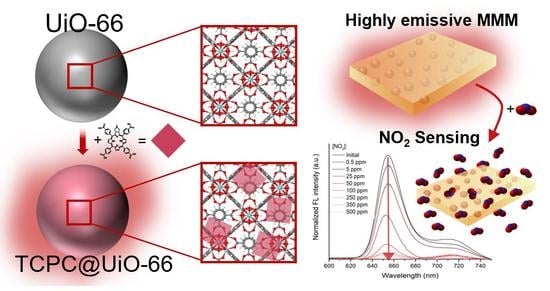
MOF-Based Materials with Sensing Potential: Pyrrolidine-Fused Chlorin at UiO-66(Hf) for Enhanced NO2 Detection
Abstract
1. Introduction
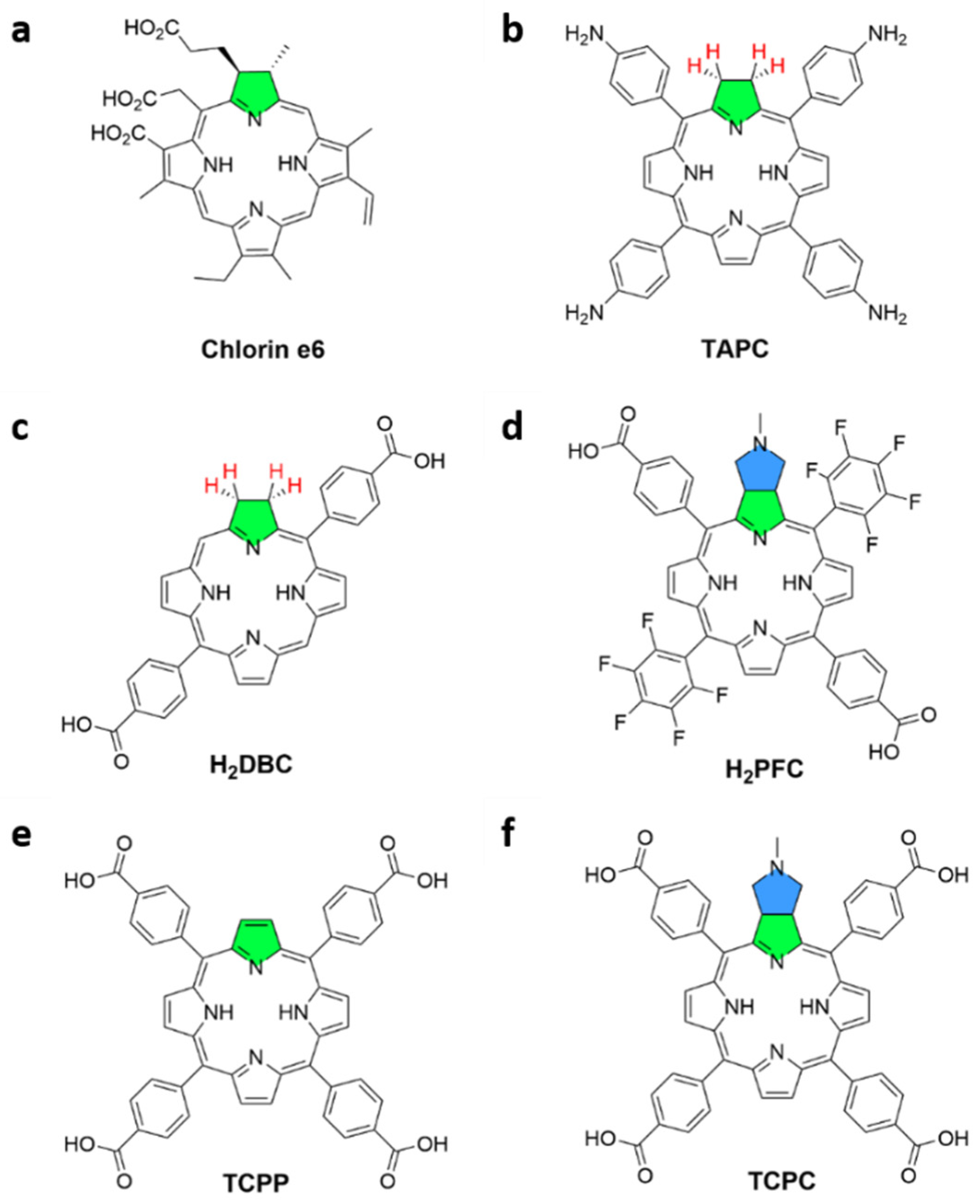
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Synthesis
2.3. Film Preparation
2.4. Time-Resolved Photoluminescence (PL) Measurements
2.5. NO2 Sensing Studies
3. Results and Discussion
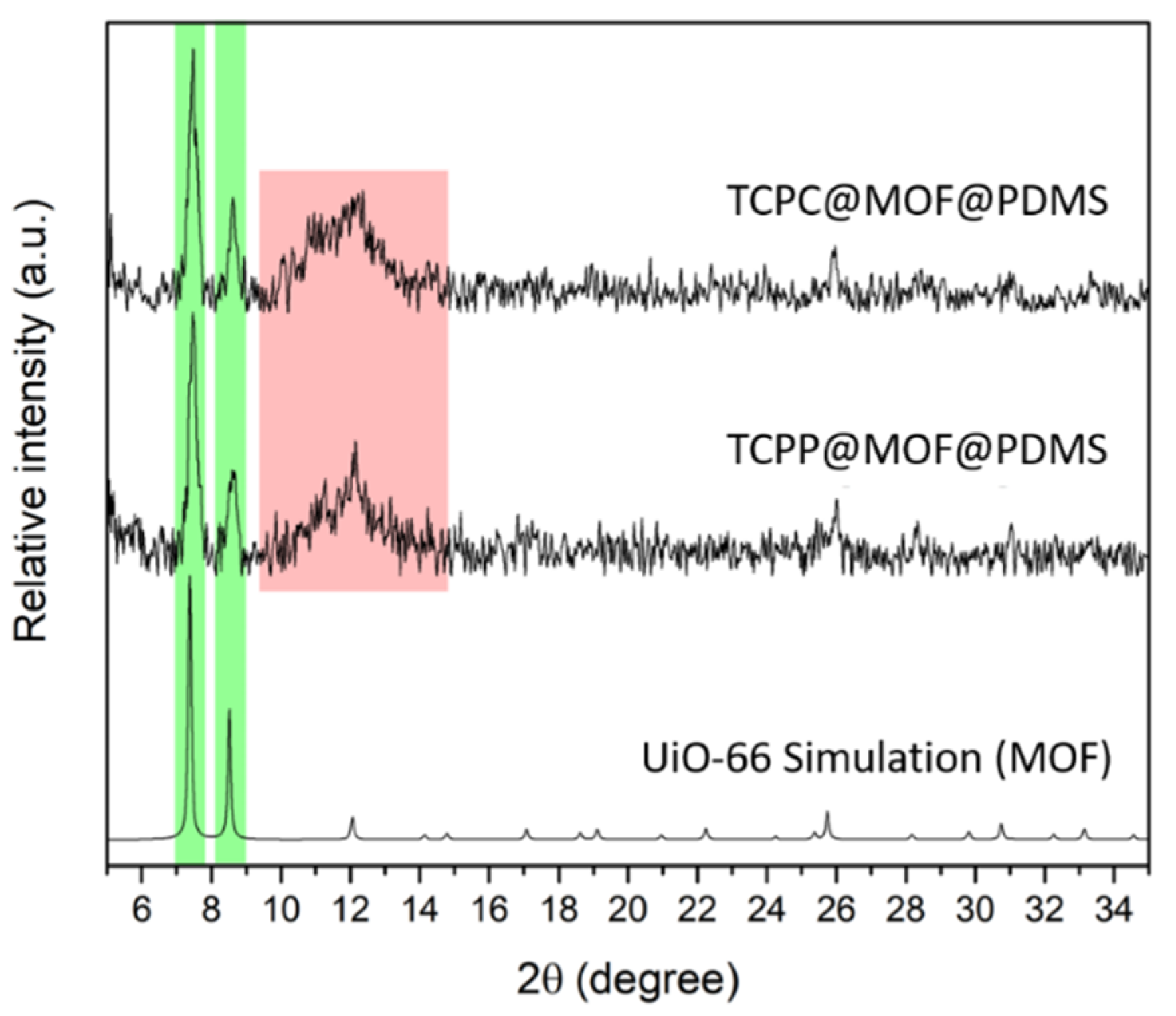
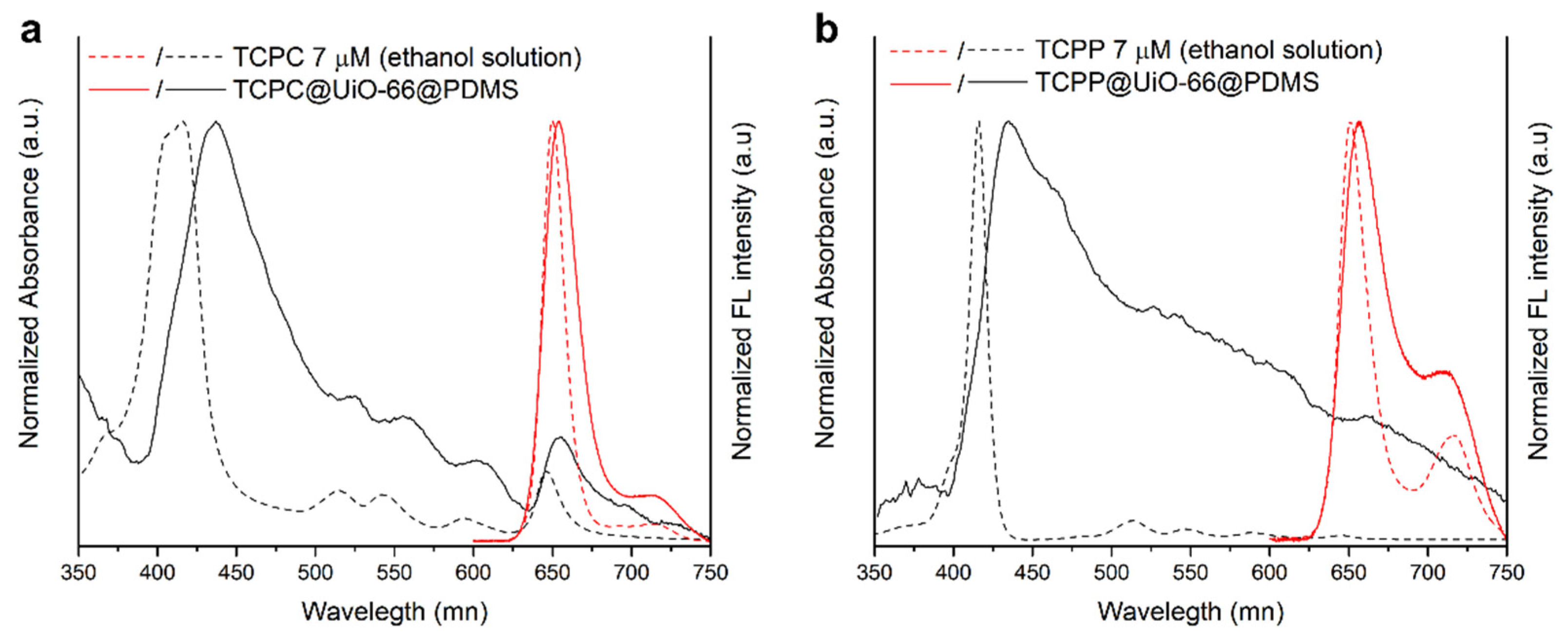
3.1. MOF-Based Materials
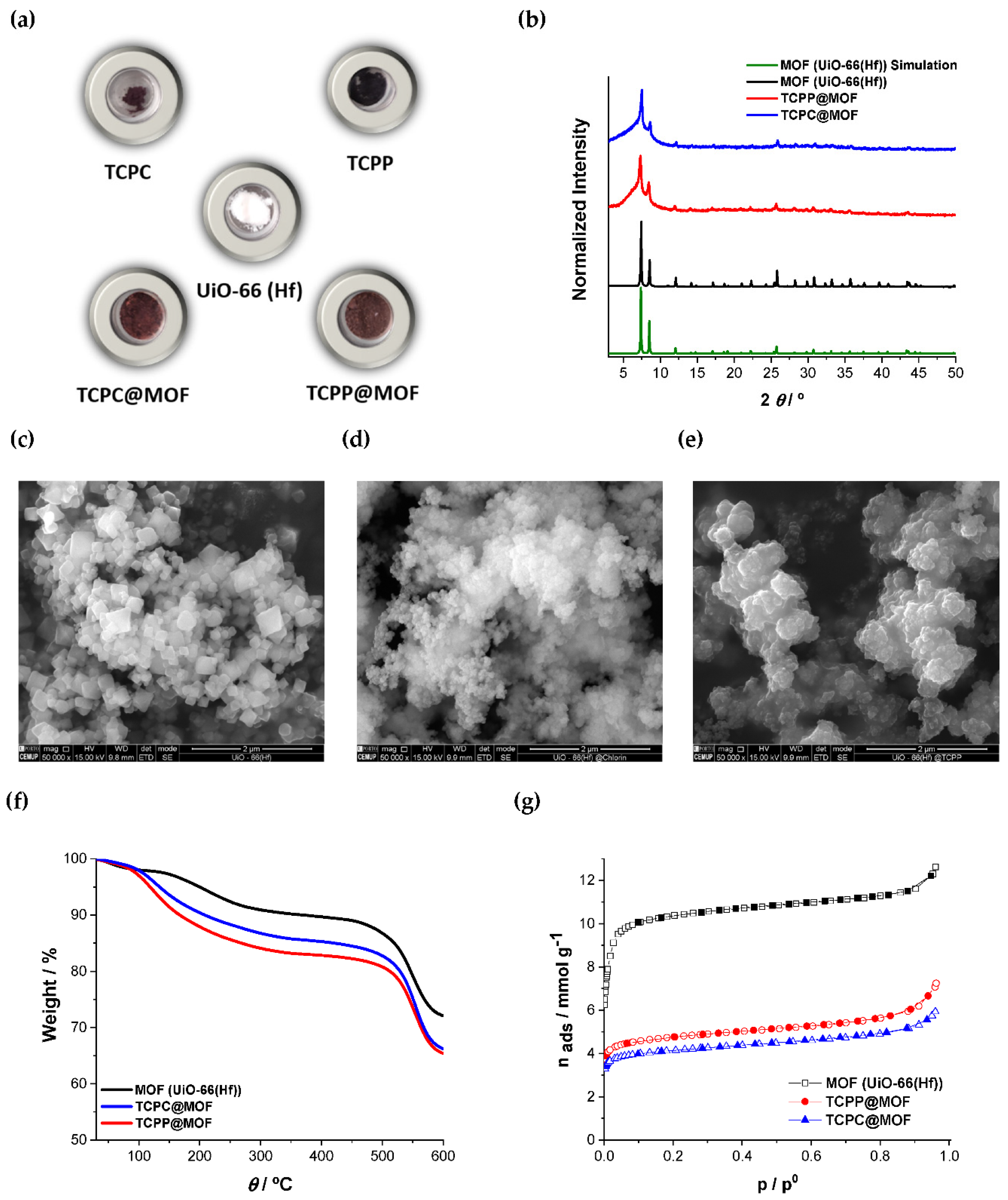
3.2. MOF-Based Membranes
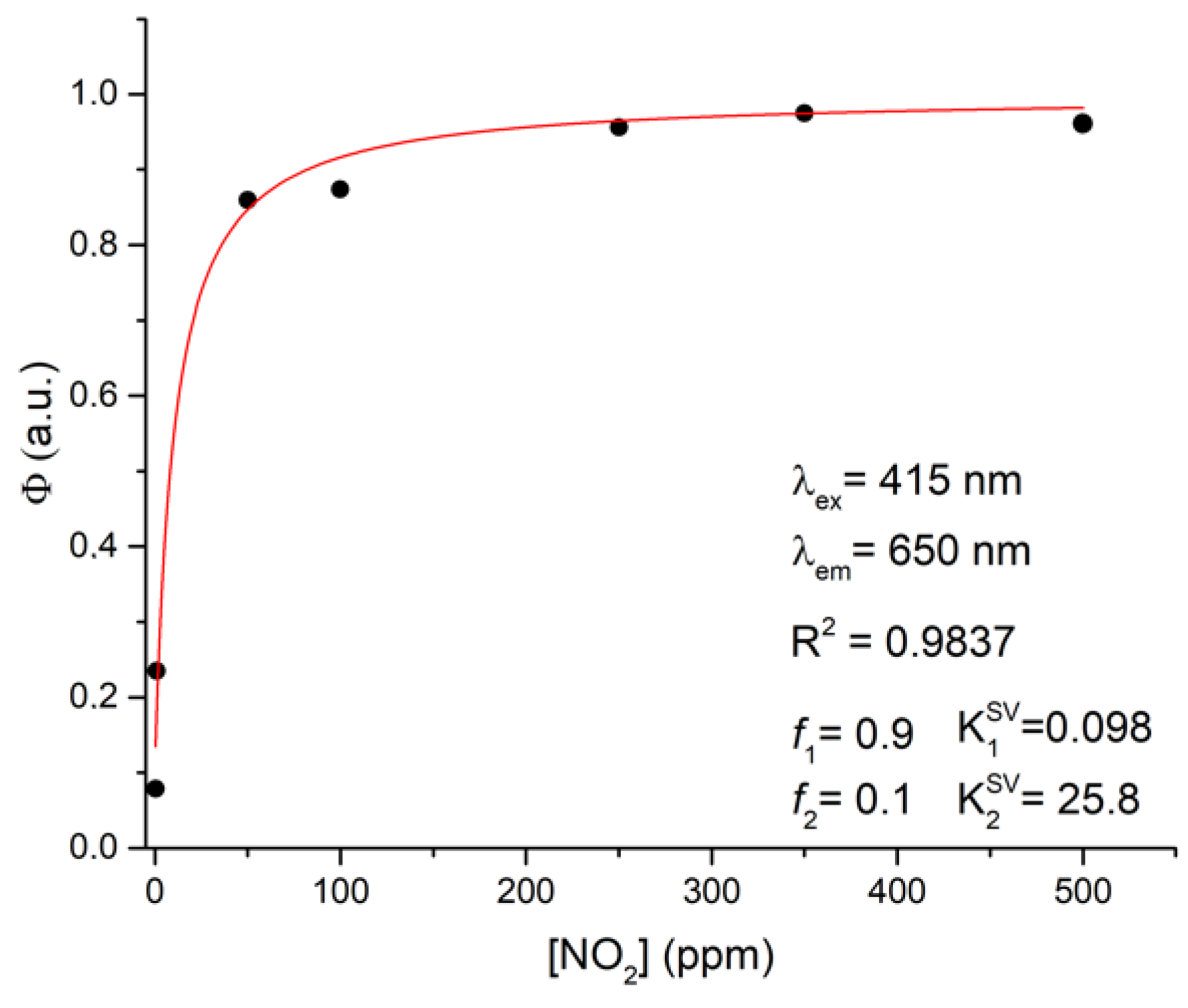
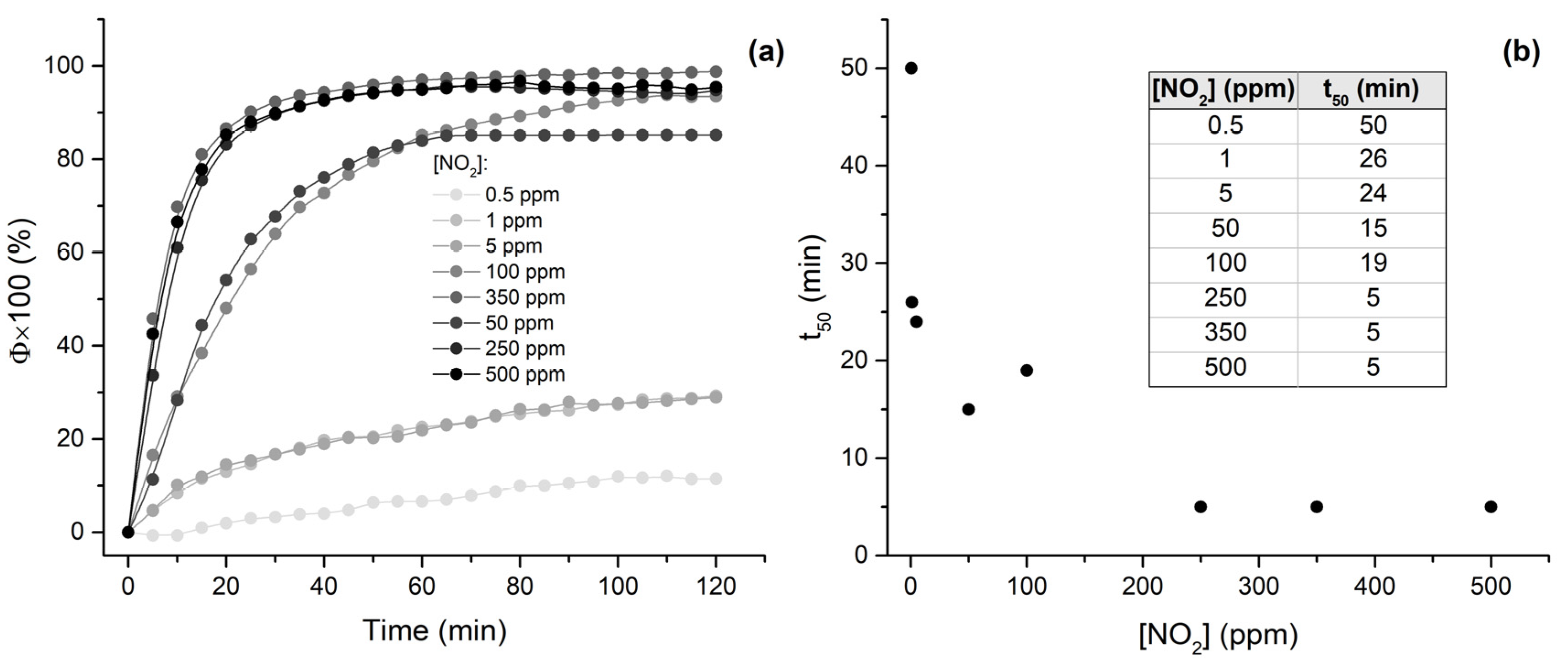
3.3. NO2 Sensing Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briefing: Europe’s Air Quality Status. 2021. Available online: https://www.eea.europa.eu/publications/air-quality-status-2021 (accessed on 12 October 2021).
- Meulenbelt, J. Nitrogen and nitrogen oxides. Medicine 2007, 35, 638. [Google Scholar] [CrossRef]
- Curtis, L. PM2.5, NO2, wildfires, and other environmental exposures are linked to higher Covid 19 incidence, severity, and death rates. Environ. Sci. Pollut. Res. 2021, 28, 54429–54447. [Google Scholar] [CrossRef] [PubMed]
- Agency, E.E. Air Quality in Europe—2020 Report. Available online: https://www.eea.europa.eu/publications/air-quality-in-europe-2020-report (accessed on 12 October 2021).
- Gutmacher, D.; Hoefer, U.; Wöllenstein, J. Gas sensor technologies for fire detection. Sens. Actuators B Chem. 2012, 175, 40–45. [Google Scholar] [CrossRef]
- Schmitt, K.; Tarantik, K.; Pannek, C.; Wöllenstein, J. Colorimetric Materials for Fire Gas Detection—A Review. Chemosensors 2018, 6, 14. [Google Scholar] [CrossRef]
- Korotcenkov, G. Properties, Advantages and Shortcomings for Applications Volume 1: Conventional Approaches. In Handbook of Gas Sensor Materials; Korotcenkov, G., Ed.; Springer-Verlag New York: New York, NY, USA, 2013; Volume 1. [Google Scholar]
- Ma, Y.; Han, X.; Xu, S.; Wang, Z.; Li, W.; da Silva, I.; Chansai, S.; Lee, D.; Zou, Y.; Nikiel, M.; et al. Atomically Dispersed Copper Sites in a Metal–Organic Framework for Reduction of Nitrogen Dioxide. J. Am. Chem. Soc. 2021, 143, 10977–10985. [Google Scholar] [CrossRef]
- Martínez-Ahumada, E.; Díaz-Ramírez, M.L.; Velásquez-Hernández, M.d.J.; Jancik, V.; Ibarra, I.A. Capture of toxic gases in MOFs: SO2, H2S, NH3 and NOx. Chem. Sci. 2021, 12, 6772–6799. [Google Scholar] [CrossRef]
- Islamoglu, T.; Chen, Z.; Wasson, M.C.; Buru, C.T.; Kirlikovali, K.O.; Afrin, U.; Mian, M.R.; Farha, O.K. Metal–Organic Frameworks against Toxic Chemicals. Chem. Rev. 2020, 120, 8130–8160. [Google Scholar] [CrossRef]
- Liu, Y.; Howarth, A.J.; Hupp, J.T.; Farha, O.K. Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal–Organic Framework. Angew. Chem. Int. Ed. 2015, 54, 9001–9005. [Google Scholar] [CrossRef]
- DeCoste, J.B.; Peterson, G.W. Metal–Organic Frameworks for Air Purification of Toxic Chemicals. Chem. Rev. 2014, 114, 5695–5727. [Google Scholar] [CrossRef]
- Barea, E.; Montoro, C.; Navarro, J.A.R. Toxic gas removal—Metal–organic frameworks for the capture and degradation of toxic gases and vapours. Chem. Soc. Rev. 2014, 43, 5419–5430. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, Y.; Ying, Y. Structural design of metal–organic frameworks with tunable colorimetric responses for visual sensing applications. Coord. Chem. Rev. 2021, 446, 214102. [Google Scholar] [CrossRef]
- Pamei, M.; Puzari, A. Luminescent transition metal–organic frameworks: An emerging sensor for detecting biologically essential metal ions. Nano-Struct. Nano-Objects 2019, 19, 100364. [Google Scholar] [CrossRef]
- Dolgopolova, E.A.; Rice, A.M.; Martin, C.R.; Shustova, N.B. Photochemistry and photophysics of MOFs: Steps towards MOF-based sensing enhancements. Chem. Soc. Rev. 2018, 47, 4710–4728. [Google Scholar] [CrossRef] [PubMed]
- Lustig, W.P.; Mukherjee, S.; Rudd, N.D.; Desai, A.V.; Li, J.; Ghosh, S.K. Metal–organic frameworks: Functional luminescent and photonic materials for sensing applications. Chem. Soc. Rev. 2017, 46, 3242–3285. [Google Scholar] [CrossRef]
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal–Organic Frameworks as Platforms for Functional Materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent Functional Metal–Organic Frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Gamonal, A.; Sun, C.; Mariano, A.L.; Fernandez-Bartolome, E.; Guerrero-SanVicente, E.; Vlaisavljevich, B.; Castells-Gil, J.; Marti-Gastaldo, C.; Poloni, R.; Wannemacher, R.; et al. Divergent Adsorption-Dependent Luminescence of Amino-Functionalized Lanthanide Metal–Organic Frameworks for Highly Sensitive NO2 Sensors. J. Phys. Chem. Lett. 2020, 11, 3362–3368. [Google Scholar] [CrossRef]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme–MOF (metal–organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J.; Verpoort, F. Metal organic frameworks mimicking natural enzymes: A structural and functional analogy. Chem. Soc. Rev. 2016, 45, 4127–4170. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Jakobsen, S.; Gianolio, D.; Wragg, D.S.; Nilsen, M.H.; Emerich, H.; Bordiga, S.; Lamberti, C.; Olsbye, U.; Tilset, M.; Lillerud, K.P. Structural determination of a highly stable metal-organic framework with possible application to interim radioactive waste scavenging: Hf-UiO-66. Phys. Rev. B 2012, 86, 125429. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Zhao, D. The chemistry and applications of hafnium and cerium(iv) metal–organic frameworks. Chem. Soc. Rev. 2021, 50, 4629–4683. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Yu, S.-S.; Kung, C.-W. Group 4 Metal-Based Metal—Organic Frameworks for Chemical Sensors. Chemosensors 2021, 9, 306. [Google Scholar] [CrossRef]
- deKrafft, K.E.; Boyle, W.S.; Burk, L.M.; Zhou, O.Z.; Lin, W. Zr- and Hf-based nanoscale metal–organic frameworks as contrast agents for computed tomography. J. Mater. Chem. 2012, 22, 18139–18144. [Google Scholar] [CrossRef]
- Nandi, S.; Biswas, S. A diamino functionalized metal-organic framework for fluorometric recognition of free chlorine in environmental water samples. Microporous Mesoporous Mater. 2020, 299, 110116. [Google Scholar] [CrossRef]
- Dalapati, R.; Biswas, S. A Pyrene-Functionalized Metal–Organic Framework for Nonenzymatic and Ratiometric Detection of Uric Acid in Biological Fluid via Conformational Change. Inorg. Chem. 2019, 58, 5654–5663. [Google Scholar] [CrossRef]
- Sk, M.; Nandi, S.; Singh, R.K.; Trivedi, V.; Biswas, S. Selective Sensing of Peroxynitrite by Hf-Based UiO-66-B(OH)2 Metal–Organic Framework: Applicability to Cell Imaging. Inorg. Chem. 2018, 57, 10128–10136. [Google Scholar] [CrossRef]
- Shahat, A.; Hassan, H.M.A.; Azzazy, H.M.E. Optical metal-organic framework sensor for selective discrimination of some toxic metal ions in water. Anal. Chim. Acta 2013, 793, 90–98. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Yan, B. Eu(III) functionalized Zr-based metal-organic framework as excellent fluorescent probe for Cd2+ detection in aqueous environment. Sens. Actuators B Chem. 2016, 222, 347–353. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.; Yan, D.; Deng, P.; Guo, Z.; Zhan, H. Europium ion post-functionalized zirconium metal–organic frameworks as luminescent probes for effectively sensing hydrazine hydrate. RSC Adv. 2018, 8, 17471–17476. [Google Scholar] [CrossRef] [PubMed]
- Xiaoxiong, Z.; Wenjun, Z.; Cuiliu, L.; Xiaohong, Q.; Chengyu, Z. Eu3+-Postdoped UIO-66-Type Metal–Organic Framework as a Luminescent Sensor for Hg2+ Detection in Aqueous Media. Inorg. Chem. 2019, 58, 3910–3915. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Hao, X.; Liu, W. Fluorescent molecule incorporated metal-organic framework for fluoride sensing in aqueous solution. Appl. Surf. Sci. 2017, 402, 129–135. [Google Scholar] [CrossRef]
- Govindaraju, S.; Puthiaraj, P.; Lee, M.-H.; Yun, K. Photoluminescent AuNCs@UiO-66 for Ultrasensitive Detection of Mercury in Water Samples. ACS Omega 2018, 3, 12052–12059. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, Y.; Liu, S.; Xu, M. The Applications of Metal−Organic Frameworks in Electrochemical Sensors. ChemElectroChem 2018, 5, 6–19. [Google Scholar] [CrossRef]
- Diamantis, S.A.; Margariti, A.; Pournara, A.D.; Papaefstathiou, G.S.; Manos, M.J.; Lazarides, T. Luminescent metal–organic frameworks as chemical sensors: Common pitfalls and proposed best practices. Inorg. Chem. Front. 2018, 5, 1493–1511. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, S.; Day, G.; Wang, X.; Yang, X.; Zhou, H.-C. Luminescent sensors based on metal-organic frameworks. Coord. Chem. Rev. 2018, 354, 28–45. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Melnikov, P.; Bobrov, A.; Marfin, Y. On the Use of Polymer-Based Composites for the Creation of Optical Sensors: A Review. Polymers 2022, 14, 4448. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.; Kim, S.-J.; Kim, Y. Porphyrinic metal–organic frameworks from custom-designed porphyrins. CrystEngComm 2016, 18, 345–368. [Google Scholar] [CrossRef]
- Yu, W.; Zhen, W.; Zhang, Q.; Li, Y.; Luo, H.; He, J.; Liu, Y. Porphyrin-Based Metal−Organic Framework Compounds as Promising Nanomedicines in Photodynamic Therapy. ChemMedChem 2020, 15, 1766–1775. [Google Scholar] [CrossRef]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef]
- Cai, H.; Huang, Y.-L.; Li, D. Biological metal–organic frameworks: Structures, host–guest chemistry and bio-applications. Coord. Chem. Rev. 2019, 378, 207–221. [Google Scholar] [CrossRef]
- Morozova, S.M.; Sharsheeva, A.; Morozov, M.I.; Vinogradov, A.V.; Hey-Hawkins, E. Bioresponsive metal–organic frameworks: Rational design and function. Coord. Chem. Rev. 2021, 431, 213682. [Google Scholar] [CrossRef]
- Fu, X.; Yang, Z.; Deng, T.; Chen, J.; Wen, Y.; Fu, X.; Zhou, L.; Zhu, Z.; Yu, C. A natural polysaccharide mediated MOF-based Ce6 delivery system with improved biological properties for photodynamic therapy. J. Mater. Chem. B 2020, 8, 1481–1488. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, Y.; Wang, H.; Liu, X.; Zhou, X.; Wang, F. DNAzyme-Loaded Metal–Organic Frameworks (MOFs) for Self-Sufficient Gene Therapy. Angew. Chem. Int. Ed. 2019, 58, 7380–7384. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Lin, X.; Lin, Z.; Wu, Y.; Liu, X.; Liu, J.; Wu, M.; Zhang, X.; Zeng, Y. Cancer Cell-Targeted Photosensitizer and Therapeutic Protein Co-Delivery Nanoplatform Based on a Metal–Organic Framework for Enhanced Synergistic Photodynamic and Protein Therapy. ACS Appl. Mater. Interfaces 2020, 12, 36906–36916. [Google Scholar] [CrossRef]
- Sheng, S.; Liu, F.; Lin, L.; Yan, N.; Wang, Y.; Xu, C.; Tian, H.; Chen, X. Nanozyme-mediated cascade reaction based on metal-organic framework for synergetic chemo-photodynamic tumor therapy. J. Control. Release 2020, 328, 631–639. [Google Scholar] [CrossRef]
- Calmeiro, J.M.D.; Dias, C.J.; Ramos, C.I.V.; Almeida, A.; Tomé, J.P.C.; Faustino, M.A.F.; Lourenço, L.M.O. Comparative photodynamic inactivation of bioluminescent E. coli by pyridinium and inverted pyridinium chlorins. Dye. Pigment. 2020, 173, 107410. [Google Scholar] [CrossRef]
- Sobotta, L.; Sniechowska, J.; Ziental, D.; Dlugaszewska, J.; Potrzebowski, M.J. Chlorins with (trifluoromethyl)phenyl substituents—Synthesis, lipid formulation and photodynamic activity against bacteria. Dye. Pigment. 2019, 160, 292–300. [Google Scholar] [CrossRef]
- Heredia, D.A.; Durantini, A.M.; Sarotti, A.M.; Gsponer, N.S.; Ferreyra, D.D.; Bertolotti, S.G.; Milanesio, M.E.; Durantini, E.N. Proton-Dependent Switching of a Novel Amino Chlorin Derivative as a Fluorescent Probe and Photosensitizer for Acidic Media. Chem. A Eur. J. 2018, 24, 5950–5961. [Google Scholar] [CrossRef]
- Almeida, J.; Zhang, G.; Wang, M.; Queirós, C.; Cerqueira, A.F.R.; Tomé, A.C.; Barone, G.; Vicente, M.G.H.; Hey-Hawkins, E.; Silva, A.M.G.; et al. Synthesis, characterization, and cellular investigations of porphyrin– and chlorin–indomethacin conjugates for photodynamic therapy of cancer. Org. Biomol. Chem. 2021, 19, 6501–6512. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.A.; Aggarwal, A.; Singh, S.; Adam, A.P.; Tome, J.P.C.; Drain, C.M. Compromising the plasma membrane as a secondary target in photodynamic therapy-induced necrosis. Bioorganic Med. Chem. 2018, 26, 5224–5228. [Google Scholar] [CrossRef]
- Hirohara, S.; Oka, C.; Totani, M.; Obata, M.; Yuasa, J.; Ito, H.; Tamura, M.; Matsui, H.; Kakiuchi, K.; Kawai, T.; et al. Synthesis, Photophysical Properties, and Biological Evaluation of trans-Bisthioglycosylated Tetrakis(fluorophenyl)chlorin for Photodynamic Therapy. J. Med. Chem. 2015, 58, 8658–8670. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Friso, E.; Miotto, G.; Jori, G.; Soncin, M.; Fabris, C.; Sibrian-Vazquez, M.; Vicente, M.G.H. Synthesis and biological investigations of tetrakis(p-carboranylthio-tetrafluorophenyl)chlorin (TPFC). Org. Biomol. Chem. 2008, 6, 3732–3740. [Google Scholar] [CrossRef]
- Castro, K.A.D.F.; Pires, S.M.G.; Ribeiro, M.A.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Schreiner, W.H.; Wypych, F.; Cavaleiro, J.A.S.; Nakagaki, S. Manganese chlorins immobilized on silica as oxidation reaction catalysts. J. Colloid Interface Sci. 2015, 450, 339–352. [Google Scholar] [CrossRef]
- Pires, S.M.G.; Paula, R.D.; Simões, M.M.Q.; Neves, M.G.P.M.S.; Santos, I.C.M.S.; Tomé, A.C.; Cavaleiro, J.A.S. A new silica-supported manganese chlorin as a biomimetic oxidation catalyst. Catal. Commun. 2009, 11, 24–28. [Google Scholar] [CrossRef]
- Marui, K.; Nomoto, A.; Akashi, H.; Ogawa, A. Green Oxidation of Amines to Imines Based on the Development of Novel Catalytic Systems Using Molecular Oxygen or Hydrogen Peroxide. Synthesis 2016, 48, 31–42. [Google Scholar]
- Guillén, M.G.; Gámez, F.; Suárez, B.; Queirós, C.; Silva, A.M.G.; Barranco, Á.; Sánchez-Valencia, J.R.; Pedrosa, J.M.; Lopes-Costa, T. Preparation and Optimization of Fluorescent Thin Films of Rosamine-SiO2/TiO2 Composites for NO2 Sensing. Materials 2017, 10, 124. [Google Scholar] [CrossRef]
- Guillén, M.G.; Suárez, B.; Roales, J.; Gámez, F.; Vargas, A.P.; Moscoso, F.G.; Lopes-Costa, T.; Queirós, C.; Silva, A.M.G.; Pedrosa, J.M. Fluorescent Rosamine/TiO2 Composite Films for the Optical Detection of NO2. J. Sens. 2018, 2018, 7954839. [Google Scholar] [CrossRef]
- Moscoso, F.G.; Almeida, J.; Sousaraei, A.; Lopes-Costa, T.; Silva, A.M.G.; Cabanillas-Gonzalez, J.; Cunha-Silva, L.; Pedrosa, J.M. Luminescent MOF crystals embedded in PMMA/PDMS transparent films as effective NO2 gas sensors. Mol. Syst. Des. Eng. 2020, 5, 1048–1056. [Google Scholar] [CrossRef]
- Sousaraei, A.; Queirós, C.; Moscoso, F.G.; Silva, A.M.G.; Lopes-Costa, T.; Pedrosa, J.M.; Cunha-Silva, L.; Cabanillas-Gonzalez, J. Reversible Protonation of Porphyrinic Metal-Organic Frameworks Embedded in Nanoporous Polydimethylsiloxane for Colorimetric Sensing. Adv. Mater. Interfaces 2021, 8, 2001759. [Google Scholar] [CrossRef]
- Moscoso, F.G.; Almeida, J.; Sousaraei, A.; Lopes-Costa, T.; Silva, A.M.G.; Cabanillas-Gonzalez, J.; Cunha-Silva, L.; Pedrosa, J.M. A lanthanide MOF immobilized in PMMA transparent films as a selective fluorescence sensor for nitroaromatic explosive vapours. J. Mater. Chem. C 2020, 8, 3626–3630. [Google Scholar] [CrossRef]
- Sousaraei, A.; Queirós, C.; Moscoso, F.G.; Lopes-Costa, T.; Pedrosa, J.M.; Silva, A.M.G.; Cunha-Silva, L.; Cabanillas-Gonzalez, J. Subppm Amine Detection via Absorption and Luminescence Turn-On Caused by Ligand Exchange in Metal Organic Frameworks. Anal. Chem. 2019, 91, 15853–15859. [Google Scholar] [CrossRef] [PubMed]
- Sakamaki, Y.; Ozdemir, J.; Heidrick, Z.; Azzun, A.; Watson, O.; Tsuji, M.; Salmon, C.; Sinha, A.; Batta-Mpouma, J.; McConnell, Z.; et al. A Bioconjugated Chlorin-Based Metal–Organic Framework for Targeted Photodynamic Therapy of Triple Negative Breast and Pancreatic Cancers. ACS Appl. Bio Mater. 2021, 4, 1432–1440. [Google Scholar] [CrossRef]
- Vargas, A.P.; Almeida, J.; Gámez, F.; Roales, J.; Queirós, C.; Rangel, M.; Lopes-Costa, T.; Silva, A.M.G.; Pedrosa, J.M. Synthesis of a highly emissive carboxylated pyrrolidine-fused chlorin for optical sensing of TATP vapours. Dye. Pigment. 2021, 195, 109721. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, D.; Yin, J.; Shang, Y.; Du, J.; Kang, Z.; Wang, R.; Chen, Y.; Sun, D.; Jiang, J. An ultrafast responsive NO2 gas sensor based on a hydrogen-bonded organic framework material. Chem. Commun. 2020, 56, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Tuerdi, G.; Yan, Y.; Yimit, A. Optical and electrochemical gas sensing applications of Cu Tetraphenylporphyrin thin films. Optik 2019, 183, 114–123. [Google Scholar] [CrossRef]
- Zhu, P.; Song, F.; Ma, P.; Wang, Y.; Chen, C.; Feng, J. Morphology-controlled self-assembly of a ferrocene–porphyrin based NO2 gas sensor: Tuning the semiconducting nature via solvent–solute interaction. J. Mater. Chem. C 2016, 4, 10471–10478. [Google Scholar] [CrossRef]
- Su, H.C.; Tran, T.-T.; Bosze, W.; Myung, N.V. Chemiresistive sensor arrays for detection of air pollutants based on carbon nanotubes functionalized with porphyrin and phthalocyanine derivatives. Sens. Actuators Rep. 2020, 2, 100011. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, P.; Song, F.; Yao, S.; Chen, C.; Zhu, P. Comparative NO2-sensing in cobalt and metal-free porphyrin nanotubes. J. Colloid Interface Sci. 2017, 490, 129–136. [Google Scholar] [CrossRef]
- Lee, J.I.; Kim, M.; Park, K.C.; Lee, C.Y.; Park, Y.D. Polythiophene hybrid film with zirconium–porphyrin metal–organic framework for improved charge carrier transport and NO2 gas sensing. Mater. Chem. Phys. 2022, 278, 125661. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, L.; Guan, Y.; Pei, Q.; Jiang, J.; Xie, Z. Integration of metal-organic framework with a photoactive porous-organic polymer for interface enhanced phototherapy. Biomaterials 2020, 235, 119792. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. A Chlorin-Based Nanoscale Metal-Organic Framework for Photodynamic Therapy of Colon Cancers. J. Am. Chem. Soc. 2015, 137, 7600–7603. [Google Scholar] [CrossRef]
- Hu, Z.; Nalaparaju, A.; Peng, Y.; Jiang, J.; Zhao, D. Modulated Hydrothermal Synthesis of UiO-66(Hf)-Type Metal–Organic Frameworks for Optimal Carbon Dioxide Separation. Inorg. Chem. 2016, 55, 1134–1141. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, L.; Liu, M.; Lei, P.; Liu, F.; Xie, Z. Nanoscale Mixed-Component Metal–Organic Frameworks with Photosensitizer Spatial-Arrangement-Dependent Photochemistry for Multimodal-Imaging-Guided Photothermal Therapy. Chem. Mater. 2018, 30, 6867–6876. [Google Scholar] [CrossRef]
- Liao, X.; Wang, X.; Wang, F.; Yao, Y.; Lu, S. Ligand Modified Metal Organic Framework UiO-66: A Highly Efficient and Stable Catalyst for Oxidative Desulfurization. J. Inorg. Organomet. Polym. Mater. 2021, 31, 756–762. [Google Scholar] [CrossRef]
- Øien, S.; Wragg, D.; Reinsch, H.; Svelle, S.; Bordiga, S.; Lamberti, C.; Lillerud, K.P. Detailed Structure Analysis of Atomic Positions and Defects in Zirconium Metal–Organic Frameworks. Cryst. Growth Des. 2014, 14, 5370–5372. [Google Scholar] [CrossRef]
- Melnikov, P.V.; Naumova, A.O.; Alexandrovskaya, A.Y.; Zaitsev, N.K. Optimizing Production Conditions for a Composite Optical Oxygen Sensor Using Mesoporous SiO2. Nanotechnologies Russ. 2018, 13, 602–608. [Google Scholar] [CrossRef]
- Ghosh, M.; Nath, S.; Hajra, A.; Sinha, S. Fluorescence self-quenching of tetraphenylporphyrin in liquid medium. J. Lumin. 2013, 141, 87–92. [Google Scholar] [CrossRef]
- Ni, M.; Gong, M.; Li, X.; Gu, J.; Li, B.; Chen, Y. Dimensions of fluorescence kinetic concentration of doped morphology homologs synthesized by TCPP and UiO-66 MOF. Appl. Mater. Today 2021, 23, 100982. [Google Scholar] [CrossRef]
- Simon-Yarza, T.; Mielcarek, A.; Couvreur, P.; Serre, C. Nanoparticles of Metal-Organic Frameworks: On the Road to In Vivo Efficacy in Biomedicine. Adv. Mater. 2018, 30, 1707365. [Google Scholar] [CrossRef]
- Dang, Y.T.; Hoang, H.T.; Dong, H.C.; Bui, K.-B.T.; Nguyen, L.H.T.; Phan, T.B.; Kawazoe, Y.; Doan, T.L.H. Microwave-assisted synthesis of nano Hf- and Zr-based metal-organic frameworks for enhancement of curcumin adsorption. Microporous Mesoporous Mater. 2020, 298, 110064. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: London, UK, 1999. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Bakuru, V.R.; Churipard, S.R.; Maradur, S.P.; Kalidindi, S.B. Exploring the Brønsted acidity of UiO-66 (Zr, Ce, Hf) metal–organic frameworks for efficient solketal synthesis from glycerol acetalization. Dalton Trans. 2019, 48, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Crivello, C.; Sevim, S.; Graniel, O.; Franco, C.; Pané, S.; Puigmartí-Luis, J.; Muñoz-Rojas, D. Advanced technologies for the fabrication of MOF thin films. Mater. Horiz. 2021, 8, 168–178. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Wöll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef]
- Roales, J.; Pedrosa, J.M.; Guillén, M.G.; Lopes-Costa, T.; Castillero, P.; Barranco, A.; González-Elipe, A.R. Free-Base Carboxyphenyl Porphyrin Films Using a TiO2 Columnar Matrix: Characterization and Application as NO2 Sensors. Sensors 2015, 15, 11118–11132. [Google Scholar] [CrossRef]
- Richardson, T.H.; Dooling, C.M.; Jones, L.T.; Brook, R.A. Development and optimization of porphyrin gas sensing LB films. Adv. Colloid Interface Sci. 2005, 116, 81–96. [Google Scholar] [CrossRef]
- Pedrosa, J.M.; Dooling, C.M.; Richardson, T.H.; Hyde, R.K.; Hunter, C.A.; Martín, M.T.; Camacho, L. Influence of Molecular Organization of Asymmetrically Substituted Porphyrins on Their Response to NO2 Gas. Langmuir 2002, 18, 7594–7601. [Google Scholar] [CrossRef]
- Gulino, A.; Mineo, P.; Scamporrino, E.; Vitalini, D.; Fragalà, I. Molecularly Engineered Silica Surfaces with an Assembled Porphyrin Monolayer as Optical NO2 Molecular Recognizers. Chem. Mater. 2004, 16, 1838–1840. [Google Scholar] [CrossRef]
- Dooling, C.M.; Worsfold, O.; Richardson, T.H.; Tregonning, R.; Vysotsky, M.O.; Hunter, C.A.; Kato, K.; Shinbo, K.; Kaneko, F. Fast, reversible optical sensing of NO2 using 5,10,15,20-tetrakis[3,4-bis(2-ethylhexyloxy)phenyl]-21H,23H-porphine assemblies. J. Mater. Chem. 2001, 11, 392–398. [Google Scholar] [CrossRef]
- Gulino, A.; Bazzano, S.; Mineo, P.; Scamporrino, E.; Vitalini, D.; Fragalà, I. Characterization, Optical Recognition Behavior, Sensitivity, and Selectivity of Silica Surfaces Functionalized with a Porphyrin Monolayer. Chem. Mater. 2005, 17, 521–526. [Google Scholar] [CrossRef]
- Abudukeremu, H.; Kari, N.; Zhang, Y.; Wang, J.; Nizamidin, P.; Abliz, S.; Yimit, A. Highly sensitive free-base-porphyrin-based thin-film optical waveguide sensor for detection of low concentration NO2 gas at ambient temperature. J. Mater. Sci. 2018, 53, 10822–10834. [Google Scholar] [CrossRef]
- Zakavi, S.; Omidyan, R.; Ebrahimi, L.; Heidarizadi, F. Substitution effects on the UV–vis and 1H NMR spectra of the dications of meso and/or β substituted porphyrins with trifluoroacetic acid: Electron-deficient porphyrins compared to the electron-rich ones. Inorg. Chem. Commun. 2011, 14, 1827–1832. [Google Scholar] [CrossRef]
- Shen, Y.; Zhong, X.; Zhang, J.; Li, T.; Zhao, S.; Cui, B.; Wei, D.; Zhang, Y.; Wei, K. In-situ growth of mesoporous In2O3 nanorod arrays on a porous ceramic substrate for ppb-level NO2 detection at room temperature. Appl. Surf. Sci. 2019, 498, 143873. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Guillén, M.G.; Gámez, F.; Lopes-Costa, T.; Cabanillas-González, J.; Pedrosa, J.M. A fluorescence gas sensor based on Förster Resonance Energy Transfer between polyfluorene and bromocresol green assembled in thin films. Sens. Actuators B Chem. 2016, 236, 136–143. [Google Scholar] [CrossRef]
- Guillén, M.G.; Gámez, F.; Lopes-Costa, T.; Castro-Smirnov, J.R.; Wannemacher, R.; Cabanillas-González, J.; Pedrosa, J.M. Amplified spontaneous emission in action: Sub-ppm optical detection of acid vapors in poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene] thin films. Sens. Actuators B Chem. 2018, 255, 1354–1361. [Google Scholar] [CrossRef]
- Demas, J.N.; DeGraff, B.A.; Xu, W. Modeling of Luminescence Quenching-Based Sensors: Comparison of Multisite and Nonlinear Gas Solubility Models. Anal. Chem. 1995, 67, 1377–1380. [Google Scholar] [CrossRef]

| Material | ABET/m2 g−1 | Vads (0.95 po)/cm3 g−1 |
|---|---|---|
| UiO-66(Hf) | 940 | 0.43 |
| TCPC@MOF | 361 | 0.20 |
| TCPP@MOF | 414 | 0.24 |
| Material | τav/ns | χ2 |
|---|---|---|
| TCPC | 0.11 | 1.388 |
| TCPP | 0.12 | 1.066 |
| UiO-66(Hf) | 2.00 | 0.872 |
| TCPC@MOF | 0.55 | 1.111 |
| TCPP@MOF | 1.03 | 0.884 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queirós, C.; Moscoso, F.G.; Almeida, J.; Silva, A.M.G.; Sousaraei, A.; Cabanillas-González, J.; Ribeiro Carrott, M.; Lopes-Costa, T.; Pedrosa, J.M.; Cunha-Silva, L. MOF-Based Materials with Sensing Potential: Pyrrolidine-Fused Chlorin at UiO-66(Hf) for Enhanced NO2 Detection. Chemosensors 2022, 10, 511. https://doi.org/10.3390/chemosensors10120511
Queirós C, Moscoso FG, Almeida J, Silva AMG, Sousaraei A, Cabanillas-González J, Ribeiro Carrott M, Lopes-Costa T, Pedrosa JM, Cunha-Silva L. MOF-Based Materials with Sensing Potential: Pyrrolidine-Fused Chlorin at UiO-66(Hf) for Enhanced NO2 Detection. Chemosensors. 2022; 10(12):511. https://doi.org/10.3390/chemosensors10120511
Chicago/Turabian StyleQueirós, Carla, Francisco G. Moscoso, José Almeida, Ana M. G. Silva, Ahmad Sousaraei, Juan Cabanillas-González, Manuela Ribeiro Carrott, Tânia Lopes-Costa, José M. Pedrosa, and Luís Cunha-Silva. 2022. "MOF-Based Materials with Sensing Potential: Pyrrolidine-Fused Chlorin at UiO-66(Hf) for Enhanced NO2 Detection" Chemosensors 10, no. 12: 511. https://doi.org/10.3390/chemosensors10120511
APA StyleQueirós, C., Moscoso, F. G., Almeida, J., Silva, A. M. G., Sousaraei, A., Cabanillas-González, J., Ribeiro Carrott, M., Lopes-Costa, T., Pedrosa, J. M., & Cunha-Silva, L. (2022). MOF-Based Materials with Sensing Potential: Pyrrolidine-Fused Chlorin at UiO-66(Hf) for Enhanced NO2 Detection. Chemosensors, 10(12), 511. https://doi.org/10.3390/chemosensors10120511