Use of Charlson Comorbidity Index and Nomogram to Predict Mortality in Elderly Patients with Late-Life Schizophrenia
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Study Population
2.2. Outcomes
2.3. Confounding Variables
2.4. Statistical Analysis
3. Results
3.1. Hospitalization
3.2. Deaths
3.3. Comorbidities
3.4. Adjusted Models of Overall Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laursen, T.M.; Munk-Olsen, T.; Gasse, C. Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS ONE 2011, 6, e24597. [Google Scholar] [CrossRef]
- Vermeulen, J.M.; van Rooijen, G.; van de Kerkhof, M.; Sutterland, A.L.; Correll, C.U.; de Haan, L. Clozapine and long-term mortality risk in patients with schizophrenia: A systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophr. Bull. 2019, 45, 315–329. [Google Scholar] [CrossRef]
- Hjorthøj, C.; Stürup, A.E.; McGrath, J.J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301. [Google Scholar] [CrossRef]
- Olfson, M.; Gerhard, T.; Huang, C.; Crystal, S.; Stroup, T.S. Premature mortality among adults with schizophrenia in the united states. JAMA Psychiatry 2015, 72, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Lêng, C.H.; Chou, M.H.; Lin, S.H.; Yang, Y.K.; Wang, J.D. Estimation of life expectancy, loss-of-life expectancy, and lifetime healthcare expenditures for schizophrenia in Taiwan. Schizophr. Res. 2016, 171, 97–102. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Yeh, L.-L.; Chan, H.-Y.; Chang, C.-K. Excess mortality and shortened life expectancy in people with major mental illnesses in Taiwan. Epidemiol. Psychiatr. Sci. 2020, 29, e156. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Rapini, G.; Olivieri, L.; Di Nicola, D.; Tomasetti, C.; Valchera, A.; Fornaro, M.; Di Fabio, F.; Perna, G.; Di Nicola, M.; et al. Safety of antipsychotics for the treatment of schizophrenia: A focus on the adverse effects of clozapine. Ther. Adv. Drug Saf. 2018, 9, 237–256. [Google Scholar] [CrossRef]
- United Nations; Department of Economic and Social Affairs; Population Division. World Population Ageing 2019: Highlights (ST/ESA/SER.A/430). Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf (accessed on 18 June 2021).
- Law & Regulation Database of The Republic of China, 2020. Senior Citizens Welfare Act. Available online: https://law.moj.gov.tw/ENG/LawClass/LawAll.aspx?pcode=D0050037 (accessed on 13 June 2021).
- Stafford, J.; Howard, R.; Kirkbride, J.B. The incidence of very late-onset psychotic disorders: A systematic review and meta-analysis, 1960–2016. Psychol. Med. 2018, 48, 1775–1786. [Google Scholar] [CrossRef]
- Cohen, C.I.; Meesters, P.D.; Zhao, J. New perspectives on schizophrenia in later life: Implications for treatment, policy, and research. Lancet Psychiatry 2015, 2, 340–350. [Google Scholar] [CrossRef]
- Cort, E.; Meehan, J.; Reeves, S.; Howard, R. Very late–onset schizophrenia-like psychosis: A clinical update. J. Psychosoc. Nurs. Ment. Health Serv. 2017, 56, 37–47. [Google Scholar] [CrossRef]
- Suen, Y.N.; Wong, S.M.Y.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.H.M.; Chang, W.C.; Chen, E.Y.H. Late-onset psychosis and very-late-onset-schizophrenia-like-psychosis: An updated systematic review. Int. Rev. Psychiatry 2019, 31, 523–542. [Google Scholar] [CrossRef] [PubMed]
- Talaslahti, T.; Alanen, H.M.; Hakko, H.; Isohanni, M.; Häkkinen, U.; Leinonen, E. Patients with very-late-onset schizophrenia-like psychosis have higher mortality rates than elderly patients with earlier onset schizophrenia. Int. J. Ger. Psychiatry 2015, 30, 453–459. [Google Scholar] [CrossRef]
- Laor, A.; Tal, S.; Guller, V.; Zbar, A.P.; Mavor, E. The Charlson comorbidity index (CCI) as a mortality predictor after surgery in elderly patients. Am. J. Surg. 2016, 82, 22–27. [Google Scholar] [CrossRef]
- Chwastiak, L.; Rosenheck, R.; McEvoy, J.P.; Keefe, R.S.; Swartz, M.S.; Lieberman, J.A. Interrelationships of psychiatric symptom severity, medical comorbidity, and functioning in schizophrenia. Psychiatr. Serv. 2006, 57, 1102–1109. [Google Scholar] [CrossRef]
- Van Assche, L.; Morrens, M.; Luyten, P.; Van de Ven, L.; Vandenbulcke, M. The neuropsychology and neurobiology of late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: A critical review. Neurosci. Biobehav. Rev. 2017, 83, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.; Rabins, P.V.; Seeman, M.V.; Jeste, D.V. Late-onset schizophrenia and very-late-onset schizophrenia-like psychosis: An international consensus. The International Late-Onset Schizophrenia Group. Am. J. Psychiatry 2000, 157, 172–178. [Google Scholar] [CrossRef]
- Brodaty, H.; Sachdev, P.; Koschera, A.; Monk, D.; Cullen, B. Long-term outcome of late-onset schizophrenia: 5-year follow-up study. Br. J. Psychiatry 2003, 183, 213–219. [Google Scholar] [CrossRef]
- Cohen, C.I. Very late-onset schizophrenia-like psychosis: Positive findings but questions remain unanswered. Lancet Psychiatry 2018, 5, 528–529. [Google Scholar] [CrossRef]
- Carney, C.P.; Jones, L.; Woolson, R.F. Medical comorbidity in women and men with schizophrenia: A population-based controlled study. J. Gen. Intern. Med. 2006, 21, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Huang, J. Schizophrenia and risk of dementia: A meta-analysis study. Neuropsychiatr. Dis. Treat. 2018, 14, 2047–2055. [Google Scholar] [CrossRef]
- Sugai, T.; Suzuki, Y.; Yamazaki, M.; Shimoda, K.; Mori, T.; Ozeki, Y.; Matsuda, H.; Sugawara, N.; Yasui-Furukori, N.; Minami, Y.; et al. High prevalence of obesity, hypertension, hyperlipidemia, and diabetes mellitus in Japanese outpatients with schizophrenia: A nationwide survey. PLoS ONE 2016, 11, e0166429. [Google Scholar] [CrossRef] [PubMed]
- Pillinger, T.; Beck, K.; Gobjila, C.; Donocik, J.G.; Jauhar, S.; Howes, O.D. Impaired glucose homeostasis in first-episode schizophrenia: A systematic review and meta-analysis. JAMA Psychiatry 2017, 74, 261–269. [Google Scholar] [CrossRef]
- Batki, S.L.; Meszaros, Z.S.; Strutynski, K.; Dimmock, J.A.; Leontieva, L.; Ploutz-Snyder, R.; Canfield, K.; Drayer, R.A. Medical comorbidity in patients with schizophrenia and alcohol dependence. Schizophr. Res. 2009, 107, 139–146. [Google Scholar] [CrossRef]
- Copeland, L.A.; Zeber, J.E.; Wang, C.P.; Parchman, M.L.; Lawrence, V.A.; Valenstein, M.; Miller, A.L. Patterns of primary care and mortality among patients with schizophrenia or diabetes: A cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv. Res. 2009, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, J.F.; Blow, F.C. Older patients with serious mental illness: Sensitivity to distance barriers for outpatient care. Med. Care 2004, 42, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Kilbourne, A.M.; Morden, N.E.; Austin, K.; Ilgen, M.; McCarthy, J.F.; Dalack, G.; Blow, F.C. Excess heart-disease-related mortality in a national study of patients with mental disorders: Identifying modifiable risk factors. Gen. Hosp. Psychiatry 2009, 31, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Bouza, C.; Lopez-Cuadrado, T.; Amate, J.M. Physical disease in schizophrenia: A population-based analysis in Spain. BMC Public Health 2010, 10, 745. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Cappucciati, M.; Rutigliano, G.; Heslin, M.; Stahl, D.; Brittenden, Z.; Caverzasi, E.; McGuire, P.; Carpenter, W.T. Diagnostic stability of ICD/DSM first episode psychosis diagnoses: Meta-analysis. Schizophr. Bull. 2016, 42, 1395–1406. [Google Scholar] [CrossRef]
- Schultze-Lutter, F.; Nenadic, I.; Grant, P. Psychosis and schizophrenia-spectrum personality disorders require early detection on different symptom dimensions. Front. Psychiatry 2019, 10, 476. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Pan, Y.J.; Kuo, K.H.; Yeh, L.L. Healthcare cost, service use and mortality in major psychiatric disorders in Taiwan. J. Affect. Disord. 2019, 246, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.J.; Malone, D. Physical health and schizophrenia. Curr. Opin. Psychiatry 2006, 19, 432–437. [Google Scholar] [CrossRef][Green Version]
- Kim, W.; Jang, S.Y.; Chun, S.Y.; Lee, T.H.; Han, K.T.; Park, E.C. Mortality in schizophrenia and other psychoses: Data from the South Korea National Health Insurance Cohort, 2002–2013. J. Korean Med. Sci. 2017, 32, 835. [Google Scholar] [CrossRef]
- Chien, I.C.; Hsu, J.H.; Lin, C.H.; Bih, S.H.; Chou, Y.J.; Chou, P. Prevalence of diabetes in patients with schizophrenia in Taiwan: A population-based National Health Insurance study. Schizophr. Res. 2009, 111, 17–22. [Google Scholar] [CrossRef]
- Jiang, Y.-D.; Chang, C.-H.; Tai, T.-Y.; Chen, J.-F.; Chuang, L.-M. Incidence and prevalence rates of diabetes mellitus in Taiwan: Analysis of the 2000–2009 Nationwide Health Insurance database. J. Formos. Med. Assoc. 2012, 111, 599–604. [Google Scholar] [CrossRef]
- Andreassen, O.A. Diabetes and schizophrenia-new findings for an old puzzle. Am. J. Psychiatry 2017, 174, 616–617. [Google Scholar] [CrossRef]
- Stubbs, B.; Vancampfort, D.; De Hert, M.; Mitchell, A.J. The prevalence and predictors of type two diabetes mellitus in people with schizophrenia: A systematic review and comparative meta-analysis. Acta Psychiatr. Scand. 2015, 132, 144–157. [Google Scholar] [CrossRef]
- Goueslard, K.; Petit, J.M.; Cottenet, J.; Chauvet-Gelinier, J.C.; Jollant, F.; Quantin, C. Increased risk of rehospitalization for acute diabetes complications and suicide attempts in patients with type 1 diabetes and comorbid schizophrenia. Diabetes Care 2018, 41, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Haroon, N.N.; Austin, P.C.; Shah, B.R.; Wu, J.; Gill, S.S.; Booth, G.L. Risk of dementia in seniors with newly diagnosed diabetes: A population-based study. Diabetes Care 2015, 38, 1868–1875. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, N.E.; Kowalchuk, C.; Agarwal, S.M.; Costa-Dookhan, K.A.; Caravaggio, F.; Gerretsen, P.; Chintoh, A.; Remington, G.J.; Taylor, V.H.; Müeller, D.J.; et al. Antipsychotics, metabolic adverse effects, and cognitive function in schizophrenia. Front. Psychiatry 2018, 9, 622. [Google Scholar] [CrossRef]
- Orsolini, L.; De Berardis, D.; Volpe, U. Up-to-date expert opinion on the safety of recently developed antipsychotics. Expert Opin. Drug Saf. 2020, 19, 981–998. [Google Scholar] [CrossRef]
- Orsolini, L.; Tomasetti, C.; Valchera, A.; Vecchiotti, R.; Matarazzo, I.; Vellante, F.; Iasevoli, F.; Buonaguro, E.F.; Fornaro, M.; Fiengo, A.L.; et al. An update of safety of clinically used atypical antipsychotics. Expert Opin. Drug Saf. 2016, 15, 1329–1347. [Google Scholar] [CrossRef]
- Sun, Y.; Lee, H.J.; Yang, S.C.; Chen, T.F.; Lin, K.N.; Lin, C.C.; Wang, P.N.; Tang, L.Y.; Chiu, M.J. A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PLoS ONE 2014, 9, e100303. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.E.; Chung, C.H.; Chen, L.F.; Chi, M.J. Increased risk of dementia in patients with Schizophrenia: A population-based cohort study in Taiwan. Eur. Psychiatry 2018, 53, 7–16. [Google Scholar] [CrossRef]
- Cooper, J.J.; Ovsiew, F. The relationship between schizophrenia and frontotemporal dementia. J. Geriatr. Psych. Neur. 2013, 26, 131–137. [Google Scholar] [CrossRef] [PubMed]
- DeMichele-Sweet, M.A.A.; Weamer, E.A.; Klei, L.; Vrana, D.T.; Hollingshead, D.J.; Seltman, H.J.; Sims, R.; Foroud, T.; Hernandez, I.; Moreno-Grau, S.; et al. Genetic risk for schizophrenia and psychosis in Alzheimer disease. Mol. Psychiatry 2017, 23, 963–972. [Google Scholar] [CrossRef]
- Berridge, M.J. Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion 2013, 7, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, L.; Van Aubel, E.; Van de Ven, L.; Bouckaert, F.; Luyten, P.; Vandenbulcke, M. The neuropsychological profile and phenomenology of late onset psychosis: A cross-sectional study on the differential diagnosis of very-late-onset schizophrenia-like psychosis, dementia with Lewy bodies and Alzheimer’s type dementia with psychosis. Arch. Clin. Neuropsychol. 2019, 34, 183–199. [Google Scholar] [CrossRef]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef]
- Ritchie, K.; Kildea, D. Is senile dementia “age-related” or “ageing-related”? Evidence from meta-analysis of dementia prevalence in the oldest old. Lancet 1995, 346, 931–934. [Google Scholar] [CrossRef]
- Wu, Y.T.; Lee, H.Y.; Norton, S.; Chen, C.; Chen, H.; He, C.; Fleming, J.; Matthews, F.E.; Brayne, C. Prevalence studies of dementia in Mainland China, Hong Kong and Taiwan: A systematic review and meta-analysis. PLoS ONE 2013, 8, e66252. [Google Scholar] [CrossRef]
- Liao, K.-M.; Ho, C.-H.; Ko, S.-C.; Li, C.-Y. Increased risk of dementia in patients with chronic obstructive pulmonary disease. Medicine 2015, 94, e930. [Google Scholar] [CrossRef]
- Bitter, I.; Czobor, P.; Borsi, A.; Fehér, L.; Nagy, B.Z.; Bacskai, M.; Rakonczai, P.; Hegyi, R.; Németh, T.; Varga, P.; et al. Mortality and the relationship of somatic comorbidities to mortality in schizophrenia. A nationwide matched-cohort study. Eur. Psychiatry 2017, 45, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Bratek, A.; Krysta, K.; Kucia, K. Psychiatric comorbidity in older adults with intellectual disability. Psychiatr. Danub. 2017, 29 (Suppl. 3), 590–593. [Google Scholar]
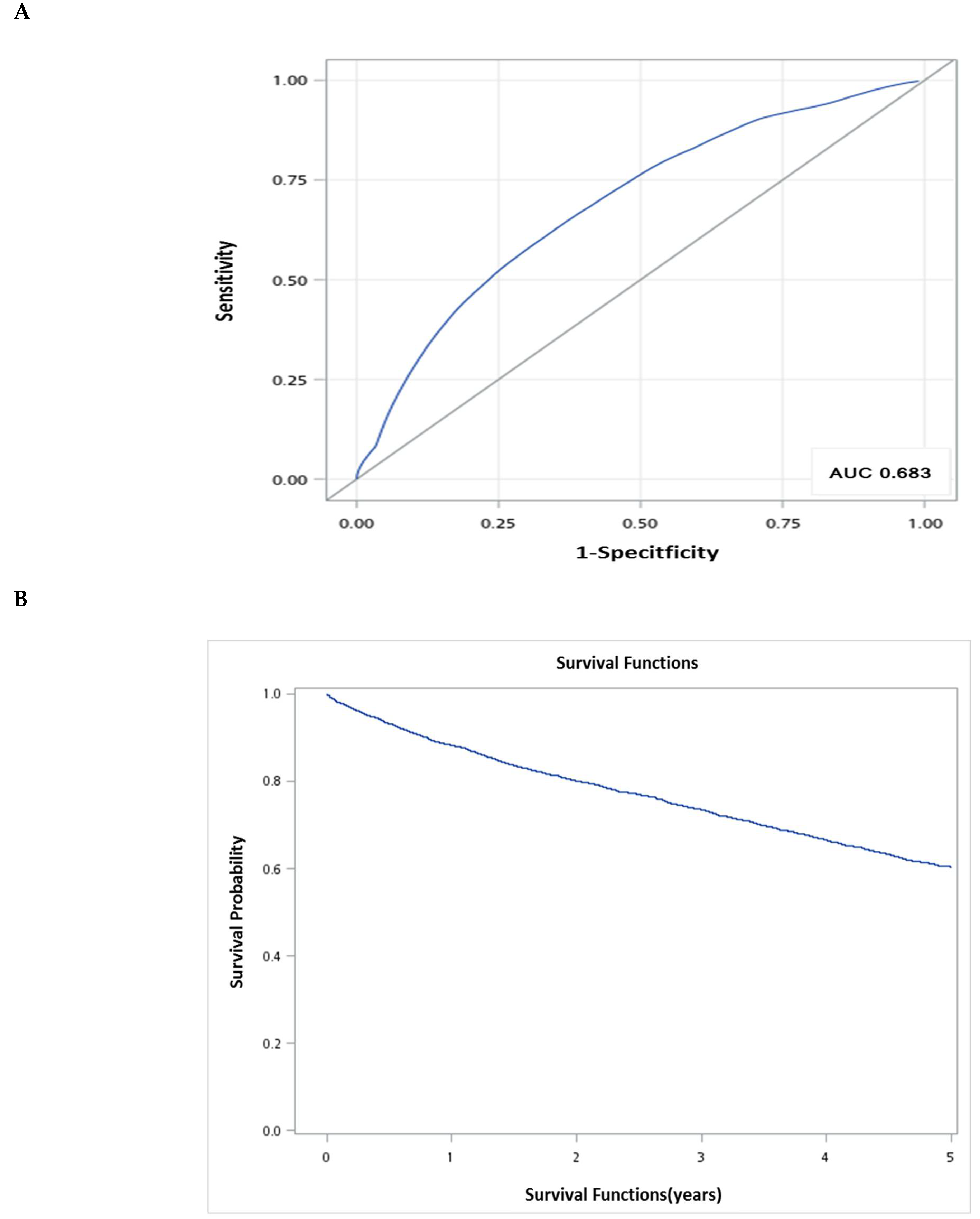
 ) survival probabilities at 5 years.
) survival probabilities at 5 years.
 ) survival probabilities at 5 years.
) survival probabilities at 5 years.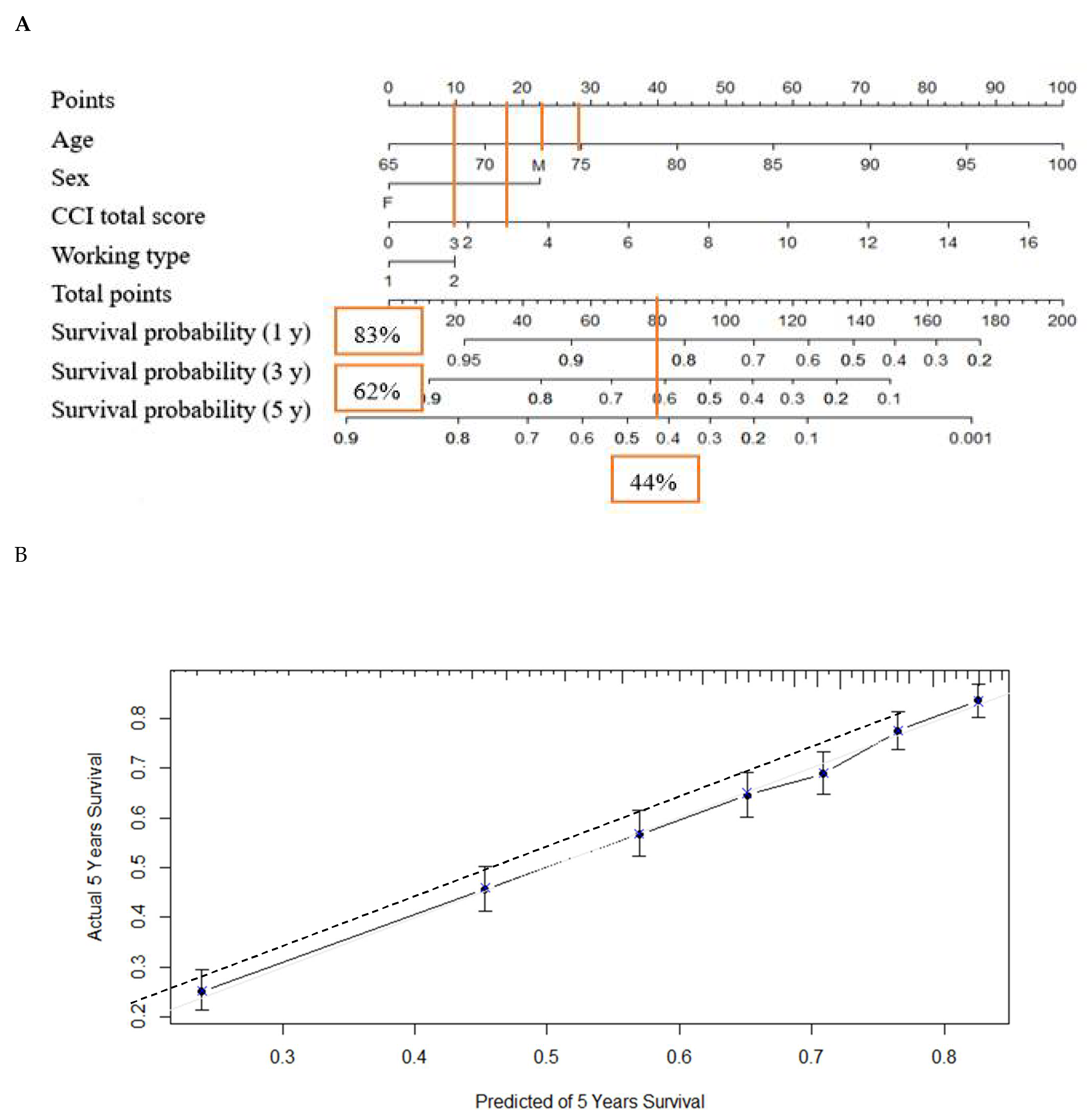
| Total (n = 3827) | Age Groups | Sex | ||||||
|---|---|---|---|---|---|---|---|---|
| 65–74 (n = 2433) 63.57% | 75–84 (n = 1166) 30.47% | 85+ (n = 228) 5.96% | p Value | Male (n = 2007) 52.44% | Female (n = 1820) 47.56% | p Value | ||
| Age distribution | ||||||||
| Mean age (years) | 72.89 ± 6.37 | 74.07 ± 6.70 | 71.60 ± 5.71 | |||||
| No. of males (%) | 2007 | 1124 (56.00) | 716 (35.68) | 167 (8.32) | <0.0001 | |||
| No. of Females (%) | 1820 | 1309 (71.92) | 450 (24.73) | 61 (3.35) | ||||
| Number of hospitalization (except first admission) | ||||||||
| SZ-related | 1.80 ± 3.37 | 2.04 ± 3.68 | 1.47 ± 2.79 | 0.95 ± 2.05 | <0.0001 | 1.91 ± 3.71 | 1.69 ± 2.94 | 0.0423 |
| Non-SZ-related | 3.58 ± 5.48 | 3.29 ± 5.66 | 4.19 ± 5.26 | 3.49 ± 4.38 | <0.0001 | 4.27 ± 6.08 | 2.81 ± 4.62 | <0.0001 |
| Hospital stay (days) of first admission due to schizophrenia | ||||||||
| Days | 26.00 | 29.00 | 20.00 | 14 | 0.0613 | 22.00 | 28.00 | 0.0110 |
| (Q1, Q3) | (9.00, 58.00) | (10.00, 60.00) | (8.00, 52.00) | (7.00, 41.50) | (8.00, 59.00) | (10.00, 56.00) | ||
| Follow-up | ||||||||
| Year | 4.11 | 6.71 | 4.43 | 2.14 | <0.0001 | 3.58 | 4.70 | <0.0001 |
| (Q1, Q3) | (1.66, 7.35) | (3.05, 11.39) | (1.74, 8.39) | (0.75, 4.38) | (1.30, 6.75) | (2.23, 7.89) | ||
| Working type | ||||||||
| Heavy labor | 614 (16.04) | 444 (18.25) | 153 (13.12) | 17 (7.46) | <0.0001 | 198 (9.87) | 416 (22.86) | <0.0001 |
| Moderate labor | 960 (25.08) | 698 (28.69) | 229 (19.64) | 33 (14.47) | 429 (21.38) | 531 (29.18) | ||
| Light labor | 2253 (58.88) | 1291 (53.06) | 784 (67.24) | 178 (78.07) | 1380 (68.75) | 873 (47.97) | ||
| Hospital location | ||||||||
| North | 1279 (33.42) | 859 (35.31) | 363 (31.13) | 57 (25.00) | <0.0001 | 528 (26.31) | 751 (41.26) | <0.0001 |
| Center | 857 (22.39) | 593 (24.37) | 222 (19.04) | 42 (18.42) | 442 (22.02) | 415 (22.80) | ||
| South | 1010 (26.39) | 676 (27.78) | 285 (24.44) | 49 (21.49) | 507 (25.26) | 503 (27.64) | ||
| East | 664 (17.35) | 297 (12.21) | 287 (24.61) | 80 (35.09) | 518 (25.81) | 146 (8.02) | ||
| Offshore | 17 (0.45) | 8 (0.33) | 9 (0.78) | - | 12 (0.60) | 5 (0.27) | ||
| Hospital level | ||||||||
| Medical centers | 608 (15.89) | 414 (17.02) | 160 (13.72) | 34 (14.91) | <0.0001 | 215 (10.71) | 393 (21.59) | <0.0001 |
| Metropolitan hospitals | 1513 (39.53) | 1087 (44.68) | 371 (31.82) | 55 (24.12) | 667 (33.23) | 846 (46.48) | ||
| Local community | 1695 (44.29) | 923 (37.94) | 633 (54.29) | 139 (60.96) | 1121 (55.85) | 574 (31.54) | ||
| Physician clinics | 11 ( 0.29) | 9 (0.37) | 2 (0.17) | - | 4 (0.21) | 7 (0.38) | ||
| Death | ||||||||
| Number of deaths (from first admission of SZ) | 1899 | 982 | 755 | 162 | 1218 | 681 | ||
| Mean age at death (years) | 73.50 ± 4.32 | 82.14 ± 3.83 | 89.32 ± 2.94 | 78.77 ± 6.68 | 77.41 ± 6.54 | |||
| (Q1, Q3) | (70.00, 76.00) | (79.00, 85.00) | (87.00, 91.00) | (74.00, 84.00) | (72.00, 82.00) | |||
| Time from 1st admission to death (years) | 4.24 ± 3.19 | 3.33 ± 2.86 | 1.87 ± 1.71 | 3.41 ± 2.97 | 4.15 ± 3.12 | |||
| (Q1, Q3) | (1.46, 6.47) | (0.945, 4.95) | (0.44, 3.06) | (0.88, 5.20) | (1.46, 6.30) | |||
| Days of hospitalization | 106.80 ± 330.15 | 92.62 ± 258.63 | 61.75 ± 158.19 | 111.49 ± 332.89 | 86.90 ± 263.52 | |||
| (Q1, Q3) | (10.00, 60.00) | (8.00, 52.00) | (7.00, 41.50) | (8.00, 59.00) | (10.00, 56.00) | |||
| Number of hospitalizations | 5.33 ± 6.90 | 5.67 ± 5.91 | 4.44 ± 4.96 | 6.18 ± 7.19 | 4.50 ± 5.55 | |||
| (Q1, Q3) | (1.00, 7.00) | (2.00, 8.00) | (1.00, 6.00) | (2.00, 8.00) | (1.00. 6.00) | |||
| CCI Scores | Total (n = 3827) | Age Group | Sex | |||
|---|---|---|---|---|---|---|
| 65–74 (n = 2433) | 75–84 (n = 1166) | 85+ (n = 228) | Male (n = 2007) | Female (n = 1820) | ||
| 0 | 1182 (30.89) | 899 (36.95) | 254 (21.78) | 29 (12.72) | 540 (26.91) | 642 (35.27) |
| 1 | 902 (23.57) | 612 (25.15) | 258 (22.13) | 32 (14.04) | 455 (22.67) | 447 (24.56) |
| 2 | 653 (17.06) | 374 (15.37) | 225 (19.30) | 54 (23.68) | 359 (17.89) | 294 (16.15) |
| 3 | 443 (11.58) | 230 (9.45) | 178 (15.27) | 35 (15.35) | 250 (12.46) | 193 (10.60) |
| 4 | 271 (7.08) | 145 (5.96) | 97 (8.32) | 29 (12.72) | 171 (8.52) | 100 (5.49) |
| 5 | 170 (4.44) | 87 (3.58) | 59 (5.06) | 24 (10.53) | 89 (4.43) | 81 (4.45) |
| 6 | 89 (2.33) | 34 (1.40) | 48 (4.12) | 7 (3.07) | 66 (3.29) | 23 (1.26) |
| 7 | 45 (1.18) | 23 (0.95) | 14 (1.20) | 8 (3.51) | 31 (1.54) | 14 (0.77) |
| 8 | 35 (0.91) | 16 (0.66) | 15 (1.29) | 4 (1.75) | 22 (1.10) | 13 (0.71) |
| 9 | 14 (0.37) | 6 (0.25) | 6 (0.51) | 2 (0.88) | 8 (0.40) | 6 (0.33) |
| 10 | 11 (0.29) | 4 (0.16) | 6 (0.51) | 1 (0.44) | 9 (0.45) | 2 (0.11) |
| 11 | 5 (0.13) | 1 (0.04) | 2 (0.17) | 2 (0.88) | 2 (0.10) | 3 (0.16) |
| 12 | 3 (0.08) | 1 (0.04) | 2 (0.17) | 0 (0.00) | 2 (0.10) | 1 (0.05) |
| 13 | 2 (0.05) | 1 (0.04) | 0 (0.00) | 1 (0.44) | 1 (0.05) | 1 (0.05) |
| 14 | 1 (0.03) | 0 (0.00) | 1 (0.09) | 0 (0.00) | 1 (0.05) | 0 (0.00) |
| 15 | 1 (0.03) | 0 (0.00) | 1 (0.09) | 0 (0.00) | 1 (0.05) | 0 (0.00) |
| Mean | 1.82 ± 1.98 | 1.52 ± 0.79 | 2.25 ± 2.14 | 2.96 ± 2.31 | 2.04 ± 2.09 | 1.59 ± 1.83 |
| <3 | 2737 (71.52) | 1885 (77.48) | 737 (63.21) | 115 (50.44) | 1354 (67.46) | 1383 (75.99) |
| ≥3 | 1090 (28.48) | 548 (22.52) | 429 (36.79) | 113 (49.56) | 653 (32.54) | 437 (24.01) |
| Comorbidity | Total (n = 3827) | Age Groups | |||
|---|---|---|---|---|---|
| 65–74 (n = 2433) | 75–84 (n = 1166) | 85+ (n = 228) | |||
| Myocardial infarction | 66 (1.72) | 36 (1.48) | 23 (1.97) | 7 (3.07) | |
| Congestive heart failure | 357 (9.33) | 151 (6.21) | 161 (13.81) | 45 (19.74) | |
| Peripheral vascular disease | 114 (2.98) | 55 (2.26) | 47 (4.03) | 12 (5.26) | |
| Cerebrovascular disease | 752 (19.65) | 401 (16.48) | 274 (23.50) | 77 (33.77) | |
| Dementia | 1294 (33.81) | 579 (23.80) | 559 (47.94) | 156 (68.42) | |
| Chronic pulmonary disease | 973 (25.42) | 468 (19.24) | 395 (33.88) | 110 (48.25) | |
| Rheumatic disease | 75 (1.96) | 46 (1.89) | 24 (2.06) | 5 (2.19) | |
| Peptic ulcer disease | 834 (21.79) | 470 (19.32) | 299 (25.64) | 65 (28.51) | |
| Mild liver disease | 442 (11.55) | 269 (11.06) | 143 (12.26) | 30 (13.16) | |
| Diabetes | 891 (22.29) | 577 (23.72) | 265 (22.72) | 49 (21.49) | |
| Hemiplegia or paraplegia | 53 (1.38) | 31 (1.27) | 17 (1.46) | 5 (2.19) | |
| Renal disease | 188 (4.91) | 91 (3.74) | 71 (6.09) | 26 (11.40) | |
| Any malignancy a | 169 (4.42) | 94 (3.86) | 61 (5.23) | 14 (6.14) | |
| Moderate or severe liver disease | 15 (0.39) | 6 (0.25) | 7 (0.60) | 2 (0.88) | |
| Metastatic solid tumor | 25 (0.65) | 13 (0.53) | 10 (0.86) | 2 (0.88) | |
| AIDS/HIV | - | - | - | ||
| 65–69 | 70–74 | 75–79 | 80–84 | 85+ | |
| Dementia | 276 (18.76) | 303 (31.50) | 315 (42.74) | 244 (56.88) | 156 (68.42) |
| Diabetes without chronic complication | 299 (20.33) | 170 (17.67) | 138 (18.72) | 76 (17.72) | 39 (17.11) |
| Diabetes with chronic complication | 71 (4.83) | 37 (3.85) | 35 (4.75) | 16 (3.73) | 10 (4.39) |
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age | 1.08 (1.08–1.09) | p < 0.0001 | 1.07 (1.06–1.08) | p < 0.0001 |
| Sex | ||||
| Male | 2.09 (1.87–2.34) | p < 0.0001 | 1.66 (1.47–1.87) | p < 0.001 |
| Female | 1 | 1 | ||
| CCI score | 1.20 (1.17–1.23) | p < 0.0001 | 1.14 (1.12–1.17) | p < 0.0001 |
| Working type | ||||
| Heavy labor | 1 | 1 | ||
| Moderate labor | 1.31 (1.07–1.59) | p = 0.0072 | 1.25 (1.03–1.52) | p = 0.0264 |
| Light labor | 1.74 (1.47–2.06) | p < 0.0001 | 1.25 (1.05–1.49) | p = 0.0136 |
| Hospital location | ||||
| North | 1 | |||
| Center | 1.06 (0.91–1.24) | NS | ||
| South | 1.14 (0.99–1.32) | NS | ||
| East and Offshore | 1.83 (1.58–2.12) | p < 0.0001 | ||
| Hospital level | ||||
| Medical Centers | 1 | |||
| Metropolitan H. | 0.91 (0.76–1.09) | NS | ||
| Local Community H. | 1.63 (1.38–1.92) | p < 0.0001 | ||
| Physician Clinics | 1.14 (0.42–3.07) | NS | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-C.; Lee, S.-C.; Ouyang, W.-C. Use of Charlson Comorbidity Index and Nomogram to Predict Mortality in Elderly Patients with Late-Life Schizophrenia. Healthcare 2021, 9, 783. https://doi.org/10.3390/healthcare9070783
Hsu M-C, Lee S-C, Ouyang W-C. Use of Charlson Comorbidity Index and Nomogram to Predict Mortality in Elderly Patients with Late-Life Schizophrenia. Healthcare. 2021; 9(7):783. https://doi.org/10.3390/healthcare9070783
Chicago/Turabian StyleHsu, Mei-Chi, Shang-Chi Lee, and Wen-Chen Ouyang. 2021. "Use of Charlson Comorbidity Index and Nomogram to Predict Mortality in Elderly Patients with Late-Life Schizophrenia" Healthcare 9, no. 7: 783. https://doi.org/10.3390/healthcare9070783
APA StyleHsu, M.-C., Lee, S.-C., & Ouyang, W.-C. (2021). Use of Charlson Comorbidity Index and Nomogram to Predict Mortality in Elderly Patients with Late-Life Schizophrenia. Healthcare, 9(7), 783. https://doi.org/10.3390/healthcare9070783






