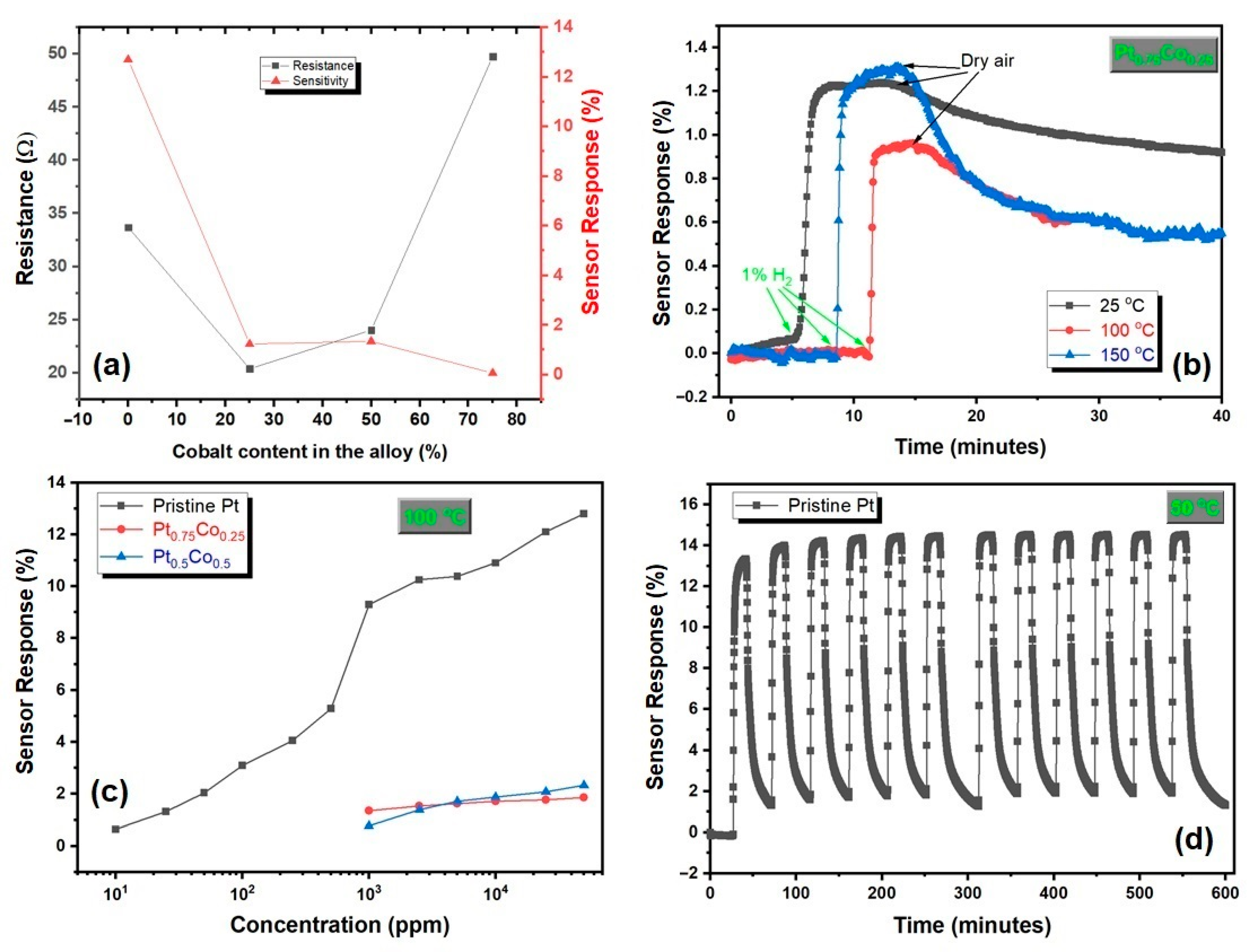
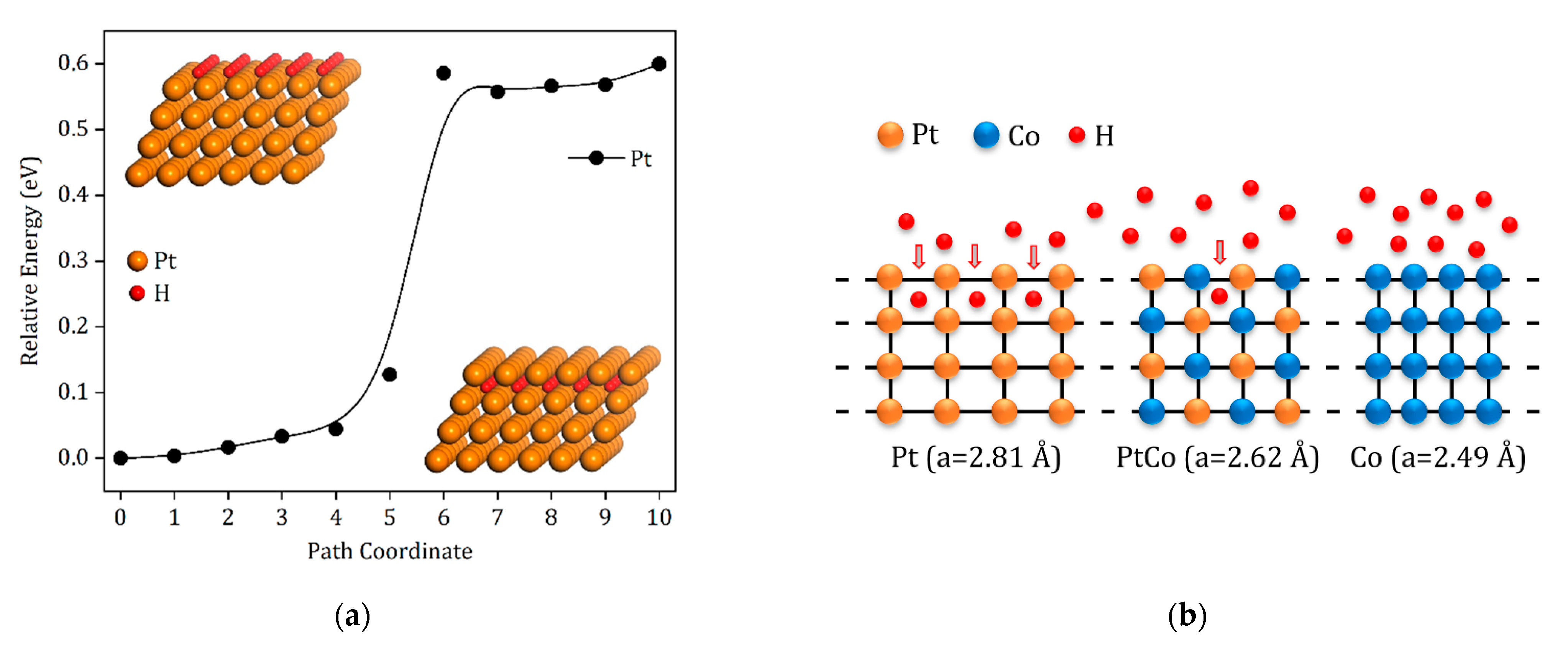
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Momirlan, M.; Veziroglu, T.N. Current status of hydrogen energy. Renew. Sust. Energ. Rev. 2002, 6, 141–179. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Hydrogen Issue. Chemsuschem 2011, 4, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Hubert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B-Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Kilinc, N. Resistive Hydrogen Sensors Based on Nanostructured Metals and Metal Alloys. Nanosci. Nanotechnol. Lett. 2013, 5, 825–841. [Google Scholar] [CrossRef]
- Sahoo, T.; Kale, P. Work Function-Based Metal-Oxide-Semiconductor Hydrogen Sensor and Its Functionality: A Review. Adv. Mater. Interfaces. 2021, 8, 2100649. [Google Scholar] [CrossRef]
- Chen, K.F.; Yuan, D.P.; Zhao, Y.Y. Review of optical hydrogen sensors based on metal hydrides: Recent developments and challenges. Opt. Laser. Technol. 2021, 137, 106808. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Bhattacharya, S. Hydrogen gas sensing methods, materials, and approach to achieve parts per billion level detection: A review. Int. J. Hydrog. Energy 2019, 44, 26076–26099. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Do Han, S.; Stetter, J.R. Review of Electrochemical Hydrogen Sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef]
- Maksymov, I.S.; Kostylev, M. Magneto-Electronic Hydrogen Gas Sensors: A Critical Review. Chemosensors 2022, 10, 49. [Google Scholar] [CrossRef]
- Shinde, P.V.; Rout, C.S. Magnetic gas sensing: Working principles and recent developments. Nanoscale Adv. 2021, 3, 1551–1568. [Google Scholar] [CrossRef]
- Majder-Lopatka, M.; Wesierski, T.; Dmochowska, A.; Salamonowicz, Z.; Polanczyk, A. The Influence of Hydrogen on the Indications of the Electrochemical Carbon Monoxide Sensors. Sustainability 2020, 12, 14. [Google Scholar] [CrossRef]
- Patel, S.V.; Gland, J.L.; Schwank, J.W. Film structure and conductometric hydrogen-gas-sensing characteristics of ultrathin platinum films. Langmuir 1999, 15, 3307–3311. [Google Scholar] [CrossRef]
- Abburi, A.; Abrams, N.; Yeh, W.J. Synthesis of nanoporous platinum thin films and application as hydrogen sensor. J. Porous. Mat. 2012, 19, 543–549. [Google Scholar] [CrossRef]
- Cao, F.; Zhao, P.F.; Wang, Z.; Zhang, X.H.; Zheng, H.; Wang, J.B.; Zhou, D.; Hu, Y.M.; Gu, H.S. An Ultrasensitive and Ultraselective Hydrogen Sensor Based on Defect-Dominated Electron Scattering in Pt Nanowire Arrays. Adv. Mater. Interfaces. 2019, 6, 1801304. [Google Scholar] [CrossRef]
- Prajapati, C.S.; Bhat, N. Self-heating oxidized suspended Pt nanowire for high performance hydrogen sensor. Sens. Actuators B-Chem. 2018, 260, 236–242. [Google Scholar] [CrossRef]
- Ding, M.N.; Liu, Y.; Wang, G.M.; Zhao, Z.P.; Yin, A.X.; He, Q.Y.; Huang, Y.; Duan, X.F. Highly Sensitive Chemical Detection with Tunable Sensitivity and Selectivity from Ultrathin Platinum Nanowires. Small 2017, 13, 1602969. [Google Scholar] [CrossRef]
- Yoo, H.W.; Cho, S.Y.; Jeon, H.J.; Jung, H.T. Well-Defined and High Resolution Pt Nanowire Arrays for a High Performance Hydrogen Sensor by a Surface Scattering Phenomenon. Anal. Chem. 2015, 87, 1480–1484. [Google Scholar] [CrossRef]
- Yang, F.; Donavan, K.C.; Kung, S.C.; Penner, R.M. The Surface Scattering-Based Detection of Hydrogen in Air Using a Platinum Nanowire. Nano. Lett. 2012, 12, 2924–2930. [Google Scholar] [CrossRef]
- Sennik, E.; Urdem, S.; Erkovan, M.; Kilinc, N. Sputtered platinum thin films for resistive hydrogen sensor application. Mater. Lett. 2016, 177, 104–107. [Google Scholar] [CrossRef]
- Tanaka, T.; Hoshino, S.; Takahashi, T.; Uchida, K. Nanoscale Pt thin film sensor for accurate detection of ppm level hydrogen in air at high humidity. Sens. Actuators B-Chem. 2018, 258, 913–919. [Google Scholar] [CrossRef]
- Tsukada, K.; Inoue, H.; Katayama, F.; Sakai, K.; Kiwa, T. Changes in Work Function and Electrical Resistance of Pt Thin Films in the Presence of Hydrogen Gas. Jpn. J. Appl. Phys. 2011, 51, 015701. [Google Scholar] [CrossRef]
- Kilinc, N. Palladium and platinum thin films for low-concentration resistive hydrogen sensor: A comparative study. J. Mater. Sci.-Mater. El. 2021, 32, 5567–5578. [Google Scholar] [CrossRef]
- Hassan, K.; Uddin, A.S.M.I.; Chung, G.S. Fast-response hydrogen sensors based on discrete Pt/Pd bimetallic ultra-thin films. Sens. Actuators B-Chem. 2016, 234, 435–445. [Google Scholar] [CrossRef]
- Tsukada, K.; Sakai, K.; Kiwa, T. Electric Characteristics of a Loop in Which Two Junctions between a Catalytic Metal and a Noncatalytic Metal Are under Different Hydrogen Gas Concentrations. Appl. Phys. Express 2012, 5, 034102. [Google Scholar] [CrossRef]
- Rajoua, K.; Baklouti, L.; Favier, F. Platinum for hydrogen sensing: Surface and grain boundary scattering antagonistic effects in Pt@Au core-shell nanoparticle assemblies prepared using a Langmuir-Blodgett method. Phys. Chem. Chem. Phys. 2018, 20, 383–394. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Yaqoob, U.; Hassan, K.; Chung, G.S. Effects of Pt shell thickness on self-assembly monolayer Pd@Pt core-shell nanocrystals based hydrogen sensing. Int. J. Hydrog. Energy 2016, 41, 15399–15410. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.J.; Shin, H.; Koo, W.T.; Jang, J.S.; Kang, J.Y.; Jeong, Y.J.; Kim, I.D. High-Resolution, Fast, and Shape-Conformable Hydrogen Sensor Platform: Polymer Nanofiber Yarn Coupled with Nanograined Pd@Pt. ACS Nano. 2019, 13, 6071–6082. [Google Scholar] [CrossRef]
- Li, X.W.; Liu, Y.; Hemminger, J.C.; Penner, R.M. Catalytically Activated Palladium@Platinum Nanowires for Accelerated Hydrogen Gas Detection. ACS Nano. 2015, 9, 3215–3225. [Google Scholar] [CrossRef]
- Kilinc, N.; Sanduvac, S.; Erkovan, M. Platinum-Nickel alloy thin films for low concentration hydrogen sensor application. J. Alloy. Compd. 2022, 892, 162237. [Google Scholar] [CrossRef]
- Nordiko. Available online: https://nordiko-tech.co.uk/ (accessed on 15 October 2022).
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Ruzsinszky, A.; Csonka, G.I.; Vydrov, O.A.; Scuseria, G.E.; Constantin, L.A.; Zhou, X.L.; Burke, K. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 2008, 100, 136406. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blochl, P.E. Projector Augmented-Wave Method. Phys. Rev. B. 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, H.; Mills, G.; Jacobsen, K.W. Nudged elastic band method for finding minimum energy paths of transitions. In Classical and Quantum Dynamics in Condensed Phase Simulations; World Scientific: Singapore, 1998; pp. 385–404. [Google Scholar]
- Chang, I.; Woo, S.; Lee, M.H.; Shim, J.H.; Piao, Y.; Cha, S.W. Characterization of porous Pt films deposited via sputtering. Appl. Surf. Sci. 2013, 282, 463–466. [Google Scholar] [CrossRef]
- Demirkan, B.; Bozkurt, S.; Savk, A.; Cellat, K.; Gulbagca, F.; Nas, M.S.; Alma, M.H.; Sen, F. Composites of Bimetallic Platinum-Cobalt Alloy Nanoparticles and Reduced Graphene Oxide for Electrochemical Determination of Ascorbic Acid, Dopamine, and Uric Acid. Sci. Rep. 2019, 9, 12258. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Li, D.W.; Gu, X.H. Dendritic PtCo alloy nanoparticles as high performance oxygen reduction catalysts. J. Colloid Interface Sci. 2012, 384, 105–109. [Google Scholar] [CrossRef]
- Lee, J.D.; Jishkariani, D.; Zhao, Y.R.; Najmr, S.; Rosen, D.; Kikkawa, J.M.; Stach, E.A.; Murray, C.B. Tuning the Electrocatalytic Oxygen Reduction Reaction Activity of Pt-Co Nanocrystals by Cobalt Concentration with Atomic-Scale Understanding. ACS Appl. Mater. Interfaces 2019, 11, 26789–26797. [Google Scholar] [CrossRef]
- Xu, J.F.; Liu, X.Y.; Chen, Y.; Zhou, Y.M.; Lu, T.H.; Tang, Y.W. Platinum-Cobalt alloy networks for methanol oxidation electrocatalysis. J. Mater. Chem. 2012, 22, 23659–23667. [Google Scholar] [CrossRef]
- O’Connor, D.J.; Sexton, B.A.; Smart, R.S.C. Surface Analysis Methods in Materials Science; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Erkovan, M.; Aköz, M.E.; Parlak, U.; Öztürk, O. The Study of Exchange Bias Effect in PtxCo1−x/CoO Bilayers. J. Supercond. Nov. Magn. 2017, 30, 2909–2913. [Google Scholar] [CrossRef]
- Sachs, C.; Hildebrand, M.; Volkening, S.; Wintterlin, J.; Ertl, G. Spatiotemporal self-organization in a surface reaction: From the atomic to the mesoscopic scale. Science 2001, 293, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Ogle, K.M.; White, J.M. The low temperature water formation reaction on Pt(111): A static SIMS and TDS study. Surf. Sci. 1984, 139, 43–62. [Google Scholar] [CrossRef]
- Gland, J.L.; Fisher, G.B.; Kollin, E.B. The hydrogen-oxygen reaction over the Pt(111) surface: Transient titration of adsorbed oxygen with hydrogen. J. Catal. 1982, 77, 263–278. [Google Scholar] [CrossRef]
- Nakamura, H.; Takase, S.; Shimizu, Y. A Cobalt-Nickel Metal-Alloy Thin-Film Sensor for Hydrogen-Phosphate Ion. Anal. Sci. 2021, 37, 337–340. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erkovan, M.; Deger, C.; Cardoso, S.; Kilinc, N. Hydrogen-Sensing Properties of Ultrathin Pt-Co Alloy Films. Chemosensors 2022, 10, 512. https://doi.org/10.3390/chemosensors10120512
Erkovan M, Deger C, Cardoso S, Kilinc N. Hydrogen-Sensing Properties of Ultrathin Pt-Co Alloy Films. Chemosensors. 2022; 10(12):512. https://doi.org/10.3390/chemosensors10120512
Chicago/Turabian StyleErkovan, Mustafa, Caner Deger, Susana Cardoso, and Necmettin Kilinc. 2022. "Hydrogen-Sensing Properties of Ultrathin Pt-Co Alloy Films" Chemosensors 10, no. 12: 512. https://doi.org/10.3390/chemosensors10120512
APA StyleErkovan, M., Deger, C., Cardoso, S., & Kilinc, N. (2022). Hydrogen-Sensing Properties of Ultrathin Pt-Co Alloy Films. Chemosensors, 10(12), 512. https://doi.org/10.3390/chemosensors10120512






