The Advances of Hydrosol–Gel Transition-Based Sensors
Abstract
:1. Introduction
2. Types of Hydrogels in the Development of Sol–Gel Transition-Based Sensors
2.1. DNA Hydrogels
2.2. Polypeptide Hydrogels
2.3. Polysaccharide Hydrogels
3. The Methods Used for the Development of Sol–Gel Transition-Based Sensors
3.1. Colorimetric Assay
3.2. Fluorescence Assay
3.3. Surface-Enhanced Raman Spectroscopy (SERS) Assay
3.4. Magnetic Relaxation Switching (MRS) Assay
3.5. pH-Based Assay
3.6. Weight Assay by Electronic Balance
3.7. Glucose Assay by Personal Glucose Meter
3.8. Distance-Based Lateral Flow Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karimi, M.; Mehrabadi, Z.; Farsadrooh, M.; Bafkary, R.; Derikvandi, H.; Hayati, P.; Mohammadi, K. Chapter 4—Metal–organic framework. In Interface Science and Technology; Ghaedi, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 33, pp. 279–387. [Google Scholar]
- Dera, R.; Diliën, H.; Billen, B.; Gagliardi, M.; Rahimi, N.; Den Akker, N.M.S.; Molin, D.G.M.; Grandfils, C.; Adriaensens, P.; Guedens, W.; et al. Phosphodiester hydrogels for cell scaffolding and drug release applications. Macromol. Biosci. 2019, 19, 1900090. [Google Scholar] [CrossRef] [PubMed]
- Pivato, R.V.; Rossi, F.; Ferro, M.; Castiglione, F.; Trotta, F.; Mele, A. β-cyclodextrin nanosponge hydrogels as drug delivery nanoarchitectonics for multistep drug release kinetics. ACS Appl. Polym. Mater. 2021, 3, 6562–6571. [Google Scholar] [CrossRef]
- Tang, J.; Qiao, Y.; Chu, Y.; Tong, Z.; Zhou, Y.; Zhang, W.; Xie, S.; Hu, J.; Wang, T. Magnetic double-network hydrogels for tissue hyperthermia and drug release. J. Mater. Chem. B 2019, 7, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Shen, J.; Sun, H.; Li, J.; Wang, X. Polymer-based hydrogels with local drug release for cancer immunotherapy. Biomed. Pharmacother. 2021, 137, 111333. [Google Scholar] [CrossRef]
- Weng, L.; Rostambeigi, N.; Zantek, N.D.; Rostamzadeh, P.; Bravo, M.; Carey, J.; Golzarian, J. An in situ forming biodegradable hydrogel-based embolic agent for interventional therapies. Acta Biomater. 2013, 9, 8182–8191. [Google Scholar] [CrossRef]
- Wang, W.; Song, H.; Zhang, J.; Li, P.; Li, C.; Wang, C.; Kong, D.; Zhao, Q. An injectable, thermosensitive and multicompartment hydrogel for simultaneous encapsulation and independent release of a drug cocktail as an effective combination therapy platform. J. Control Release 2015, 203, 57–66. [Google Scholar] [CrossRef]
- Guilbaud-Chéreau, C.; Dinesh, B.; Wagner, L.; Chaloin, O.; Ménard-Moyon, C.; Bianco, A. Aromatic dipeptide homologue-based hydrogels for photocontrolled drug release. Nanomaterials 2022, 12, 1643. [Google Scholar] [CrossRef]
- Hu, J.; Chen, Y.; Li, Y.; Zhou, Z.; Cheng, Y. A thermo-degradable hydrogel with light-tunable degradation and drug release. Biomaterials 2016, 112, 133–140. [Google Scholar] [CrossRef]
- Lou, C.; Tian, X.; Deng, H.; Wang, Y.; Jiang, X. Dialdehyde-β-cyclodextrin-crosslinked carboxymethyl chitosan hydrogel for drug release. Carbohydr. Polym. 2019, 231, 115678. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-based hydrogels for tissue engineering applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef]
- Khuu, N.; Kheiri, S.; Kumacheva, E. Structurally anisotropic hydrogels for tissue engineering. Trends Chem. 2021, 3, 1002–1026. [Google Scholar] [CrossRef]
- Yi, Y.; Xie, C.; Liu, J.; Zheng, Y.; Wang, J.; Lu, X. Self-adhesive hydrogels for tissue engineering. J. Mater. Chem. B 2021, 9, 8739–8767. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, M.; Zhang, Y.; Yin, J.; Pei, R. Nanocomposite hydrogels for tissue engineering applications. Nanoscale 2020, 12, 14976–14995. [Google Scholar] [CrossRef]
- Fu, L.; Wang, A.; Lyu, F.; Lai, G.; Yu, J.; Lin, C.-T.; Liu, Z.; Yu, A.; Su, W. A solid-state electrochemical sensing platform based on a supramolecular hydrogel. Sens. Actuators B Chem. 2018, 262, 326–333. [Google Scholar] [CrossRef]
- Sun, X.; He, S.; Qin, Z.; Li, J.; Yao, F. Fast self-healing zwitterion nanocomposite hydrogel for underwater sensing. Compos. Commun. 2021, 26, 100784. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wan, J.; Jiang, S.; Wu, Y.; Wang, W.; Gong, X.; Wang, H. Quantum dots-based hydrogels for sensing applications. Chem. Eng. J. 2020, 408, 127351. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sarkar, R.; Nandi, S.; Porgador, A.; Jelinek, R. Detection of reactive oxygen species by a carbon-dot–ascorbic acid hydrogel. Anal. Chem. 2016, 89, 830–836. [Google Scholar] [CrossRef]
- Chang, W.-H.; Lee, Y.-F.; Liu, Y.-W.; Willner, I.; Liao, W.-C. Stimuli-responsive hydrogel microcapsules for the amplified detection of microRNAs. Nanoscale 2021, 13, 16799–16808. [Google Scholar] [CrossRef]
- Ma, Y.; Mao, Y.; An, Y.; Tian, T.; Zhang, H.; Yan, J.; Zhu, Z.; Yang, C.J. Target-responsive DNA hydrogel for non-enzymatic and visual detection of glucose. Analyst 2018, 143, 1679–1684. [Google Scholar] [CrossRef]
- Vassalini, I.; Ribaudo, G.; Gianoncelli, A.; Casula, M.F.; Alessandri, I. Plasmonic hydrogels for capture, detection and removal of organic pollutants. Environ. Sci. Nano 2020, 7, 3888–3900. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, V.V.; Hoogenboom, R. Bioinspired double network hydrogels: From covalent double network hydrogels via hybrid double network hydrogels to physical double network hydrogels. Mater. Horiz. 2020, 8, 1173–1188. [Google Scholar] [CrossRef]
- Gao, Y.; Gu, S.; Jia, F.; Wang, Q.; Gao, G. “All-in-one” hydrolyzed keratin protein-modified polyacrylamide composite hydrogel transducer. Chem. Eng. J. 2020, 398, 125555. [Google Scholar] [CrossRef]
- Xie, H.; Yu, Q.; Mao, J.; Wang, S.; Hu, Y.; Guo, Z. A conductive polyacrylamide/double bond chitosan/polyaniline hydrogel for flexible sensing. J. Mater. Sci. Mater. Electron. 2020, 31, 10381–10389. [Google Scholar] [CrossRef]
- Hayashi, T.; Takinoue, M.; Onoe, H. DNA aptamer-linked structural-color hydrogel for repeatable biochemical sensing. In Proceedings of 20th International Conference on Solid-State Sensors, Actuators and Microsystems and Eurosensors XXXIII (TRANSDUCERS and EUROSENSORS), Berlin, Germany, 23–27 June 2019; pp. 582–585. [Google Scholar]
- Dai, J.; Zhang, H.; Huang, C.; Chen, Z.; Han, A. A Gel-Based Separation-Free Point-of-Care Device for Whole Blood Glucose Detection. Anal. Chem. 2020, 92, 16122–16129. [Google Scholar] [CrossRef]
- Erfkamp, J.; Guenther, M.; Gerlach, G. Enzyme-Functionalized Piezoresistive Hydrogel Biosensors for the Detection of Urea. Sensors 2019, 19, 2858. [Google Scholar] [CrossRef] [Green Version]
- Qin, T.; Liao, W.; Yu, L.; Zhu, J.; Wu, M.; Peng, Q.; Han, L.; Zeng, H. Recent progress in conductive self-healing hydrogels for flexible sensors. J. Polym. Sci. 2022, 60, 2607–2634. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Mi, Y.; Zhao, S.; Qi, S.; Sun, M.; Peng, B.; Xu, Q.; Niu, Y.; Zhou, Y. Transparent stretchable hydrogel sensors: Materials, design and applications. J. Mater. Chem. C 2022, 10, 13351–13371. [Google Scholar] [CrossRef]
- Ata, S.; Banerjee, S.L.; Singha, N.K. Polymer nano-hybrid material based on graphene oxide/POSS via surface initiated atom transfer radical polymerization (SI-ATRP): Its application in specialty hydrogel system. Polymer 2016, 103, 46–56. [Google Scholar] [CrossRef]
- Ping, J.; Wu, W.; Qi, L.; Liu, J.; Liu, J.; Zhao, B.; Wang, Q.; Yu, L.; Lin, J.M.; Hu, Q. Hydrogel-assisted paper-based lateral flow sensor for the detection of trypsin in human serum. Biosens. Bioelectron. 2021, 192, 113548. [Google Scholar] [CrossRef]
- Zhao, L.; Yin, S.; Ma, Z. Ca(2+)-triggered pH-response sodium alginate hydrogel precipitation for amplified sandwich-type impedimetric immunosensor of tumor marker. ACS Sens. 2019, 4, 450–455. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Ge, J.; Jie, G. Versatile fluorescence detection of microRNA based on novel DNA hydrogel-amplified signal probes coupled with DNA walker amplification. Chem. Commun. 2019, 55, 3919–3922. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xie, S.; Zhang, J.; Tang, D.; Tang, Y. An electrochemical impedance biosensor for Hg2+ detection based on DNA hydrogel by coupling with DNAzyme-assisted target recycling and hybridization chain reaction. Biosens. Bioelectron. 2017, 98, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.A.; Park, J.C.; Na, H.; Jeon, H.; Nam, Y.S. Short DNA-catalyzed formation of quantum dot-DNA hydrogel for enzyme-free femtomolar specific DNA assay. Biosens. Bioelectron. 2021, 182, 113110. [Google Scholar] [CrossRef]
- Mao, X.; Pan, S.; Zhou, D.; He, X.; Zhang, Y. Fabrication of DNAzyme-functionalized hydrogel and its application for visible detection of circulating tumor DNA. Sens. Actuators B Chem. 2019, 285, 385–390. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, P.; Guo, Y.; Wang, L.; Luo, F.; Qiu, B.; Guo, L.; Su, X.; Lin, Z.; Chen, G. Detection of aflatoxin B1 in food samples based on target-responsive aptamer-cross-linked hydrogel using a handheld pH meter as readout. Talanta 2018, 176, 34–39. [Google Scholar] [CrossRef]
- Han, C.; Yuan, X.; Shen, Z.; Xiao, Y.; Wang, X.; Khan, M.; Liu, S.; Li, W.; Hu, Q.; Wu, W. A paper-based lateral flow sensor for the detection of thrombin and its inhibitors. Anal. Chim. Acta 2022, 1205, 339756. [Google Scholar] [CrossRef]
- Li, Z.; Tang, C.; Huang, D.; Qin, W.; Luo, F.; Wang, J.; Guo, L.; Qiu, B.; Lin, Z. Sensitive hyaluronidase biosensor based on target-responsive hydrogel using electronic balance as readout. Anal. Chem. 2019, 91, 11821–11826. [Google Scholar] [CrossRef]
- Danish, E.Y.; Bakhsh, E.M.; Akhtar, K. Design of chitosan nanocomposite hydrogel for sensitive detection and removal of organic pollutants. Int. J. Biol. Macromol. 2020, 159, 276–286. [Google Scholar] [CrossRef]
- Li, C.; Duan, L.; Cheng, X. Facile method to synthesize fluorescent chitosan hydrogels for selective detection and adsorption of Hg2+/Hg+. Carbohydr. Polym. 2022, 288, 119417. [Google Scholar] [CrossRef]
- Adnan, P.K.M.; Sreejith, L. Compact poly-electrolyte complex hydrogels of gelatin and sodium alginate for sensing wound status. Mater. Lett. 2022, 313, 131705. [Google Scholar] [CrossRef]
- Lengert, E.; Saveleva, M.; Abalymov, A.; Atkin, V.; Wuytens, P.C.; Kamyshinsky, R.; Vasiliev, A.L.; Gorin, D.A.; Sukhorukov, G.B.; Skirtach, A.G.; et al. Silver alginate hydrogel micro- and nanocontainers for theranostics: Synthesis, encapsulation, remote release, and detection. ACS Appl. Mater. Interfaces 2017, 9, 21949–21958. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Z.; Feng, C.; Xianyu, Y.; Chen, Y. Direct transverse relaxation time biosensing strategy for detecting foodborne pathogens through enzyme-mediated sol−gel transition of hydrogels. Anal. Chem. 2021, 93, 6613–6619. [Google Scholar] [CrossRef]
- Yao, S.; Xiang, L.; Wang, L.; Gong, H.; Chen, F.; Cai, C. pH-responsive DNA hydrogels with ratiometric fluorescence for accurate detection of miRNA-21. Anal. Chim. Acta 2022, 1207, 339795. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.; Zhang, J.; Peng, Y.; Li, S.; Han, D.; Ren, S.; Qin, K.; Li, S.; Gao, Z. Stimuli-responsive DNA-based hydrogels for biosensing applications. J. Nanobiotechnol. 2022, 20, 40. [Google Scholar] [CrossRef]
- Chu, J.; Chen, C.; Li, X.; Yu, L.; Li, W.; Cheng, M.; Tang, W.; Xiong, Z. A responsive pure DNA hydrogel for label-free detection of lead ion. Anal. Chim. Acta 2021, 1157, 338400. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, R.; Hou, Y.; Qin, Y.; Li, S.; Yang, S.; Gao, Z. DNA hydrogels combined with microfluidic chips for melamine detection. Anal. Chim. Acta 2022, 1228, 340312. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, X.; Sun, Y.; Dai, Y.; Sun, W.; Zhu, X.; Liu, H.; Han, R.; Gao, D.; Luo, C. A chemiluminescent biosensor for ultrasensitive detection of adenosine based on target-responsive DNA hydrogel with Au@HKUST-1 encapsulation. Sens. Actuators B Chem. 2019, 289, 56–64. [Google Scholar] [CrossRef]
- Liu, C.; Gou, S.; Bi, Y.; Gao, Q.; Sun, J.; Hu, S.; Guo, W. Smart DNA-gold nanoparticle hybrid hydrogel film based portable, cost-effective and storable biosensing system for the colorimetric detection of lead (II) and uranyl ions. Biosens. Bioelectron. 2022, 210, 114290. [Google Scholar] [CrossRef]
- Ji, X.; Lv, H.; Sun, X.; Ding, C. Green-emitting carbon dot loaded silica nanoparticles coated with DNA-cross-linked hydrogels for sensitive carcinoembryonic antigen detection and effective targeted cancer therapy. Chem. Commun. 2019, 55, 15101–15104. [Google Scholar] [CrossRef]
- Ji, X.; Wang, J.; Niu, S.; Ding, C. Size-controlled DNA-cross-linked hydrogel coated silica nanoparticles served as a ratiometric fluorescent probe for the detection of adenosine triphosphate in living cells. Chem. Commun. 2019, 55, 5243–5246. [Google Scholar] [CrossRef]
- Qiao, Y.; Liu, X.; Zhou, X.; Zhang, H.; Zhang, W.; Xiao, W.; Pan, G.; Cui, W.; Santos, H.A.; Shi, Q. Gelatin templated polypeptide co-cross-linked hydrogel for bone tegeneration. Adv. Healthc. Mater. 2020, 9, 1901239. [Google Scholar] [CrossRef]
- Cheng, L.; Cai, Z.; Ye, T.; Yu, X.; Chen, Z.; Yan, Y.; Qi, J.; Wang, L.; Liu, Z.; Cui, W.; et al. Injectable polypeptide-protein hydrogels for promoting infected wound healing. Adv. Funct. Mater. 2020, 30, 2001196. [Google Scholar] [CrossRef]
- Jandl, B.; Sedghiniya, S.; Carstens, A.; Astakhova, K. Peptide–fluorophore hydrogel as a signal boosting approach in rapid detection of cancer DNA. ACS Omega 2019, 4, 13889–13895. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.X.; Xie, T.J.; Li, C.H.; Ye, Q.C.; Tian, L.L.; Li, Y.F.; Huang, C.Z.; Zhen, S.J. A crosslinked submicro-hydrogel formed by DNA circuit-driven protein aggregation amplified fluorescence anisotropy for biomolecules detection. Anal. Chim. Acta 2021, 1154, 338319. [Google Scholar] [CrossRef]
- Ahmad, N.; Colak, B.; Zhang, D.-W.; Gibbs, M.J.; Watkinson, M.; Becer, C.R.; Gautrot, J.E.; Krause, S. Peptide cross-linked poly (ethylene glycol) hydrogel films as biosensor coatings for the detection of collagenase. Sensors 2019, 19, 1677. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Hu, X.; Xu, J.; Liu, X.; Yang, L. Single-step In situ acetylcholinesterase-mediated alginate hydrogelation for enzyme encapsulation in CE. Anal. Chem. 2018, 90, 4071–4078. [Google Scholar] [CrossRef]
- Hu, X.; Yang, J.; Chen, C.; Khan, H.; Guo, Y.; Yang, L. Capillary electrophoresis-integrated immobilized enzyme microreactor utilizing single-step in-situ penicillinase-mediated alginate hydrogelation: Application for enzyme assays of penicillinase. Talanta 2018, 189, 377–382. [Google Scholar] [CrossRef]
- Sun, Y.; Li, S.; Chen, R.; Wu, P.; Liang, J. Ultrasensitive and rapid detection of T-2 toxin using a target-responsive DNA hydrogel. Sens. Actuators B Chem. 2020, 311, 127912. [Google Scholar] [CrossRef]
- Gao, L.; Li, Y.; Huang, Z.Z.; Tan, H. Visual detection of alkaline phosphatase based on ascorbic acid-triggered gel-sol transition of alginate hydrogel. Anal. Chim. Acta 2021, 1148, 238193. [Google Scholar] [CrossRef]
- Hao, L.; Liu, X.; Xu, S.; An, F.; Gu, H.; Xu, F. A novel aptasensor based on DNA hydrogel for sensitive visual detection of ochratoxin A. Mikrochim. Acta 2021, 188, 395. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Z.Z.; Weng, Y.; Tan, H. Pyrophosphate ion-responsive alginate hydrogel as an effective fluorescent sensing platform for alkaline phosphatase detection. Chem. Commun. 2019, 55, 11450–11453. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, W.; Shen, X.; Wang, S.; Li, Q.; An, F.; Wu, S. A fluorescent DNA hydrogel aptasensor based on the self-assembly of rolling circle amplification products for sensitive detection of ochratoxin A. J. Agric. Food Chem. 2020, 68, 369–375. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhou, X.; Liu, W.; Liu, Y.; Wang, X. Flexible DNA hydrogel SERS active biofilms for conformal ultrasensitive detection of uranyl Ions from aquatic products. Langmuir 2020, 36, 2930–2936. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Jiang, N.; Wang, J.; Yu, M.; Zhuang, X. Preparation of aptamer responsive DNA functionalized hydrogels for the sensitive detection of alpha-fetoprotein using SERS method. Bioconjug. Chem. 2020, 31, 813–820. [Google Scholar] [CrossRef]
- Tang, L.; Huang, Y.; Lin, C.; Qiu, B.; Guo, L.; Luo, F.; Lin, Z. Highly sensitive and selective aflatoxin B1 biosensor based on Exonuclease I-catalyzed target recycling amplification and targeted response aptamer-crosslinked hydrogel using electronic balances as a readout. Talanta 2020, 214, 120862. [Google Scholar] [CrossRef]
- Dong, N.; Cai, Q.; Li, Z.; Xu, L.; Wu, H.; Lin, Z.; Qiu, B.; Li, C.; Lin, Z. Convenient hyaluronidase biosensors based on the target-trigger enhancing of the permeability of a membrane using an electronic balance as a readout. Analyst 2021, 146, 3299–3304. [Google Scholar] [CrossRef]
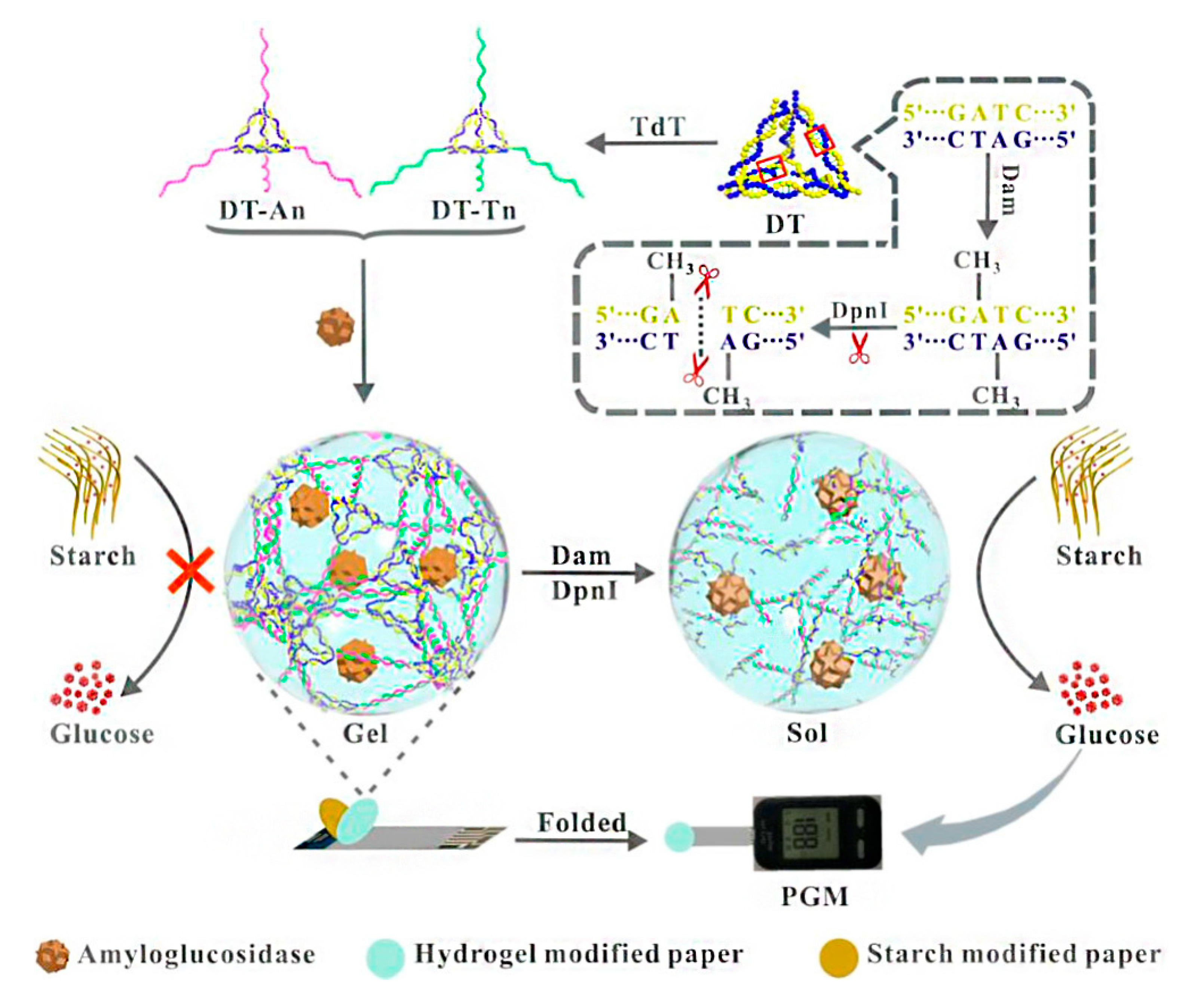
- Gao, X.; Li, X.; Sun, X.; Zhang, J.; Zhao, Y.; Liu, X.; Li, F. DNA tetrahedra-cross-linked hydrogel functionalized paper for onsite analysis of DNA methyltransferase activity using a personal glucose meter. Anal. Chem. 2020, 92, 4592–4599. [Google Scholar] [CrossRef]
- Xu, J.; Hu, X.; Khan, H.; Tian, M.; Yang, L. Converting solution viscosity to distance-readout on paper substrates based on enzyme-mediated alginate hydrogelation: Quantitative determination of organophosphorus pesticides. Anal. Chim. Acta 2019, 1071, 1–7. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Khan, H.; Yang, L. A dual-readout paper-based sensor for on-site detection of penicillinase with a smartphone. Sens. Actuators B Chem. 2021, 335, 129707. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Qian, Z.M.; Wang, S.; Yang, F.Q. Glucose oxidase-mediated sodium alginate gelation: Equipment-Free detection of glucose in fruit samples. Enzym. Microb. Technol. 2021, 148, 109805. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Y.; Wang, H.; Chen, D.; Wen, Y. A portable visual capillary sensor based on functional DNA crosslinked hydrogel for point-of-care detection of lead ion. Sens. Actuators B Chem. 2019, 307, 127625. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Y.; Li, M.; Jia, L.; Zhang, L.; Zhu, J. Gelatin-based photonic hydrogels for visual detection of pathogenic Pseudomonas aeruginosa. Sens. Actuators B Chem. 2020, 329, 129137. [Google Scholar] [CrossRef]
- Mao, Y.; Li, J.; Yan, J.; Ma, Y.; Song, Y.; Tian, T.; Liu, X.; Zhu, Z.; Zhou, L.; Yang, C. A portable visual detection method based on a target-responsive DNA hydrogel and the color change of gold nanorods. Chem. Commun. 2017, 53, 6375–6378. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Zhang, Y.; Liu, Q.; Huang, H.; Wang, X.; Shi, Z.; Li, Y.; Liu, S.; Xue, L.; Lei, Y. A smart hydrogel system for visual detection of glucose. Biosens. Bioelectron. 2019, 142, 111547. [Google Scholar] [CrossRef]
- Wu, W.-Z.; Huang, M.-X.; Huang, Q.-D.; Lyu, C.-H.; Lai, J.-P.; Sun, H. Molecularly imprinted photonic hydrogels for visual detection of methylanthranilate in wine. Chin. J. Anal. Chem. 2019, 47, 1330–1336. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Liu, S.; Li, B. A label-free visual aptasensor for zearalenone detection based on target-responsive aptamer-cross-linked hydrogel and color change of gold nanoparticles. Food Chem. 2022, 389, 133078. [Google Scholar] [CrossRef]
- Lu, S.; Wang, S.; Zhao, J.; Sun, J.; Yang, X. A pH-controlled bidirectionally pure DNA hydrogel: Reversible self-assembly and fluorescence monitoring. Chem. Commun. 2018, 54, 4621–4624. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.; He, R.; Zhang, Y.; He, Y.; Wang, C.; Lu, Z.; Liu, Y.; Ju, H. Fluorescence hydrogel array based on interfacial cation exchange amplification for highly sensitive microRNA detection. Anal. Chim. Acta 2019, 1080, 206–214. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, A.; Zhang, Q.; Cui, D. Nanomaterial-based SERS sensing technology for biomedical application. J. Mater. Chem. B 2019, 7, 3755–3774. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, J.; He, X.; Yang, S.; Wu, H.; Qin, Z.; Chu, M.; Zhang, Z.; Liao, J.; Wang, X. Research progress of SERS on uranyl ions and uranyl compounds: A review. J. Mater. Chem. C 2022, 10, 4006–4018. [Google Scholar] [CrossRef]
- Alcantara, D.; Lopez, S.; García-Martin, M.L.; Pozo, D. Iron oxide nanoparticles as magnetic relaxation switching (MRSw) sensors: Current applications in nanomedicine. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1253–1262. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; An, W.; Wei, Y.; Liu, N.; Chen, Y.; Shuang, S. Magnetic relaxation switch immunosensor for the rapid detection of the foodborne pathogen Salmonella enterica in milk samples. Food Control 2015, 55, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xie, M. A magnetic relaxation switching immunosensor for one-step detection of salbutamol based on gold nanoparticle–streptavidin conjugate. RSC Adv. 2015, 5, 95401–95404. [Google Scholar] [CrossRef]
- Jia, F.; Xu, L.; Yan, W.; Wu, W.; Yu, Q.; Tian, X.; Dai, R.; Li, X. A magnetic relaxation switch aptasensor for the rapid detection of Pseudomonas aeruginosa using superparamagnetic nanoparticles. Microchim. Acta 2017, 184, 1539–1545. [Google Scholar] [CrossRef]
- Alqurashi, Y.; Elsherif, M.; Hendi, A.; Essa, K.; Butt, H. Optical Hydrogel Detector for pH Measurements. Biosensors 2022, 12, 40. [Google Scholar] [CrossRef]
- Han, B.; Yan, Q.; Liu, Q.; Li, D.; Chen, Y.; He, G. Bright green emission non-conjugated polymer dots: pH trigged hydrogel for specific adsorption of anionic dyes and visual detection of tert-butylhydroquinone. Sep. Purif. Technol. 2022, 292, 121023. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, Y.-N.; Han, B.; Zhao, Q.; Zhang, A.; Fu, D. In-line Mach-Zehnder interferometer and FBG with smart hydrogel for simultaneous pH and temperature detection. IEEE Sens. J. 2018, 18, 7499–7504. [Google Scholar] [CrossRef]
- Scarpa, E.; Mastronardi, V.M.; Guido, F.; Algieri, L.; Qualtieri, A.; Fiammengo, R.; Rizzi, F.; De Vittorio, M. Wearable piezoelectric mass sensor based on pH sensitive hydrogels for sweat pH monitoring. Sci. Rep. 2020, 10, 10854. [Google Scholar] [CrossRef]
- Zhao, L.; Li, G.; Gan, J.; Yang, Z. Hydrogel optical fiber based ratiometric fluorescence sensor for highly sensitive pH detection. J. Light. Technol. 2021, 39, 6653–6659. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, Z.; Zou, Y.; Huang, Y.; Liu, D.; Jia, S.; Xu, D.; Wu, M.; Zhou, Y.; Zhou, S.; et al. Target-responsive “sweet” hydrogel with glucometer readout for portable and quantitative detection of non-glucose targets. J. Am. Chem. Soc. 2013, 135, 3748–3751. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jia, S.; Zhang, H.; Ma, Y.; Guan, Z.; Li, J.; Zhu, Z.; Ji, T.; Yang, C.J. Integrating target-responsive hydrogel with pressuremeter readout enables simple, sensitive, user-friendly, quantitative point-of-care testing. ACS Appl. Mater. Interfaces 2017, 9, 22252–22258. [Google Scholar] [CrossRef] [PubMed]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2018, 126, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, B.; Chen, L.; Qin, W. A three-dimensional origami paper-based device for potentiometric biosensing. Angew. Chem. Int. Ed. 2016, 55, 13033–13037. [Google Scholar] [CrossRef]
- Liang, B.; Zhu, Q.; Fang, L.; Cao, Q.; Liang, X.; Ye, X. An origami paper device for complete elimination of interferents in enzymatic electrochemical biosensors. Electrochem. Commun. 2017, 82, 43–46. [Google Scholar] [CrossRef]
- Xue, W.; Dan Zhao, N.; Zhang, Q.; Chang, Y.; Liu, M. An origami paper-based analytical device for DNA damage analysis. Chem. Commun. 2021, 57, 11465–11468. [Google Scholar] [CrossRef]
- Chauhan, A.; Toley, B.J. Barrier-free microfluidic paper analytical devices for multiplex colorimetric detection of analytes. Anal. Chem. 2021, 93, 8954–8961. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chong, K.-Y.; Lei, K.F. Analysis of the internal hypoxic environment in solid tumor tissue using a folding paper system. ACS Appl. Mater. Interfaces 2021, 13, 33885–33893. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, L.; Jia, J.; Chen, H.; Fu, H.; She, Y. Development of a triple channel colorimetric paper sensor array based on quantum dots: A robust tool for process monitoring and quality control of basic liquors of Baijiu. Sens. Actuators B Chem. 2020, 319, 128260. [Google Scholar] [CrossRef]
- Pomili, T.; Donati, P.; Pompa, P.P. Paper-based multiplexed colorimetric device for the simultaneous detection of salivary biomarkers. Biosensors 2021, 11, 443. [Google Scholar] [CrossRef]
- Xiong, X.; Zhang, J.; Wang, Z.; Liu, C.; Xiao, W.; Han, J.; Shi, Q. Simultaneous multiplexed detection of protein and metal ions by a colorimetric microfluidic paper-based analytical device. BioChip J. 2020, 14, 429–437. [Google Scholar] [CrossRef]
- Vaquer, A.; Barón, E.; de la Rica, R. Dissolvable polymer valves for sweat chrono-sampling in wearable paper-based analytical devices. ACS Sens. 2022, 7, 488–494. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Do, J.Y.; Hong, C.A. Target DNA- and pH-responsive DNA hydrogel–based capillary assay for the optical detection of short SARS-CoV-2 cDNA. Microchim. Acta 2021, 189, 34. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, F.; Zhang, K.; Min, T.; Chen, D.; Wen, Y. Distance-based biosensor for ultrasensitive detection of uracil-DNA glycosylase using membrane filtration of DNA hydrogel. ACS Sens. 2021, 6, 2395–2402. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Y.; Jiao, X.; Li, T.; Lv, Z.; Yang, C.J.; Zhang, X.; Wen, Y. Control of capillary behavior through target-responsive hydrogel permeability alteration for sensitive visual quantitative detection. Nat. Commun. 2019, 10, 1036. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Luo, L.; Guo, Y.; Zhao, B.; Chen, X.; Shi, X.; Khan, M.; Lin, J.M.; Hu, Q. Viscosity-based flow sensor on paper for quantitative and label-free detection of alpha-amylase and Its inhibitor. ACS Sens. 2022, 7, 593–600. [Google Scholar] [CrossRef]
- Zhao, B.; Qi, L.; Tai, W.; Zhao, M.; Chen, X.; Yu, L.; Shi, J.; Wang, X.; Lin, J.M.; Hu, Q. Paper-based flow sensor for the detection of hyaluronidase via an enzyme hydrolysis-induced viscosity change in a polymer solution. Anal. Chem. 2022, 94, 4643–4649. [Google Scholar] [CrossRef]
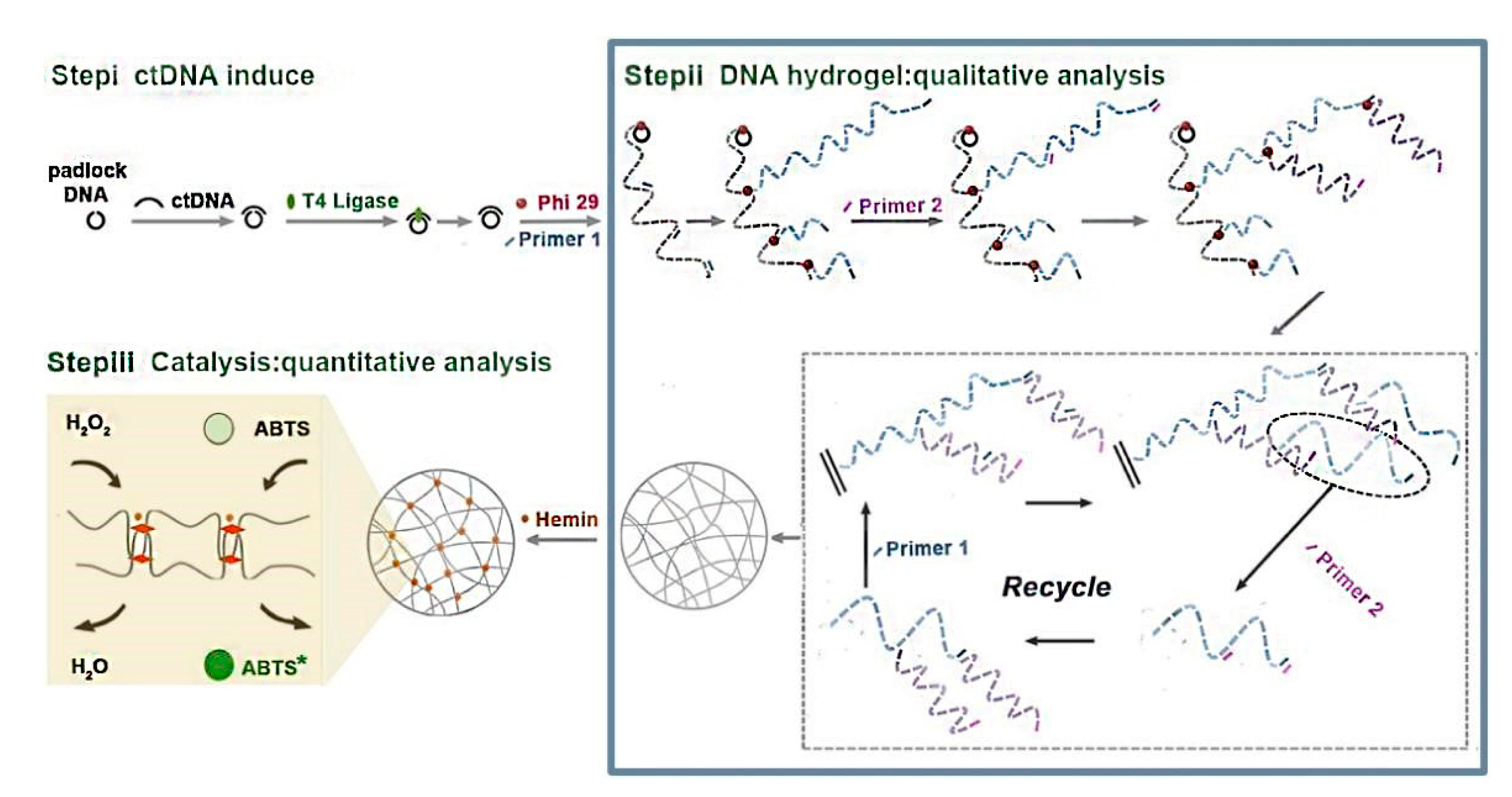
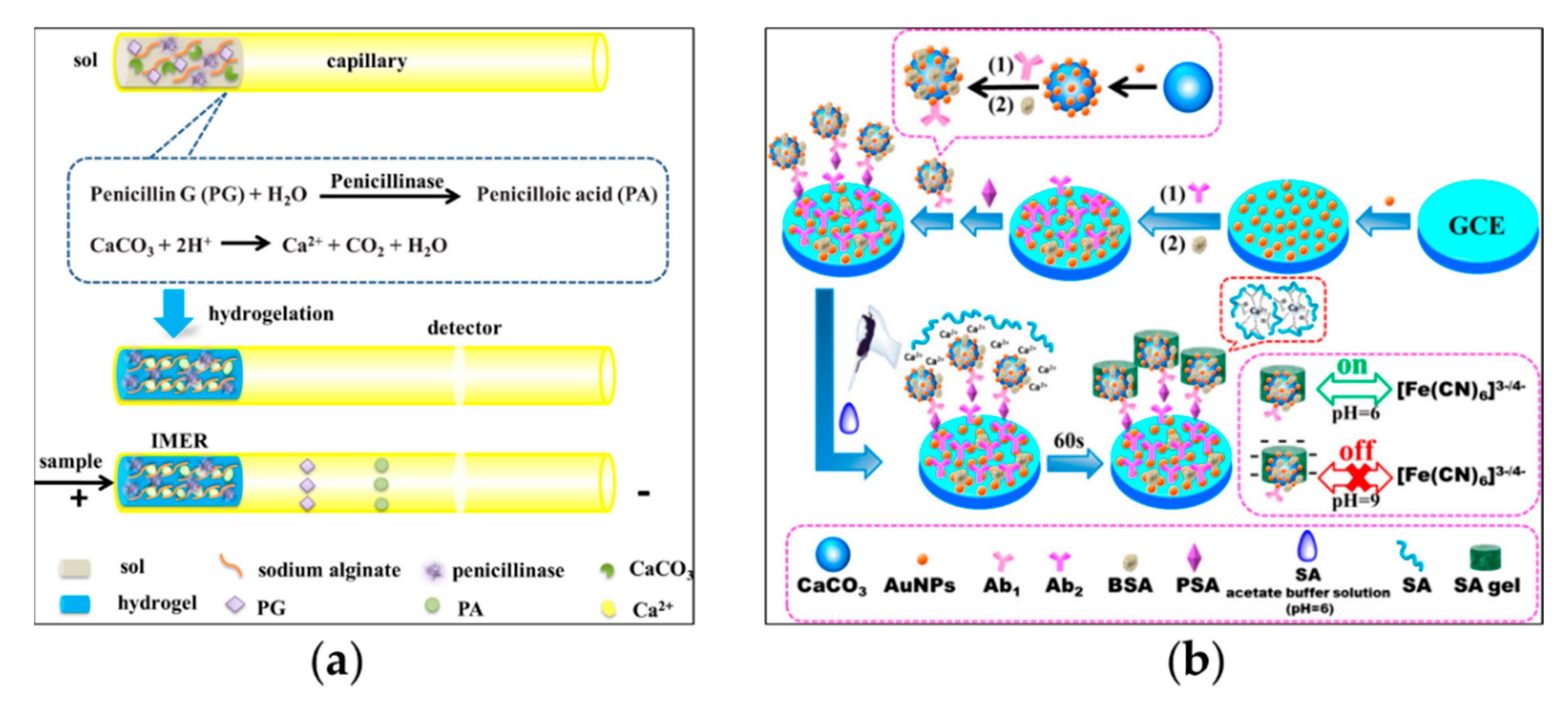



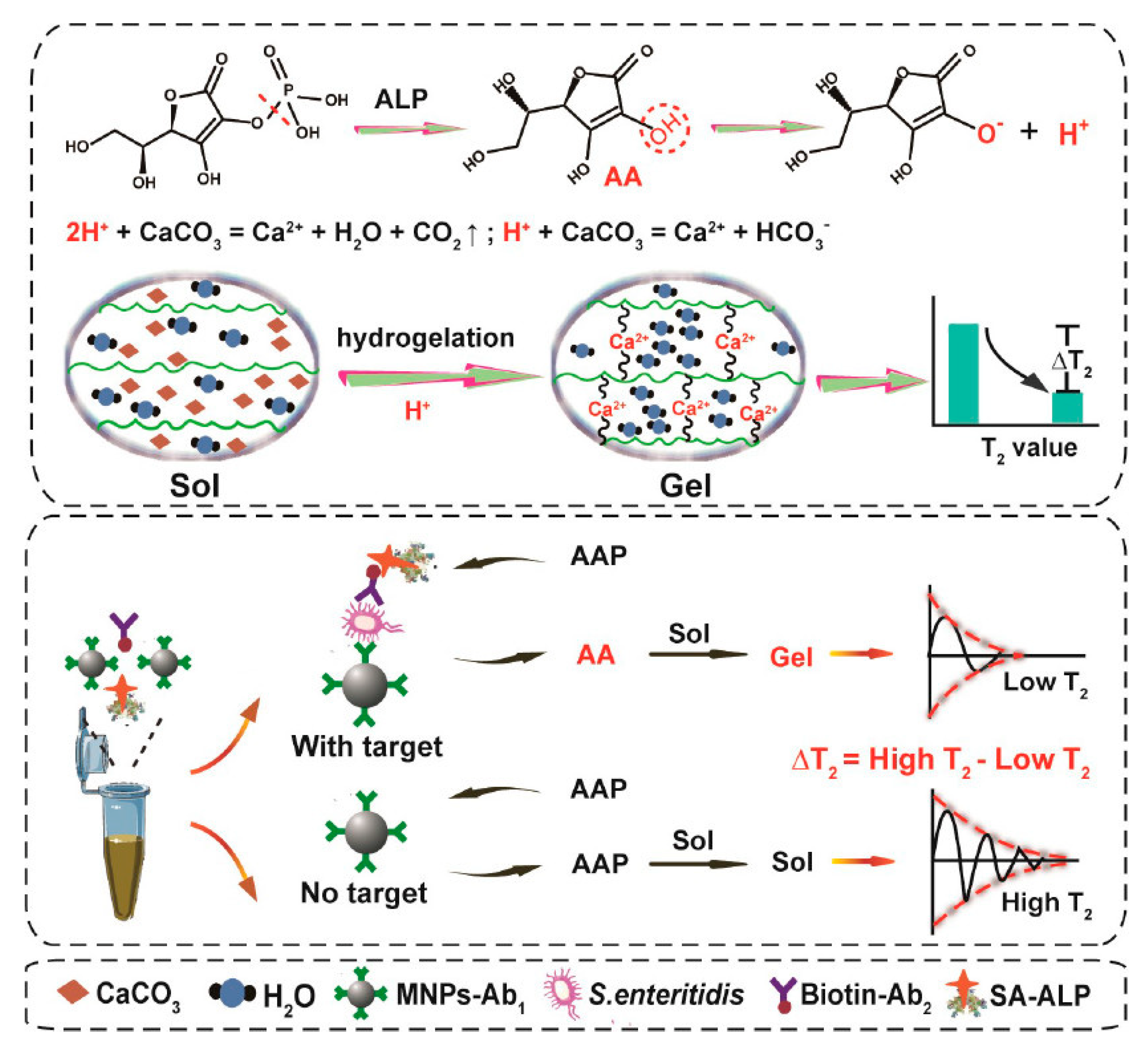

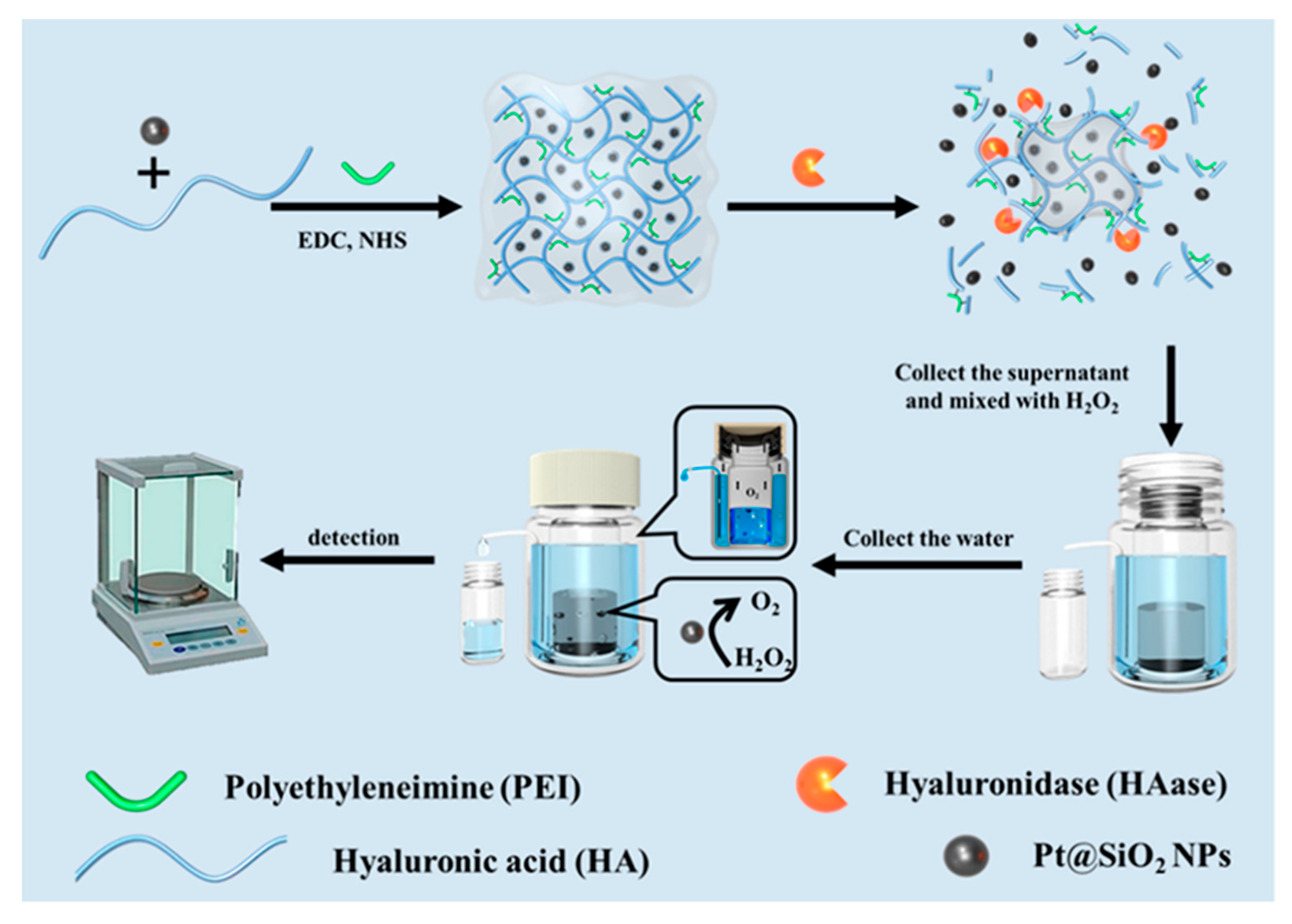



| Type of Hydrogels | Output Signal | Target | Recognition Unit | Reference |
|---|---|---|---|---|
| Hybrid/pure DNA hydrogels | Fluorescence | MicroRNA-141 | DNA probe | [34] |
| Color | Glucose | Target aptamer strand | [20] | |
| Electricity | Hg2+ | DNAzyme/Hairpin DNA | [35] | |
| Fluorescence | Drug-resistant bacteria gene | Hairpin DNA | [36] | |
| Color | Circulating tumor DNA | G-quadruplex | [37] | |
| pH | Aflatoxin B1 | Target aptamer strand | [38] | |
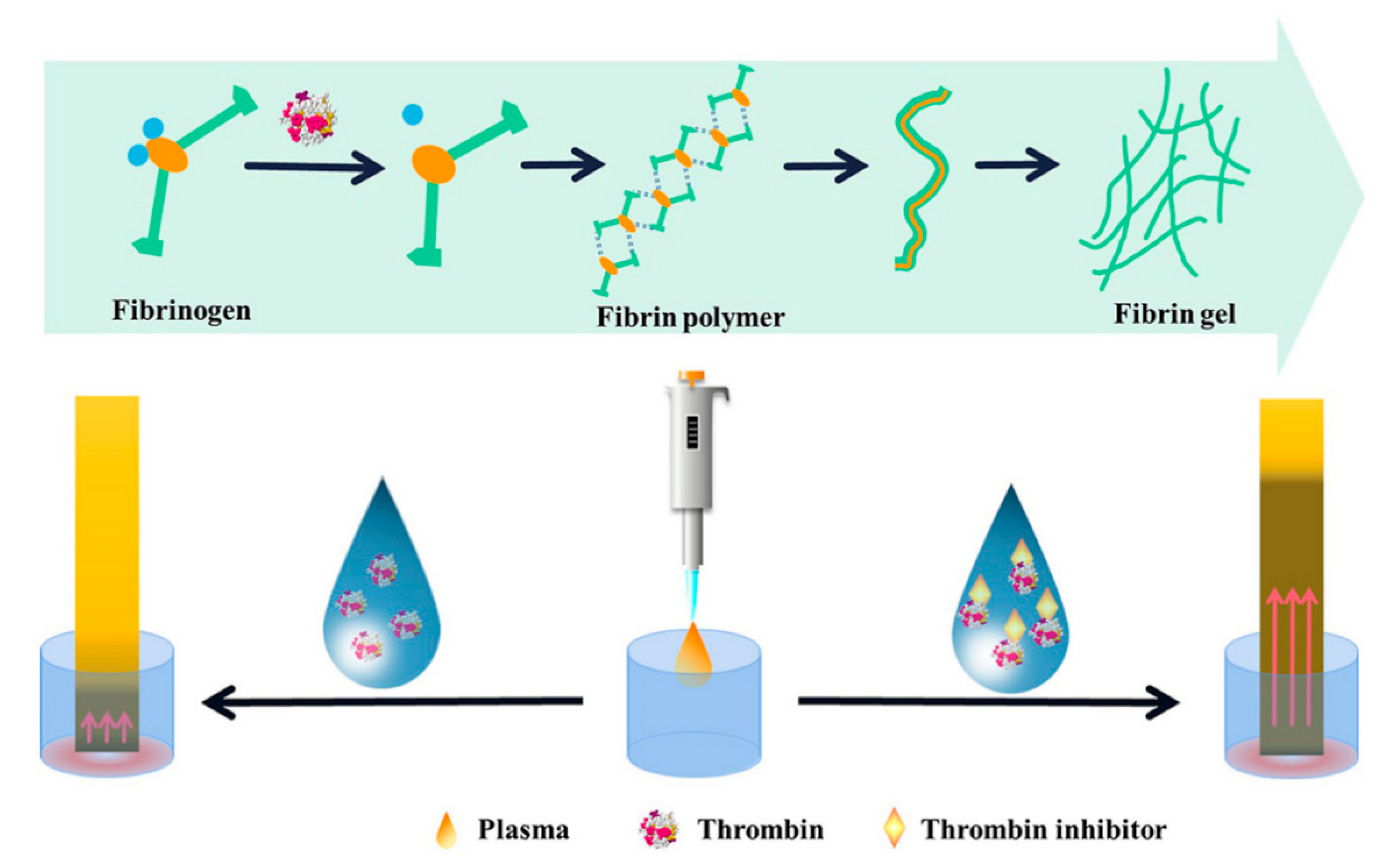
| Polypeptide hydrogels | Distance | Thrombin/inhibitors | Thrombin | [39] |
| Weight | Hyaluronidase | Pt@SiO2NPs | [40] | |
| Polysaccharide hydrogels | Electrochemistry | 4-Nitrophenol | Glutaraldehyde | [41] |
| Fluorescence | Hg2+/Hg+ | Glutaraldehyde | [42] | |
| Color | pH | Ca2+ crosslinker | [43] | |
| Surface-Enhanced Raman Scattering | BSA-tetramethylrhodamine | Ag-nanoparticle crosslinker | [44] | |
| Magnetic Resonance Spectroscopy | Foodborne pathogens | ALP-modified antibody | [43,45] |
| Method | Detection Application | Limit of Detection | Reference |
|---|---|---|---|
| Colorimetric assay | T-2 mycotoxin | 0.87 pg/mL | [61] |
| Alkaline phosphatase | 0.37 mU/mL | [62] | |
| Ochratoxin A | 0.005 ng/mL | [63] | |
| Fluorescence assay | Alkaline phosphatase | 21.3 μM | [64] |
| Ochratoxin A | 0.01 ng/mL | [65] | |
| Surface-enhanced Raman spectroscopy (SERS) assay | UO22+ | 8.38 × 10−13 M | [66] |
| α-fetoprotein | 50 pg/mL | [67] | |
| Magnetic relaxation switching (MRS) assay | Foodborne pathogens | 50 CFU/mL | [45] |
| pH-based assay | Aflatoxin B1 | 0.1 μM | [38] |
| Weight assay by electronic balance | Hyaluronidase | 0.2 U/mL | [40] |
| AFB1 | 9.4 μg/kg | [68] | |
| Hyaluronidase | 0.35 U mL−1 | [69] | |
| Glucose assay by personal glucose meter | DNA adenine methyltransferase | 0.001 U/mL | [70] |
| Distance-based lateral flow assay | Organophosphorus pesticides | 3.3 ng/mL | [71] |
| Penicillinase | 2.67 × 10−3 mU/μL 2.67 × 10−2 mU/μL | [72] | |
| Glucose | 1.4 mM | [73] | |
| Pb2+ | 10 nM | [74] | |
| Thrombin and inhibitor | 16.1 mU/mL | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Ding, S.; Zhao, M.; Hu, Q. The Advances of Hydrosol–Gel Transition-Based Sensors. Chemosensors 2022, 10, 415. https://doi.org/10.3390/chemosensors10100415
Song H, Ding S, Zhao M, Hu Q. The Advances of Hydrosol–Gel Transition-Based Sensors. Chemosensors. 2022; 10(10):415. https://doi.org/10.3390/chemosensors10100415
Chicago/Turabian StyleSong, Haoyang, Shichao Ding, Mei Zhao, and Qiongzheng Hu. 2022. "The Advances of Hydrosol–Gel Transition-Based Sensors" Chemosensors 10, no. 10: 415. https://doi.org/10.3390/chemosensors10100415
APA StyleSong, H., Ding, S., Zhao, M., & Hu, Q. (2022). The Advances of Hydrosol–Gel Transition-Based Sensors. Chemosensors, 10(10), 415. https://doi.org/10.3390/chemosensors10100415








