What Is the Value of Ultrasound in Individuals ‘At-Risk’ of Rheumatoid Arthritis Who Do Not Have Clinical Synovitis?
Abstract
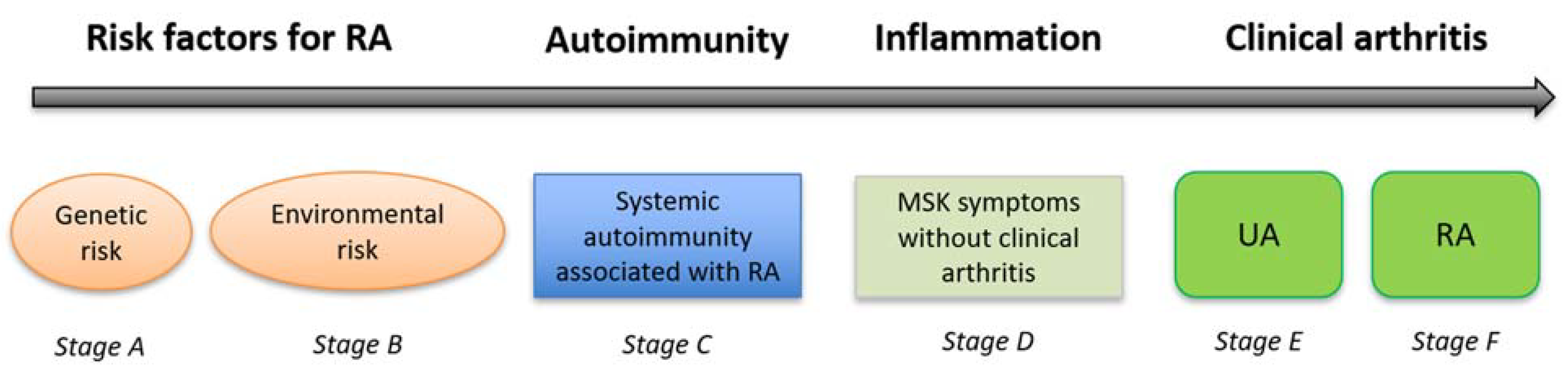
1. Introduction
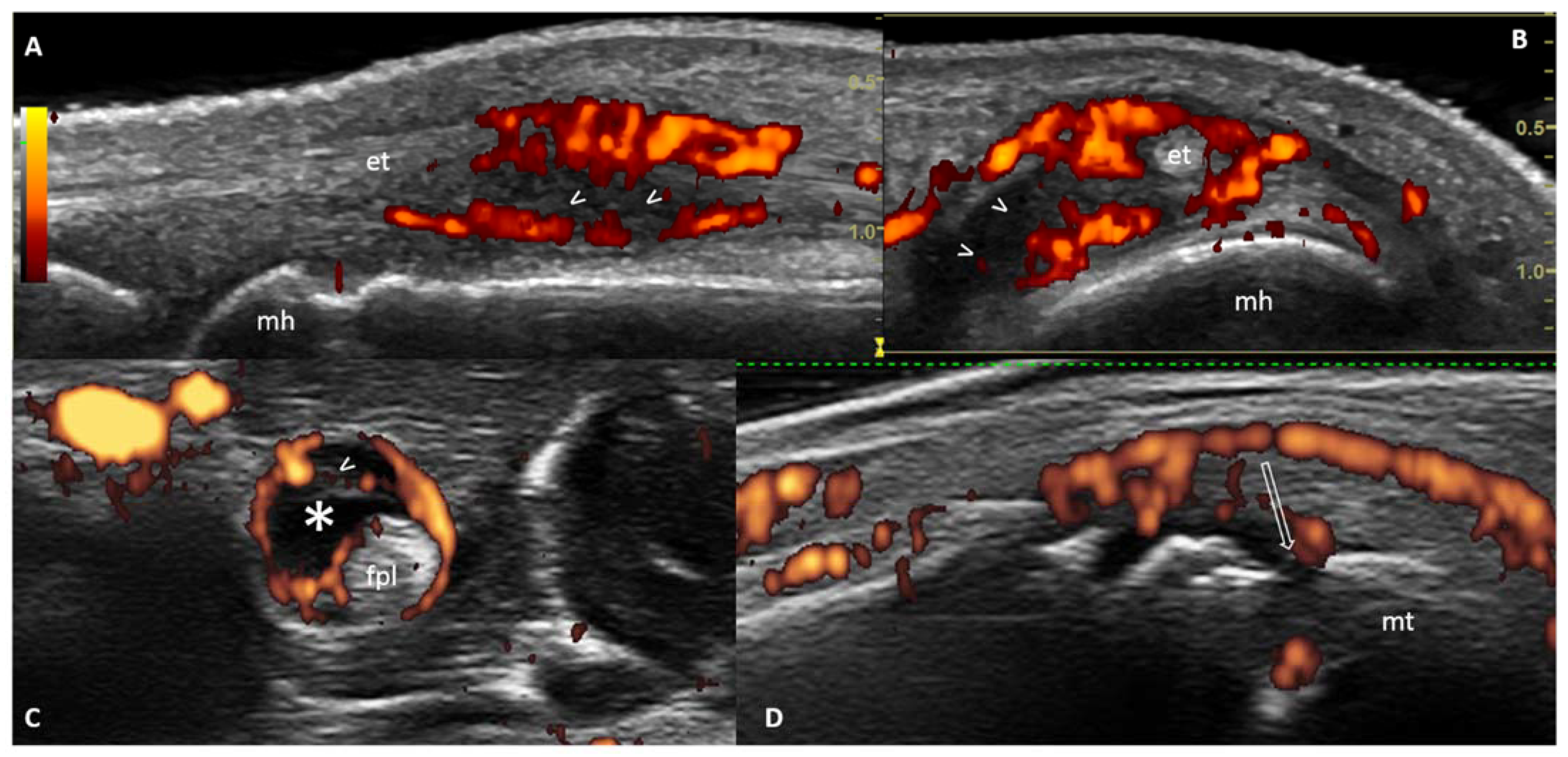
2. The Role of Ultrasound (US) in Predicting Inflammatory Arthritis in ‘At-Risk’ Individuals
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- van der Helm-van Mil, A.H.M.; Landewé, R.B.M. The earlier, the better or the worse? Towards accurate management of patients with arthralgia at risk for RA. Ann. Rheum. Dis. 2020, 79, 312–315. [Google Scholar] [CrossRef]
- Deane, K.D.; Holers, V.M. Rheumatoid Arthritis: Pathogenesis, Prediction and Prevention–An Emerging Paradigm Shift. Arthritis Rheumatol. 2020, 73, 181–193. [Google Scholar] [CrossRef]
- Gerlag, D.M.; Raza, K.; van Baarsen, L.G.; Brouwer, E.; Buckley, C.D.; Burmester, G.R.; Gabay, C.; Catrina, A.I.; Cope, A.P.; Cornelis, F.; et al. EULAR recommendations for terminology and research in individuals at risk of rheumatoid arthritis: Report from the Study Group for Risk Factors for Rheumatoid Arthritis. Ann. Rheum. Dis. 2012, 71, 638–641. [Google Scholar] [CrossRef]
- Mankia, K.; Emery, P. Preclinical Rheumatoid Arthritis: Progress toward Prevention. Arthritis Rheumatol. 2016, 68, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Bumester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Nielen, M.M.; van Schaardenburg, D.; Reesink, H.W.; van de Stadt, R.J.; van der Horst-Bruinsma, I.E.; de Koning, M.H.; Habibuw, M.R.; Vandenbroucke, J.P.; Dijkmans, B.A. Specific autoantibodies precede the symptoms of rheumatoid arthritis: A study of serial measurements in blood donors. Arthritis Rheum. 2004, 50, 380–386. [Google Scholar] [CrossRef]
- Bos, W.H.; Wolbink, G.J.; Boers, M.; van der Horst-Bruinsma, I.E.; Tak, P.P.; van de Stadt, R.J.; van der Laken, C.J.; Dijkmans, B.A.; van Schaardenburg, D. Arthritis development in patients with arthralgia is strongly associated with anti-citrullinated protein antibody status: A prospective cohort study. Ann. Rheum. Dis. 2010, 69, 490–494. [Google Scholar] [CrossRef] [PubMed]
- van de Stadt, L.A.; van der Horst, A.R.; de Koning, M.H.; Bos, W.H.; Wolbink, G.J.; van de Stadt, R.J.; Pruijn, G.J.M.; Dijkmans, B.A.; van Schaardenburg, D.; Hamann, D. The extent of the anti-citrullinated protein antibody repertoire is associated with arthritis development in patients with seropositive arthralgia. Ann. Rheum. Dis. 2011, 70, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Ten Brinck, R.M.; van Steenbergen, H.W.; van Delft, M.A.M.; Verheul, M.K.; Toes, R.E.M.; Trouw, L.A.; van der Helm-van Mil, A.H.M. The risk of individual autoantibodies, autoantibody combinations and levels for arthritis development in clinically suspect arthralgia. Rheumatology 2017, 56, 2145–2153. [Google Scholar] [CrossRef]
- Di Matteo, A.; Mankia, K.; Duquenne, L.; Mahler, M.; Corscadden, D.; Mbara, K.; Garcia-Montoya, L.; Nam, J.L.; Emery, P.l. Third-Generation Anti-Cyclic Citrullinated Peptide Antibodies Improve Prediction of Clinical Arthritis in Individuals at Risk of Rheumatoid Arthritis. Arthritis Rheumatol. 2020, 72, 1820–1828. [Google Scholar] [CrossRef]
- Mankia, K.; Di Matteo, A.; Emery, P. Prevention and cure: The major unmet needs in the management of rheumatoid arthritis. J. Autoimmun. 2020, 110, 102399. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Mankia, K.; Azukizawa, M.; Wakefield, R.J. The Role of Musculoskeletal Ultrasound in the Rheumatoid Arthritis Continuum. Curr. Rheumatol. Rep. 2020, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Duquenne, L.; Chowdhury, R.; Mankia, K.; Emery, P. The Role of Ultrasound Across the Inflammatory Arthritis Continuum: Focus on "at-Risk" Individuals. Front. Med. 2020, 7, 587827. [Google Scholar] [CrossRef] [PubMed]
- Pratt, A.G.; Lorenzi, A.R.; Wilson, G.; Platt, P.N.; Isaacs, J.D. Predicting persistent inflammatory arthritis amongst early arthritis clinic patients in the UK: Is musculoskeletal ultrasound required? Arthritis Res. Ther. 2013, 15, R118. [Google Scholar] [CrossRef]
- Salaffi, F.; Ciapetti, A.; Gasparini, S.; Carotti, M.; Filippucci, E.; Grassi, W. A clinical prediction rule combining routine assessment and power doppler ultrasonography for predicting progression to rheumatoid arthritis from early-onset undifferentiated arthritis. Clin. Exp. Rheumatol. 2010, 28, 686–694. [Google Scholar]
- Sahbudin, I.; Pickup, L.; Nightingale, P.; Allen, G.; Cader, Z.; Singh, R.; de Pablo, P.; Buckley, C.D.; Raza, K.; Filer, A. The role of ultrasound-defined tenosynovitis and synovitis in the prediction of rheumatoid arthritis development. Rheumatology 2018, 57, 1243–1252. [Google Scholar] [CrossRef]
- Filer, A.; De Pablo, P.; Allen, G.; Nightingale, P.; Jordan, A.; Jobanputra, P.; Bowman, S.; Buckley, C.D.; Raza, K. Utility of ultrasound joint counts in the prediction of rheumatoid arthritis in patients with very early synovitis. Ann. Rheum. Dis. 2011, 70, 500–507. [Google Scholar] [CrossRef]
- Iqbal, K.; Lendrem, D.W.; Hargreaves, B.; Isaacs, J.D.; Thompson, B.; Pratt, A.G. Routine musculoskeletal ultrasound findings impact diagnostic decisions maximally in autoantibody-seronegative early arthritis patients. Rheumatology 2019, 58, 1268–1273. [Google Scholar] [CrossRef]
- Freeston, J.E.; Wakefield, R.J.; Conaghan, P.G.; Hensor, E.M.A.; Stewart, S.P.; Emery, P. A diagnostic algorithm for persistence of very early inflammatory arthritis: The utility of power Doppler ultrasound when added to conventional assessment tools. Ann. Rheum. Dis. 2010, 69, 417–419. [Google Scholar] [CrossRef]
- Nam, J.L.; D’Agostino, M.A. Role of ultrasound imaging in individuals at risk of RA. Best Pract. Res. Clin. Rheumatol. 2017, 31, 71–79. [Google Scholar] [CrossRef]
- Hunt, L.; Eugénio, G.; Grainger, A.J. Magnetic resonance imaging in individuals at risk of rheumatoid arthritis. Best. Pract. Res. Clin. Rheumatol. 2017, 31, 80–89. [Google Scholar] [CrossRef]
- van de Stadt, L.A.; Bos, W.H.; Meursinge Reynders, M.; Wieringa, H.; Turkstra, F.; van der Laken, C.J.; van Schaardenburg, D. The value of ultrasonography in predicting arthritis in auto-antibody positive arthralgia patients: A prospective cohort study. Arthritis Res. Ther. 2010, 12, R98. [Google Scholar] [CrossRef] [PubMed]
- Rakieh, C.L.; Nam, J.; Hunt, L.; Hensor, E.M.A.; Das, S.; Bissell, L.-A.; Villeneuve, E.; McGonagle, D.; Hodgson, R.; Grainger, A.; et al. Predicting the development of clinical arthritis in anti-CCP positive individuals with non-specific musculoskeletal symptoms: A prospective observational cohort study. Ann. Rheum. Dis. 2015, 74, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.L.; Hensor, E.M.A.; Hunt, L.; Conaghan, P.G.; Wakefield, R.J.; Emery, P. Ultrasound findings predict progression to inflammatory arthritis in anti-CCP antibody-positive patients without clinical synovitis. Ann. Rheum. Dis. 2016, 75, 2060–2067. [Google Scholar] [CrossRef]
- Wakefield, R.J.; Balint, P.V.; Szkudlarek, M.; Backhaus, M.; D’Agostino, M.-A.; Naredo Sanchez, E.; Iagnocco, A.; Schmidt, W.A.; Bruyn, G.A.W.; Kane, D.; et al. Musculoskeletal ultrasound including definitions for ultrasonographic pathology. J. Rheumatol. 2005, 32, 2485–2487. [Google Scholar]
- D’Agostino, M.; Wakefield, R.J.; Filippucci, E. Intra- and inter-observer reliability of ultrasonography for detecting and scoring synovitis in rheumatoid arthritis: A report of a EULAR ECSISIT task force. Ann. Rheum. Dis. 2005, 64, 62. [Google Scholar]
- Zufferey, P.; Rebell, C.; Benaim, C.; Ziswiler, H.R.; Dumusc, A.; So, A. Ultrasound can be useful to predict an evolution towards rheumatoid arthritis in patients with inflammatory polyarthralgia without anticitrullinated antibodies. Jt. Bone Spine 2017, 84, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Zufferey, P.; Tamborrini, G.; Gabay, C.; Krebs, A.; Kyburz, D.; Michel, B.; Moser, U.; Villiger, P.M.; So, A.; Ziswiler, H.R. Recommendations for the use of ultrasound in rheumatoid arthritis: Literature review and SONAR score experience. Swiss Med. Wkly. 2013, 143, w13861. [Google Scholar] [CrossRef]
- van der Ven, M.; van der Veer-Meerkerk, M.; Ten Cate, D.F.; Rasappu, N.; Kok, M.R.; Csakvari, D.; Hazes, J.M.W.; Gerards, A.H.; Luime, J.J. Absence of ultrasound inflammation in patients presenting with arthralgia rules out the development of arthritis. Arthritis Res. Ther. 2017, 19, 1–7. [Google Scholar] [CrossRef]
- Van Beers-Tas, M.H.; Blanken, A.B.; Nielen, M.M.J.; Turkstra, F.; van der Laken, C.J.; Meursinge Reynders, M.; van Schaardenburg, D. The value of joint ultrasonography in predicting arthritis in seropositive patients with arthralgia: A prospective cohort study. Arthritis Res. Ther. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Szkudlarek, M.; Court-Payen, M.; Jacobsen, S.; Klarlund, M.; Thomsen, H.S.; Østergaard, M. Inter-observer agreement in ultrasonography of the finger and toe joint in rheumatoid arthritis. Arthritis Rheum. 2003, 48, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, A.; Mankia, K.; Duquenne, L.; Cipolletta, E.; Wakefield, R.J.; Garcia-Montoya, L.; Nam, J.L.; Emery, P. Ultrasound erosions in the feet best predict progression to inflammatory arthritis in anti-CCP positive at-risk individuals without clinical synovitis. Ann. Rheum. Dis. 2020, 79, 901–907. [Google Scholar] [CrossRef]
- Di Matteo, A.; Mankia, K.; Nam, J.L.; Cipolletta, E.; Garcia-Montoya, L.; Duquenne, L.; Rowbotham, E.; Emery, P. In anti-CCP+ at-risk individuals, radiographic bone erosions are uncommon and are not associated with the development of clinical arthritis. Rheumatology 2020, keaa761. [Google Scholar] [CrossRef] [PubMed]
- Mankia, K.; Briggs, C.; Emery, P. How Are Rheumatologists Managing Anticyclic Citrullinated Peptide Antibodies-positive Patients Who Do Not Have Arthritis? J. Rheumatol. 2020, 47, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Duquenne, L.; Mankia, K.; Nam, J.; Pentony, P.; Garcia-Montoya, L.; Di Matteo, A.; Hunt, L.; Emery, P. THU0072 Ultrasound predicts imminent progression to arthritis in anti-ccp positive at-risk individuals. Ann. Rheum. Dis. 2019, 78, 304–305. [Google Scholar]
- Nam, J.L.; Hunt, L.; Hensor, E.M.; Emery, P. Enriching case selection for imminent RA: The use of anti-CCP antibodies in individuals with new non-specific musculoskeletal symptoms-a cohort study. Ann. Rheum. Dis. 2016, 75, 1452–1456. [Google Scholar] [CrossRef]
- Rogier, C.; Wouters, F.; van Boheemen, L.; Schaardenburg, D.; de Jong, H.P.P.; van der Helm-van Mil, A.H.M. Subclinical synovitis in arthralgia: How often does it result in clinical arthritis? Reflecting on starting points for disease-modifying anti-rheumatic drug treatment. Rheumatology 2020, keaa774. [Google Scholar] [CrossRef] [PubMed]
- van Steenbergen, H.W.; Mangnus, L.; Reijnierse, M.; Huizinga, T.W.; van der Helm-van, A.H. Clinical factors, anticitrullinated peptide antibodies and MRI-detected subclinical inflammation in relation to progression from clinically suspect arthralgia to arthritis. Ann. Rheum. Dis. 2016, 75, 1824–1830. [Google Scholar] [CrossRef]
- Padovano, I.; Costantino, F.; Breban, M.; D’Agostino, M.A. Prevalence of ultrasound synovial inflammatory findings in healthy subjects. Ann. Rheum. Dis. 2016, 75, 1819–1823. [Google Scholar] [CrossRef]
- Iagnocco, A. Ultrasound in osteoarthritis. Clin. Exp. Rheumatol. 2014, 32 (Suppl. 80), S48–S52. [Google Scholar]
- Gent, Y.Y.J.; Ter Wee, M.M.; Ahmadi, N.; van Kuijk, C.; Voskuyl, A.E.; van der Laken, C.J.; Dowling, C.; van de Stadt, L.A.; van Schaardenburg, D. Three-year clinical outcome following baseline magnetic resonance imaging in anti-citrullinated protein antibody-positive arthralgia patients: An exploratory study. Arthritis Rheumatol. 2014, 266, 2909–2910. [Google Scholar] [CrossRef] [PubMed]
- Van Steenbergen, H.W.; Van Nies, J.A.B.; Huizinga, T.W.J.; Bloem, J.L.; Reijnierse, M.; van der Helm-van Mil, A.H.M. Characterising arthralgia in the preclinical phase of rheumatoid arthritis using MRI. Ann. Rheum. Dis. 2015, 74, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.A.; Terslev, L.; Aegerter, P.; Backhaus, M.; Balint, P.; Bruyn, G.A.; Filippucci, E.; Grassi, W.; Iagnocco, A.; Jousse-Joulin, S.; et al. Scoring ultrasound synovitis in rheumatoid arthritis: A EULAR-OMERACT ultrasound taskforce—Part 1: Definition and development of a standardised, consensus-based scoring system. RMD Open 2017, 3, e000428. [Google Scholar] [CrossRef] [PubMed]
- Hresko, A.; Lin, T.C.; Solomon, D.H. Medical Care Costs Associated with Rheumatoid Arthritis in the US: A Systematic Literature Review and Meta-Analysis. Arthritis Care Res. 2018, 70, 1431–1438. [Google Scholar] [CrossRef]
- Hsieh, P.H.; Wu, O.; Geue, C.; McIntosh, E.; McInnes, I.B.; Siebert, S. Economic burden of rheumatoid arthritis: A systematic review of literature in biologic era. Ann. Rheum. Dis. 2020, 79, 771–777. [Google Scholar] [CrossRef]
- Deane, K.D. Preclinical Rheumatoid Arthritis and Rheumatoid Arthritis Prevention. Curr. Rheumatol. Rep. 2018, 20, 50. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year of Publication | Study Design | Number of ‘At-Risk’ Individuals | ACPA Positive | RF Positive | ACPA or RF Positive | MSK Involvement | Control Group | Proportion of Progressors to IA | Median Time of Progression to IA | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| van de Stadt et al. [22] | 2010 | PS | 192 | 69% | 63% | 100% | Arthralgia (i.e., non-traumatic pain in any joint) | Y (9 HC) | 23% | Mean: 11 M (SD ± 9) | US findings associated with clinical arthritis development at joint level but not at patient level |
| Rakieh et al. [23] | 2014 | PS | 100 | 100% | 46% | 100% | New onset MSK symptoms | N | 50% | Median: 7.9 M (IQR 3.2, 14.5) | PD signal predictive for IA development in the multivariable analysis |
| Nam et al. [24] | 2016 | PS | 136 | 100% | 45% | 100% | New onset MSK symptoms | Y (48 HC) | 41.9% | Median: 18.3 M (range 0.1–79.6) | US findings (especially PD) predictive for progression to IA |
| Zufferey et al. [27] | 2016 | RS | 80 | 0% | 0% | 0% | Polyarthralgia | N | 8.7% | Median: 18 M | US only independent predictor to IA development in the multivariable analysis |
| van der Ven et al. [29] | 2017 | PS | 159 | 15% | 26% | NR | Inflammatory arthralgia | N | 16% | 1 year follow-up | PD in combination with clinical parameters is associated with IA development |
| van Beers-Tas et al. [30] | 2018 | PS | 163 | 46% | 73% | 100% | Arthralgia | N | 31% | Median 12 M (IQR 5–24) | US synovial thickening predicted IA development (excluding MTP joints). PD signal infrequent and not predictive |
| Di Matteo et al. [32] | 2020 | PS | 419 | 100% | 38% | 100% | New onset MSK symptoms | N | 30.7% | Median: 9.9 M (IQR 3.6–26.7) | BE in >1 joint, and BE in combination with US synovitis in the MTP5 joints, most predictive for the development of IA |
| Authors | Sonographer Inter-Observer Agreement | Sonographer Blind to Clinical Data | Scanned Areas | Number of Joints Evaluated | US Probe Frequencies | US Elementary Lesions Assessed (Prevalence %) | US Definitions Used | Grading of the US Findings | Baseline or Longitudinal Scans | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GS (MHz) | PD (MHz) | SH | SE | PD | BE | TS | ||||||||
| van de Stadt et al. [22] | Y | Y | Painful, adjacent and contralateral joints | Variable for each patient | 8–15 | 7.3–8.9 | Y (12.5) | Y (11.4) | Y (17.1) | N | Y (6.7) | Szkudlarek | Semiquantitative | Baseline |
| Rakieh et al. [23] | Y | N | Bilateral wrists, 1–5 MCP and 1–5 PIP joints | 22 | 8–15 | NR | N | N | Y (33) | N | N | Naredo/Torp-Pedersen | Dichotomic | Baseline |
| Nam et al. [24] | Y | N | Bilateral wrists, 1–5 MCP, 1–5 PIP, 1–5 MTP joints | 32 and other joints if symptomatic | * | * | Y (95.5) | N | Y (33) | Y (20.5) | N | OMERACT | Semiquantitative | Baseline |
| Zufferey et al. [27] | N | N | Bilateral elbows, wrists, 2–5 MCP, 2–5 PIP and knee joints | 22 | 7–13 | NR | Y (25) | N | N | N | N | SONAR | Semiquantitative | Baseline |
| van der Ven et al. [29] | N | Y | Bilateral wrists, 2–5 MCP, 2–5 PIP, 2–5 MTP joints | 26 | 10–18 | NR | N | Y (35.6) | Y (14.9) | N | N | OMERACT modified version by The Spanish Society of Rheumatology | Semiquantitative | Baseline |
| van Beers-Tas et al. [30] | Single operator | Y | Bilateral wrists, 2,3 MCP, 2,3 PIP, 2,3,5 MTP joints | 16 | 11.4 | 8.9 | Y (30) | N | Y (4) | N | N | Szkudlarek | Semiquantitative | Baseline |
| Di Matteo et al. [32] | Y | Y | Bilateral 2,5 MCP and 5 MTP joints | 6 | * | * | Y (NR) | N | Y (22.9) | Y (9.8) | N | EULAR-OMERACT | Dichotomic | Baseline |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Matteo, A.; Corradini, D.; Mankia, K. What Is the Value of Ultrasound in Individuals ‘At-Risk’ of Rheumatoid Arthritis Who Do Not Have Clinical Synovitis? Healthcare 2021, 9, 752. https://doi.org/10.3390/healthcare9060752
Di Matteo A, Corradini D, Mankia K. What Is the Value of Ultrasound in Individuals ‘At-Risk’ of Rheumatoid Arthritis Who Do Not Have Clinical Synovitis? Healthcare. 2021; 9(6):752. https://doi.org/10.3390/healthcare9060752
Chicago/Turabian StyleDi Matteo, Andrea, Davide Corradini, and Kulveer Mankia. 2021. "What Is the Value of Ultrasound in Individuals ‘At-Risk’ of Rheumatoid Arthritis Who Do Not Have Clinical Synovitis?" Healthcare 9, no. 6: 752. https://doi.org/10.3390/healthcare9060752
APA StyleDi Matteo, A., Corradini, D., & Mankia, K. (2021). What Is the Value of Ultrasound in Individuals ‘At-Risk’ of Rheumatoid Arthritis Who Do Not Have Clinical Synovitis? Healthcare, 9(6), 752. https://doi.org/10.3390/healthcare9060752






