Enhanced Sensing Performance of Electrospun Tin Dioxide Nanofibers Decorated with Cerium Dioxide Nanoparticles for the Detection of Liquefied Petroleum Gas
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Material Synthesis and Sensor Fabrication
2.3. Gas-Sensing Experimental Setup
3. Results and Discussion
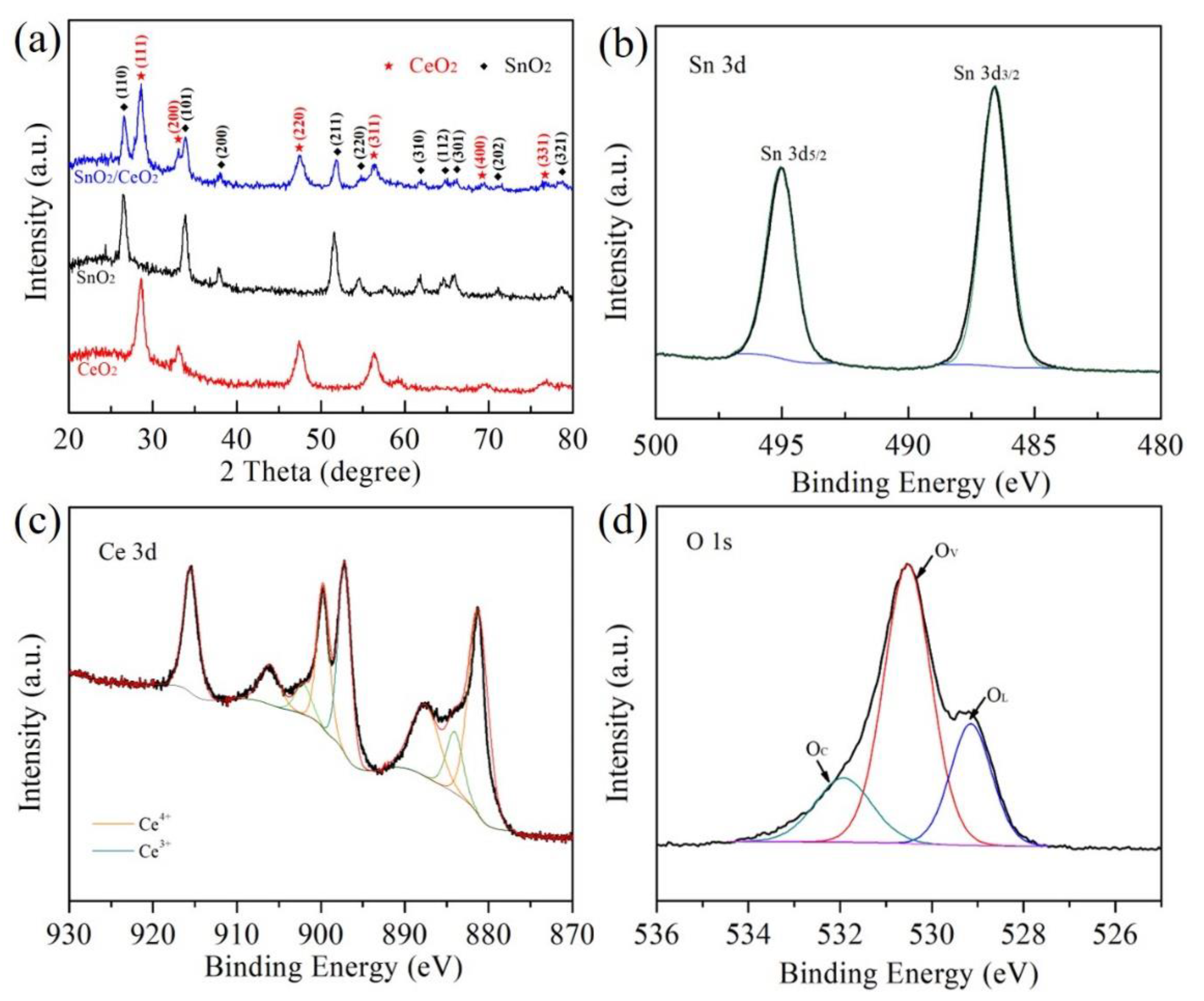
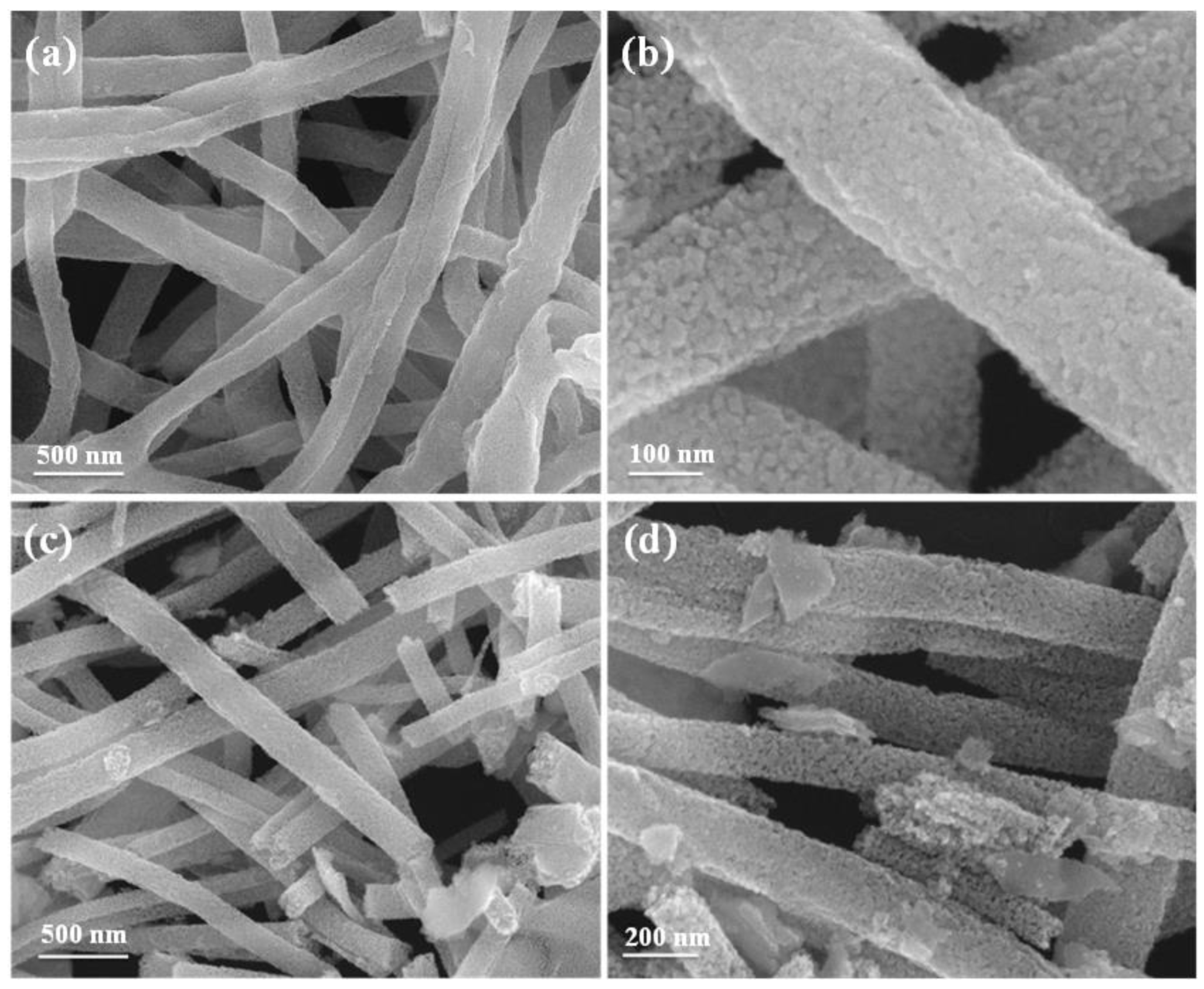
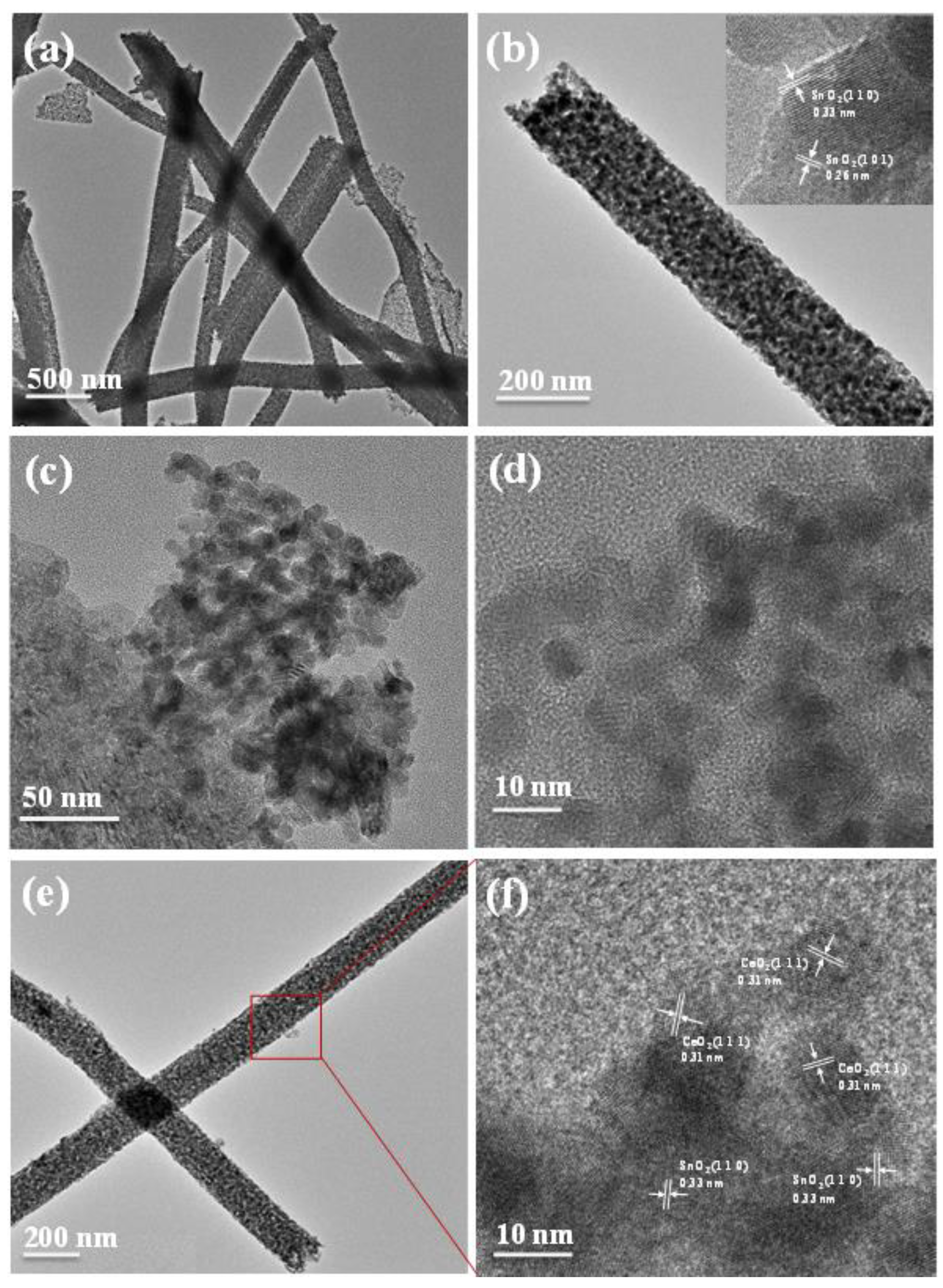
3.1. Structure Characterization
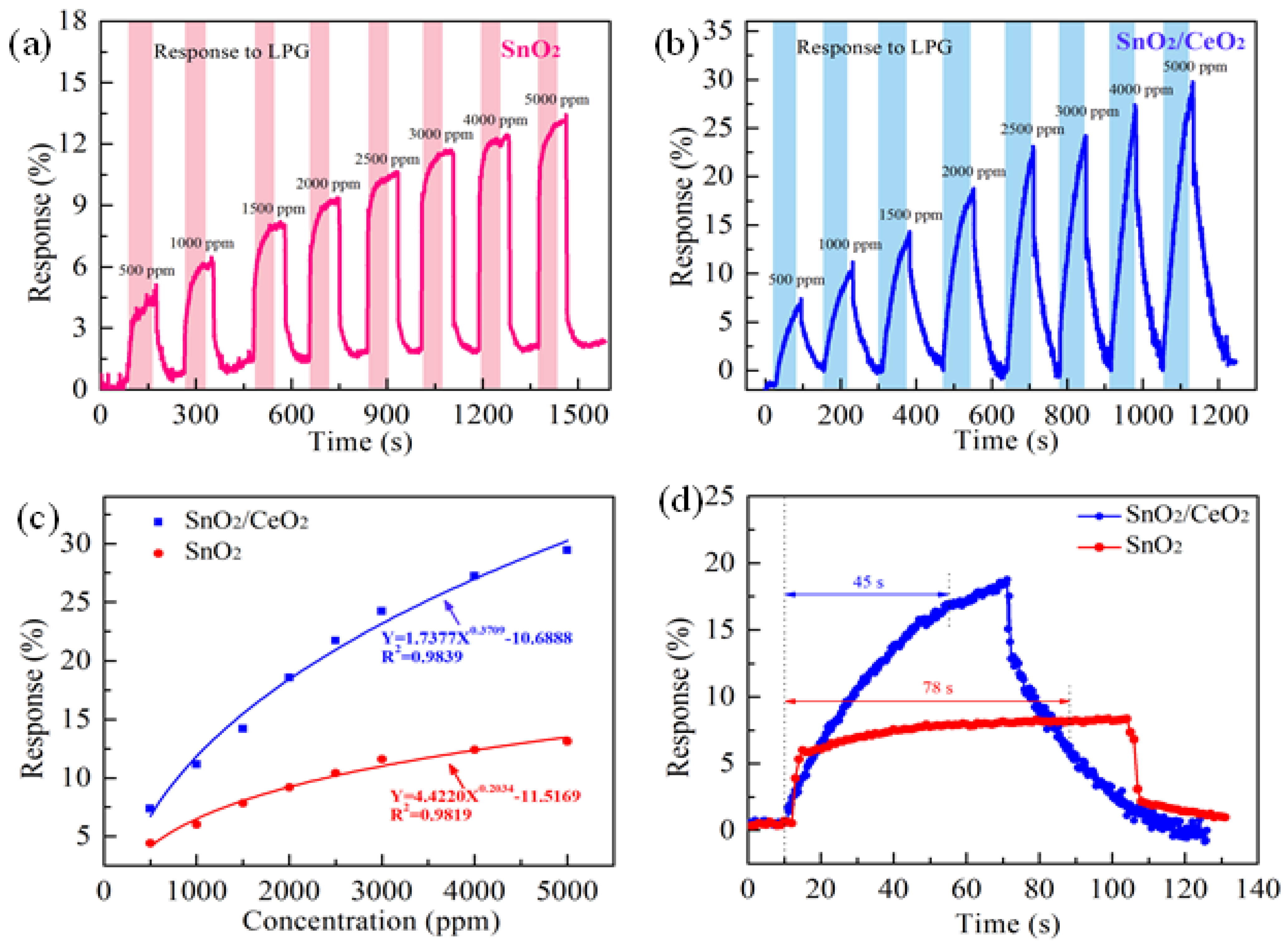
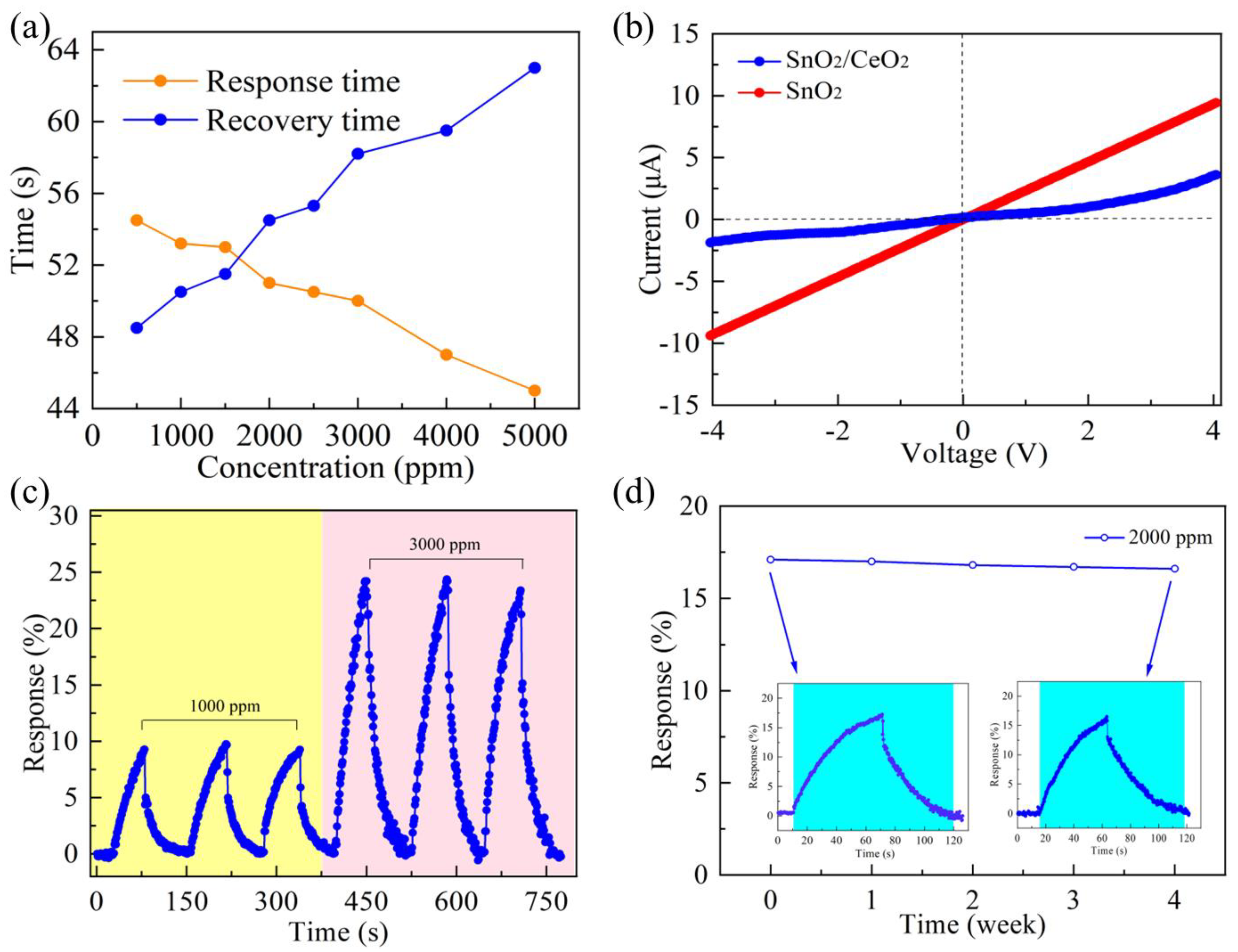
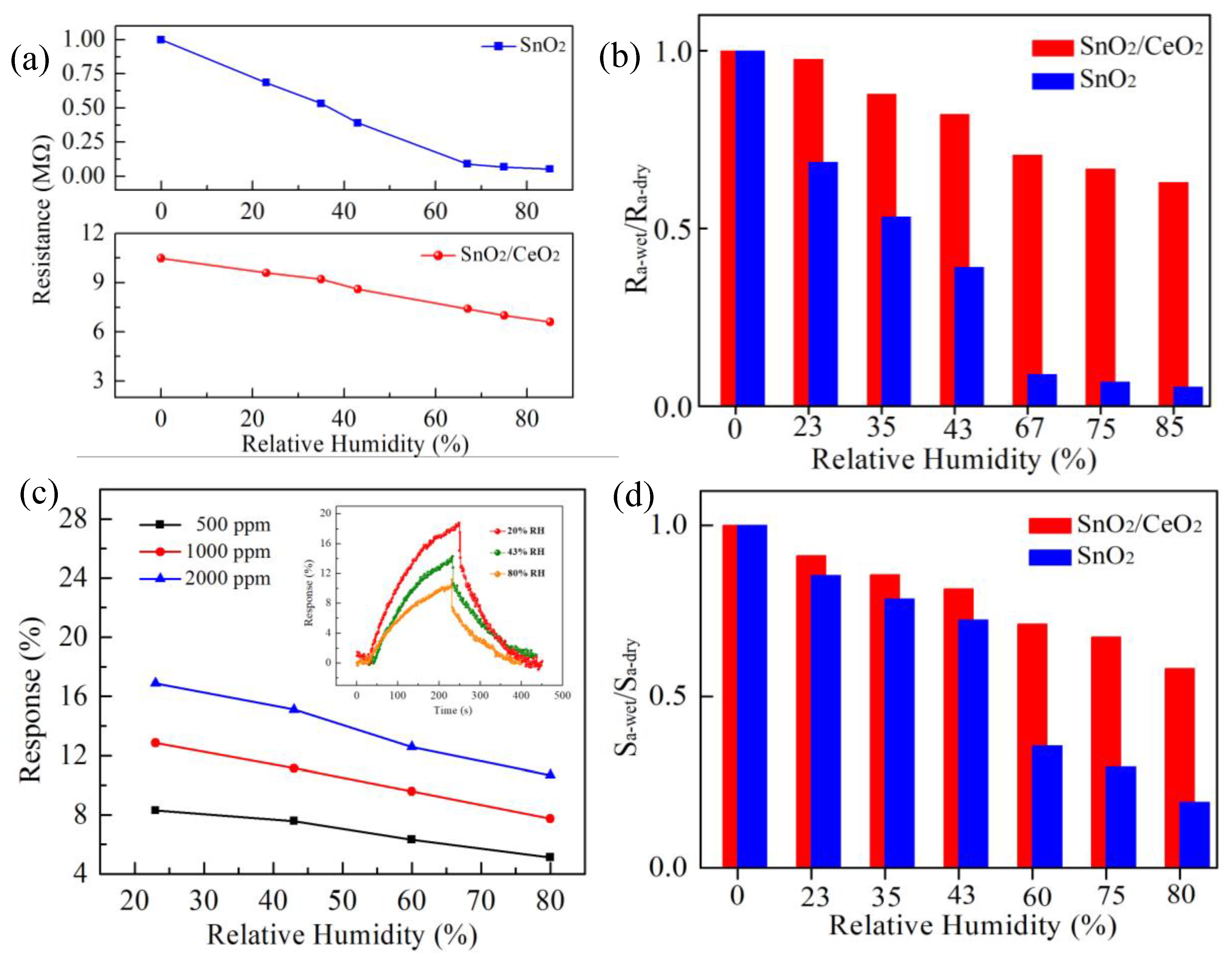
3.2. LPG Sensing Properties
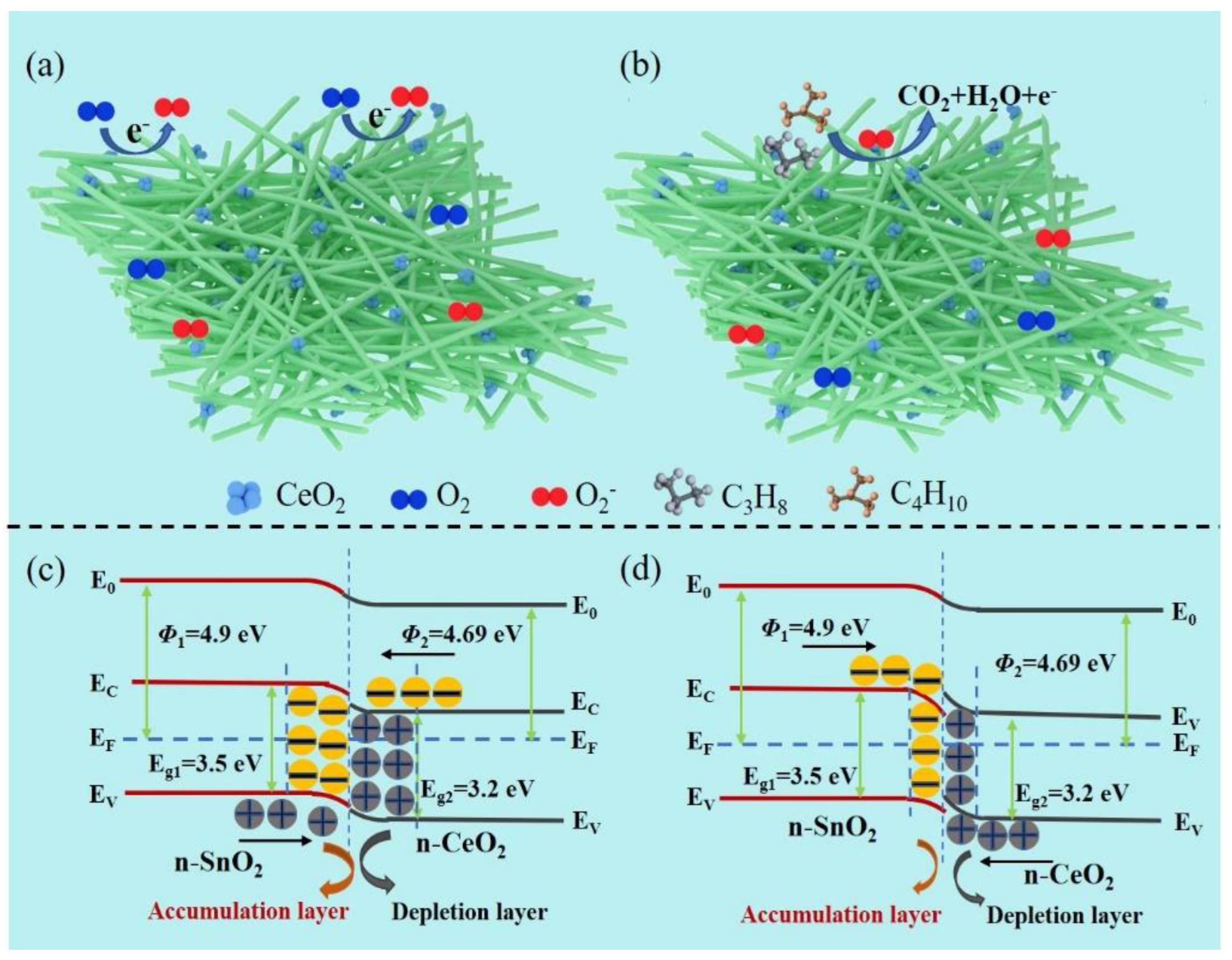
3.3. LPG Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.; Zhang, D.; Zhang, B.; Wang, D.Y.; Tang, M. Wearable pressure sensor array with layer-by-layer assembled MXene nanosheets/Ag nanoflowers for motion monitoring and human-machine interfaces. ACS Appl. Mater. Interfaces 2022, 14, 48907–48916. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Saxena, P.; Madhwal, D.; Bhardwaj, N.; Jain, V.K. Battery-operated resistive sensor based on electrochemically exfoliated pencil graphite core for room temperature detection of LPG. Sens. Actuators B 2021, 343, 130133. [Google Scholar] [CrossRef]
- Choudhary, S.; Annapoorni, S.; Malik, R. Facile strategy to synthesize donut-shaped α-Fe2O3 nanoparticles for enhanced LPG detection. Sens. Actuators B 2021, 334, 129668. [Google Scholar] [CrossRef]
- Yadav, B.C.; Singh, S.; Yadav, A. Nanonails structured ferric oxide thick film as room temperature liquefied petroleum gas (LPG) sensor. Appl. Surf. Sci. 2011, 257, 1960–1966. [Google Scholar] [CrossRef]
- Gupta, P.; Pandey, N.K.; Kumar, K.; Yadav, B.C. Structural, optical and LPG sensing properties of zinc-doped nickel oxide pellets operated at room temperature. Sens. Actuators A 2021, 319, 112484. [Google Scholar] [CrossRef]
- Kang, Z.J.; Zhang, D.Z.; Li, T.T.; Liu, X.H.; Song, X.S. Polydopamine-modified SnO2 nanofiber composite coated QCM gas sensor for high-performance formaldehyde sensing. Sens. Actuators B 2021, 345, 130299. [Google Scholar] [CrossRef]
- Wang, L.; Ma, S.Y.; Xu, X.L.; Li, J.P.; Yang, T.T.; Cao, P.F.; Yun, P.D.; Wang, S.Y.; Han, T. Oxygen vacancy-based Tb-doped SnO2 nanotubes as an ultra-sensitive sensor for ethanol detection. Sens. Actuators B 2021, 344, 130111. [Google Scholar] [CrossRef]
- Meng, F.J.; Guo, X.M. Tuning the oxygen defects and Fermi levels via In3+ doping in SnO2-In2O3 nanocomposite for efficient CO detection. Sens. Actuators B 2022, 357, 131412. [Google Scholar] [CrossRef]
- Mnethu, O.; Nkosi, S.S.; Kortidis, I.; Motaung, D.E.; Kroon, R.E.; Swart, H.C.; Ntsasa, N.G.; Tshilongo, J.; Moyo, T. Ultra-sensitive and selective p-xylene gas sensor at low operating temperature utilizing Zn doped CuO nanoplatelets: Insignificant vestiges of oxygen vacancies. J. Colloid Interface Sci. 2020, 576, 364–375. [Google Scholar] [CrossRef]
- Bulemo, P.M.; Kim, D.H.; Kim, I.D. Controlled synthesis of electrospun hollow Pt-loaded SnO2 microbelts for acetone sensing. Sens. Actuators B 2021, 344, 130208. [Google Scholar] [CrossRef]
- Wang, T.T.; Cheng, L. Hollow hierarchical TiO2-SnO2-TiO2 composite nanofibers with increased active-sites and charge transfer for enhanced acetone sensing performance. Sens. Actuators B 2021, 334, 129644. [Google Scholar] [CrossRef]
- Paulo, V.M.; Pedro, H.S.; Ranilson, A.S.; Marcelo, O.O. High gas sensor performance of WO3 nanofibers prepared by electrospinning. J. Alloys Compd. 2021, 864, 158745. [Google Scholar]
- Shingange, K.; Swart, H.C.; Mhlongo, G.H. Design of porous p-type LaCoO3 nanofibers with remarkable response and selectivity to ethanol at low operating temperature. Sens. Actuators B 2020, 308, 127670. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhang, D.Z.; Zhang, H.B.; Gong, L.K.; Yang, Y.; Zhao, W.H.; Yu, S.J.; Yin, Y.D.; Sun, D.F. In situ polymerized polyaniline/MXene (V2C) as building blocks of supercapacitor and ammonia sensor self-powered by electromagnetic-triboelectric hybrid generator. Nano Energy 2021, 88, 106242. [Google Scholar] [CrossRef]
- Cai, Z.C.; Goo, E.; Park, S. Synthesis of tin dioxide (SnO2) hollow nanospheres and its ethanol-sensing performance augmented by gold nanoparticle decoration. J. Alloys Compd. 2021, 883, 160868. [Google Scholar] [CrossRef]
- Choi, M.S.; Ahn, J.; Kim, M.Y.; Mirzaei, A.; Choi, S.M.; Chun, D.W.; Jin, C.; Lee, K.H. Changes in the crystal structure of SnO2 nanoparticles and improved H2S gas-sensing characteristics by Al doping. Appl. Surf. Sci. 2021, 565, 150493. [Google Scholar] [CrossRef]
- Liu, A.; Lv, S.Y.; Zhao, L.J.; Liu, F.M.; Wang, J.; You, R.; Yang, Z.J.; He, J.M.; Jiang, L.; Wang, C.G.; et al. Room temperature flexible NH3 sensor based on polyaniline coated Rh-doped SnO2 hollow nanotubes. Sens. Actuators B 2021, 330, 129313. [Google Scholar] [CrossRef]
- Li, P.P.; Wang, B.; Qin, C.; Han, C.; Sun, L.; Wang, Y.D. Band-gap-tunable CeO2 nanoparticles for room-temperature NH3 gas sensors. Ceram. Int. 2020, 46, 19232–19240. [Google Scholar] [CrossRef]
- Wang, D.Y.; Zhang, D.Z.; Tang, M.C.; Zhang, H.; Sun, T.H.; Yang, C.Q.; Mao, R.Y.; Li, K.S.; Wang, J.H. Ethylene chlorotrifluoroethylene/hydrogel-based liquid-solid triboelectric nanogenerator driven self-powered MXene-based sensor system for marine environmental monitoring. Nano Energy 2022, 100, 107509. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Wang, C.; Zhao, L.J.; Liu, F.M.; Sun, X.Y.; Hu, X.L.; Lu, G.Y. Preparation of Ce-doped SnO2 cuboids with enhanced 2-butanone sensing performance. Sens. Actuators B 2021, 341, 130039. [Google Scholar] [CrossRef]
- Ortega, P.; Hangai, B.; Moreno, H.; Rocha, L.; Ramirez, M.; Ponce, M.; Longo, E.; Simoes, A. Tuning structural, optical, and gas sensing properties of ceria-based materials by rare-earth doping. J. Alloys Compd. 2021, 888, 161517. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, B.Y.; Li, Y.; Zhang, S.M.; Zhang, Y.X.; Hua, X.H.; Liu, G.; Li, B.S.; Zhou, J.Y.; Xie, E.Q. Methanol gas detection of electrospun CeO2 nanofibers by regulating Ce3+/Ce4+ mole ratio via Pd doping. Sens. Actuators B 2020, 307, 127638. [Google Scholar] [CrossRef]
- Yoon, J.W.; Kim, J.S.; Kim, T.H.; Hong, Y.J.; Kang, Y.C.; Lee, J.H. A new strategy for humidity independent oxide chemiresistors: Dynamic self-refreshing of In2O3 sensing surface assisted by layer-by-layer coated CeO2 nanoclusters. Small 2016, 12, 4229–4240. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lv, H.; Liu, Z.; Chen, J.K.; Wang, J.; Sun, B.H.; Zhang, Y.; Wang, R.H.; Shi, K.Y. Thin-layered MoS2 nanoflakes vertically grown on SnO2 nanotubes as highly effective room-temperature NO2 gas sensor. J. Hazard. Mater. 2021, 416, 125830. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Yang, K.; Xiao, C.L.; Jia, L.C. Electrospun Bi-doped SnO2 porous nanosheets for highly sensitive nitric oxide detection. J. Hazard. Mater. 2021, 416, 126118. [Google Scholar] [CrossRef]
- Liu, J.S.; Song, B.Y.; Huang, J.; Deng, Z.P.; Zhang, X.F.; Xu, Y.M.; Huo, L.H.; Gao, S. Absorbent cotton derived mesoporous CeO2 hollow tubule for enhanced detection of p-xylene at low energy consumption. J. Hazard. Mater. 2021, 873, 159774. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, L.X.; Xing, Y.; Zhang, P.; Chen, J.J.; Yin, J.; Bie, L.J. Morphology-controlled synthesis of CeO2 nanocrystals and their facet-dependent gas sensing properties. Sens. Actuators B 2021, 330, 129374. [Google Scholar] [CrossRef]
- Tammanoon, N.; Wisitsoraat, A.; Tuantranont, A.; Liewhiran, C. Flame-made Zn-substituted SnO2 nanoparticulate compound for ultra-sensitive formic acid gas sensing. J. Alloys Compd. 2021, 871, 159547. [Google Scholar] [CrossRef]
- Zito, C.A.; Perfecto, T.M.; Dippel, A.C.; Volanti, D.P.; Koziej, D. Low-temperature carbon dioxide gas sensor based on yolk−shell ceria nanospheres. ACS Appl. Mater. Interfaces 2020, 12, 17745–17751. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Gas sensors based on CeO2 nanoparticles prepared by chemical precipitation method and their temperature-dependent selectivity towards H2S and NO2 gases. Appl. Surf. Sci. 2020, 505, 144356. [Google Scholar] [CrossRef]
- Li, Q.Y.; Huang, N.; Cui, Y.H.; Lin, J.S.; Zhao, C.; Ding, L. Synthesis of porous rod-like In2O3 nanomaterials and its selective detection of NO at room temperature. J. Alloys Compd. 2022, 902, 163632. [Google Scholar] [CrossRef]
- Choi, M.S.; Mirzaei, A.; Na, H.G.; Kim, S.; Kim, D.E.; Lee, K.H.; Jin, C.; Choi, S.W. Facile and fast decoration of SnO2 nanowires with Pd embedded SnO2-x nanoparticles for selective NO2 gas sensing. Sens. Actuators B 2021, 340, 129984. [Google Scholar] [CrossRef]
- Lv, Y.K.; Li, Y.Y.; Yao, H.C.; Li, Z.J. Nitrogen-doped graphene quantum dots-modified mesoporous SnO2 hierarchical hollow cubes for low temperature detection of nitrogen dioxide. Sens. Actuators B 2021, 339, 129882. [Google Scholar] [CrossRef]
- Zhou, S.Q.; Lu, Q.J.; Chen, M.P.; Li, B.; Wei, H.T.; Zi, B.Y.; Zeng, J.Y.; Zhang, Y.M.; Zhang, J.; Zhu, Z.Q.; et al. Platinum-supported cerium-doped indium oxide for highly sensitive triethylamine gas sensing with good antihumidity. ACS Appl. Mater. Interfaces 2020, 12, 42962–42970. [Google Scholar] [CrossRef]
- Sun, Y.J.; Zhao, Z.T.; Zhou, R.; Li, P.W.; Zhang, W.D.; Suematsu, K.; Hu, J. Synthesis of In2O3 nanocubes, nanocube clusters, and nanocubes-embedded Au nanoparticles for conductometric CO sensors. Sens. Actuators B 2021, 345, 130433. [Google Scholar] [CrossRef]
- Luo, Y.B.; An, B.X.; Bai, J.L.; Wang, Y.R.; Cheng, X.; Wang, Q.; Li, J.P.; Yang, Y.F.; Wu, Z.K.; Xie, E. Ultrahigh-response hydrogen sensor based on PdO/NiO co-doped In2O3 nanotubes. J. Colloid Interface Sci. 2021, 599, 533–542. [Google Scholar] [CrossRef]
- Li, H.K.; Yang, Z.Y.; Ling, W.Y.; Zhu, D.C.; Pu, Y. UV excited gas sensing SnO2-ZnO aerogels to ppb-level ethanol detection. Sens. Actuators B 2021, 337, 129815. [Google Scholar] [CrossRef]
- Haldar, T.; Kumar, U.; Yadav, B.C.; Kumar, V.V.R.K. Hierarchical flower-like Bi2SiO5/MWCNT nanocomposites for highly sensitive LPG sensor at room temperature. J. Alloys Compd. 2021, 856, 158157. [Google Scholar] [CrossRef]
- Phuoc, P.H.; Viet, N.N.; Thong, L.V.; Hung, C.M.; Hoa, N.D.; Duy, N.V.; Hong, H.S.; Hieu, N.V. Comparative study on the gas-sensing performance of ZnO/SnO2 external and ZnO-SnO2 internal heterojunctions for ppb H2S and NO2 gases detection. Sens. Actuators B 2021, 334, 129606. [Google Scholar] [CrossRef]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced gas sensing properties of SnO2 hollow spheres decorated with CeO2 nanoparticles heterostructure composite materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef]
- Liu, Z.C.; Gui, Y.G.; Xu, L.G.; Chen, X.P. Adsorption and sensing performances of transition metal (Ag, Pd, Pt, Rh, and Ru) modified WSe2 monolayer upon SF6 decomposition gases (SOF2 and SO2F2). Appl. Surf. Sci. 2022, 581, 152365. [Google Scholar] [CrossRef]
- Haq, M.; Din, S.U.; Baohui, D.; Khan, S.; Zhu, L.P. Low-Temperature Detection of Sulfur-Hexafluoride Decomposition Products Using Octahedral Co3O4-Modified NiSnO3 Nanofibers. ACS Appl. Mater. Interfaces 2022, 14, 9292–9306. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, A.; Rizwan, K.; Farooq, U.; Iqbal, S.; Altaf, A.A. Advanced polymeric/inorganic nanohybrids: An integrated platform for gas sensing applications. Chemosphere 2022, 294, 133772. [Google Scholar] [CrossRef]
- Yang, Z.M.; Zhang, D.Z.; Wang, D.Y. Carbon monoxide gas sensing properties of metal-organic frameworks-derived tin dioxide nanoparticles/molybdenum diselenide nanoflowers. Sens. Actuators B 2020, 304, 127369. [Google Scholar] [CrossRef]
- Hu, J.; Sun, Y.J.; Xue, Y.; Zhang, M.; Li, P.W.; Lian, K.; Zhuiykov, S.; Zhang, W.D.; Chen, Y. Highly sensitive and ultra-fast gas sensor based on CeO2-loaded In2O3 hollow spheres for ppb-level hydrogen detection. Sens. Actuators B 2018, 257, 124–135. [Google Scholar] [CrossRef]
- Cheng, T.; Yang, W.M.; Hao, W.J.; Yang, Y.; Li, X.; Wei, T.H. A colorimetry-impedance dual-mode ceruloplasmin detection strategy based on dendritic mesoporous silicon nanoparticles with CeO2 encapsulation inside and horseradish peroxidase enrichment outside. Sens. Actuators B 2022, 355, 131312. [Google Scholar] [CrossRef]
- Gong, L.; Wang, X.; Zhang, D.; Ma, X.; Yu, S. Flexible wearable humidity sensor based on cerium oxide/graphitic carbon nitride nanocomposite self-powered by motion-driven alternator and its application for human physiological detection. J. Mater. Chem. A 2021, 9, 5619–5629. [Google Scholar] [CrossRef]
- Zhang, J.; Li, T.; Guo, J.; Hu, Y.; Zhang, D. Two-step hydrothermal fabrication of CeO2-loaded MoS2 nanoflowers for ethanol gas sensing application. Appl. Surf. Sci. 2021, 568, 150942. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Y.K.; Song, P.; Sun, J.; Wang, Q. Enhanced trimethylamine gas-sensing performance of CeO2 nanoparticles-decorated MoO3 nanorods. J. Mater. Sci. Mater. Electron. 2022, 33, 3453–3464. [Google Scholar] [CrossRef]
- Bao, H.M.; Guo, Y.J.; Zhang, T.; Fu, H.; Zhu, S.Y.; Zhou, L.; Zhang, H.W.; Li, Y.; Cai, W.P. Microporous-ceria-wrapped gold nanoparticles for conductometric and SERS dual monitoring of hazardous gases at room temperature. Adv. Mater. Interfaces 2022, 9, 2102107. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Diversiform metal oxide-based hybrid nanostructure for gas sensing with versatile prospects. Coord. Chem. Rev. 2020, 413, 213272. [Google Scholar] [CrossRef]
- Mou, H.R.; Sun, Y.; Zeng, Z.G.; Zhao, H.B.; An, B.L.; Xu, J.Q.; Wang, X.H. Low-temperature hydrogen detection sensor based on CeO2-DOPED SnO2. J. Mater. Sci. Mater. Electron. 2020, 31, 15785–15793. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhang, J.; Zhang, H.; Chen, C.; Zhang, D. Enhanced Sensing Performance of Electrospun Tin Dioxide Nanofibers Decorated with Cerium Dioxide Nanoparticles for the Detection of Liquefied Petroleum Gas. Chemosensors 2022, 10, 497. https://doi.org/10.3390/chemosensors10120497
Liu X, Zhang J, Zhang H, Chen C, Zhang D. Enhanced Sensing Performance of Electrospun Tin Dioxide Nanofibers Decorated with Cerium Dioxide Nanoparticles for the Detection of Liquefied Petroleum Gas. Chemosensors. 2022; 10(12):497. https://doi.org/10.3390/chemosensors10120497
Chicago/Turabian StyleLiu, Xichen, Jianhua Zhang, Hao Zhang, Can Chen, and Dongzhi Zhang. 2022. "Enhanced Sensing Performance of Electrospun Tin Dioxide Nanofibers Decorated with Cerium Dioxide Nanoparticles for the Detection of Liquefied Petroleum Gas" Chemosensors 10, no. 12: 497. https://doi.org/10.3390/chemosensors10120497
APA StyleLiu, X., Zhang, J., Zhang, H., Chen, C., & Zhang, D. (2022). Enhanced Sensing Performance of Electrospun Tin Dioxide Nanofibers Decorated with Cerium Dioxide Nanoparticles for the Detection of Liquefied Petroleum Gas. Chemosensors, 10(12), 497. https://doi.org/10.3390/chemosensors10120497





