Diabetic Retinopathy Screening and Registration in Europe—Narrative Review
Abstract
1. Introduction
2. Materials and Methods
Eligibility Criteria
- Country/Region/City of the screening program/registry
- First author
- Year of publication
- Aim of the article
- Name of screening program/register
- Time period of register/screening functioning
- Defined geographical area covered
- Target population in which cases arise
- Number of the population from which DR cases are identified (where indicated)
- Age group of target population
- Sources of information for cases of diabetes (where indicated)
- Screening method
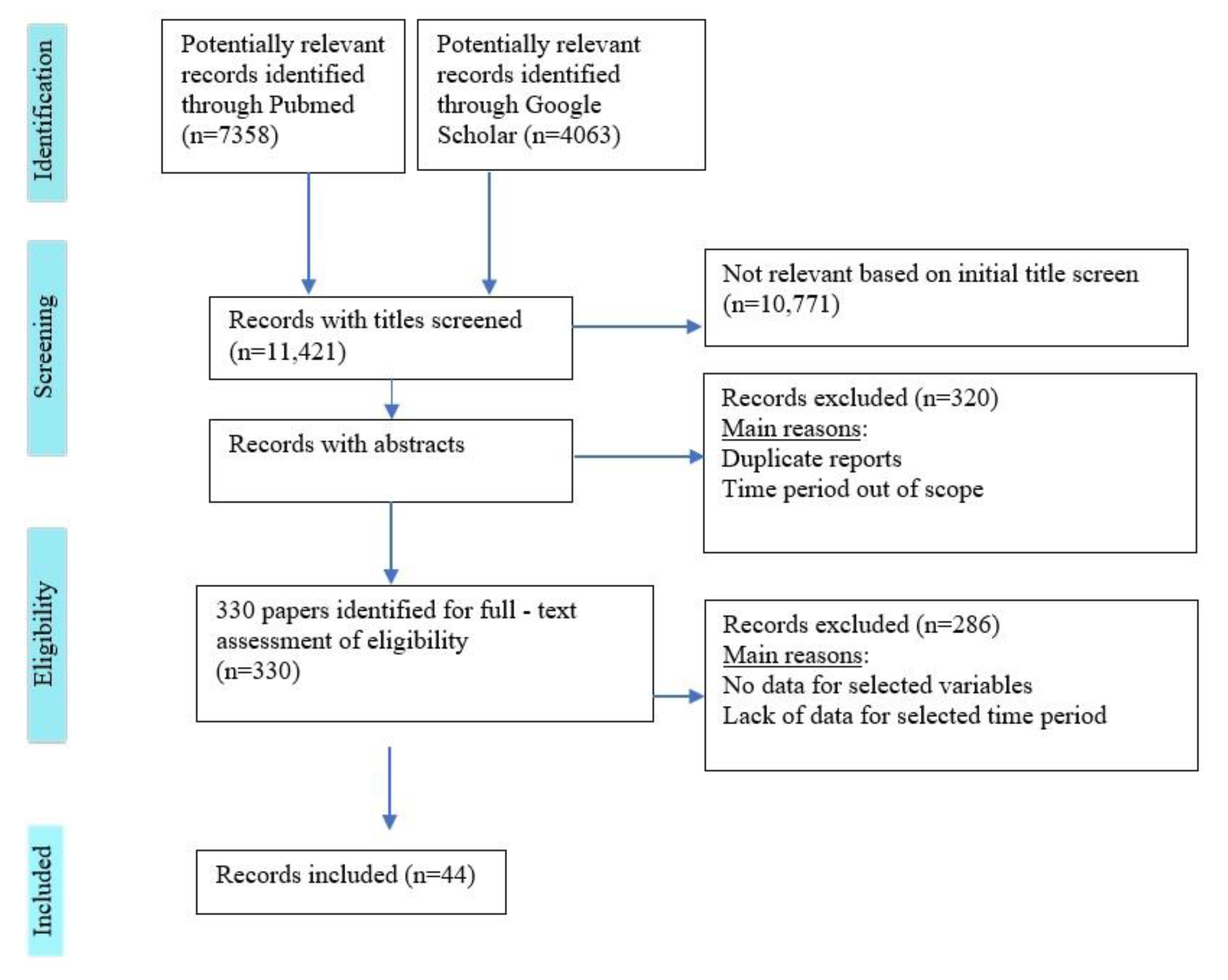
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diabetes. Available online: https://www.who.int/westernpacific/health-topics/diabetes (accessed on 20 January 2021).
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of Diabetic Retinopathy, Diabetic Macular Edema and Related Vision Loss. Eye Vis. Lond. Eng. 2015, 2, 17. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.A.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef]
- Thomas, R.; Halim, S.; Gurudas, S.; Sivaprasad, S.; Owens, D. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res. Clin. Pr. 2019, 157, 107840. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Airey, M.; Baxter, H.; Forrester, J.; Kennedy-Martin, T.; Girach, A. Epidemiology of diabetic retinopathy and macular oedema: A systematic review. Eye 2004, 18, 963–983. [Google Scholar] [CrossRef] [PubMed]
- Tackling COVID-19 with Telemedicine. Available online: https://theophthalmologist.com/subspecialties/tackling-covid-19-with-telemedicine (accessed on 20 February 2021).
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease. Available online: https://apps.who.int/iris/handle/10665/37650 (accessed on 23 January 2021).
- Pieczynski, J.; Grzybowski, A. Diabetic Retinopathy Screening Methods and Programmes Adopted in Different Parts of the World—Further Insights. Eur. Ophthalmic Rev. 2015, 9, 161. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 1–7. [Google Scholar] [CrossRef]
- Laitinen, A.; Laatikainen, L.; Härkänen, T.; Koskinen, S.; Reunanen, A.; Aromaa, A. Prevalence of major eye diseases and causes of visual impairment in the adult Finnish population: A nationwide population-based survey. Acta Ophthalmol. 2010, 88, 463–471. [Google Scholar] [CrossRef]
- Hautala, N.; Aikkila, R.; Korpelainen, J.; Keskitalo, A.; Kurikka, A.; Falck, A.; Bloigu, R.; Alanko, H. Marked reductions in visual impairment due to diabetic retinopathy achieved by efficient screening and timely treatment. Acta Ophthalmol. 2014, 92, 582–587. [Google Scholar] [CrossRef]
- Laatikainen, L.; Ojamo, M.; Rudanko, S.; Summanen, P.; Keinänen-Kiukaanniemi, S.; Tuomilehto, J.; Herrala, S.; Uusitalo, H. Improving visual prognosis of the diabetic patients during the past 30 years based on the data of the Finnish Register of Visual Impairment. Acta Ophthalmol. 2016, 94, 226–231. [Google Scholar] [CrossRef]
- Massin, P.; Chabouis, A.; Erginay, A.; Viens-Bitker, C.; Lecleire-Collet, A.; Meas, T.; Guillausseau, P.-J.; Choupot, G.; André, B.; Denormandie, P. OPHDIAT: A telemedical network screening system for diabetic retinopathy in the Île-de-France. Diabetes Metab. 2008, 34, 227–234. [Google Scholar] [CrossRef]
- Schulze-Döbold, C.; Erginay, A.; Robert, N.; Chabouis, A.; Massin, P. Ophdiat®: Five-year experience of a telemedical screening programme for diabetic retinopathy in Paris and the surrounding area. Diabetes Metab. 2012, 38, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Kelliher, C.; Kenny, D.; O’Brien, C. Trends in blind registration in the adult population of the Republic of Ireland 1996-2003. Br. J. Ophthalmol. 2006, 90, 367–371. [Google Scholar] [CrossRef]
- James, M.; Goodchild, C.; Bashir, S.; Mannix, M. Report on the creation of a diabetes register and retinopathy screening outcomes in the Mid-West of Ireland. Ir. J. Med Sci. 2016, 185, 151–159. [Google Scholar] [CrossRef]
- Tracey, M.; McHugh, S.; Fitzgerald, A.; Buckley, C.; Canavan, R.; Kearney, P. Trends in blindness due to diabetic retinopathy among adults aged 18–69 years over a decade in Ireland. Diabetes Res. Clin. Pr. 2016, 121, 1–8. [Google Scholar] [CrossRef]
- Andersen, N.; Hjortdal, J.Ø.; Schielke, K.C.; Bek, T.; Grauslund, J.; Laugesen, C.S.; Lund-Andersen, H.; Cerqueira, C.S.; Andresen, J. The Danish Registry of Diabetic Retinopathy. Clin. Epidemiol. 2016, 8, 613–619. [Google Scholar] [CrossRef]
- Is the Rule of Halves Framework Relevant for Diabetes Care in Copenhagen Today? A Register-Based Cross-Sectional Study—Abstract—Europe PMC. Available online: https://europepmc.org/article/PMC/6252698 (accessed on 24 January 2021).
- Looker, H.C.; Nyangoma, S.O.; Cromie, D.T.; Olson, J.A.; Leese, G.P.; Black, M.W.; Doig, J.; Lee, N.; Lindsay, R.S.; McKnight, J.A.; et al. Rates of Referable Eye Disease in the Scottish National Diabetic Retinopathy Screening Programme. Br. J. Ophthalmol. 2017, 98, 790–795. Available online: https://bjo.bmj.com/content/98/6/790 (accessed on 15 February 2021). [CrossRef]
- Thomas, R.L.; Dunstan, F.D.; Luzio, S.D.; Chowdhury, S.R.; North, R.; Hale, S.L.; Gibbins, R.L.; Owens, D.R. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br. J. Ophthalmol. 2015, 99, 64–68. [Google Scholar] [CrossRef]
- Heintz, E.; Wirehn, A.-B.; Peebo, B.B.; Rosenqvist, U.; Levin, L.-A. Prevalence and healthcare costs of diabetic retinopathy: A population-based register study in Sweden. Diabetologia 2010, 53, 2147–2154. [Google Scholar] [CrossRef]
- About Diabetic RetinaScreen. Available online: https://www2.hse.ie/screening-and-vaccinations/diabetic-retina-screening/diabetic-retina-screening.html (accessed on 26 January 2021).
- Icks, A.; Trautner, C.; Haastert, B.; Berger, M.; Giani, G. Blindness Due to Diabetes: Population-based Age- and Sex-specific Incidence Rates. Diabet. Med. J. Br. Diabet. Assoc. 1997, 14, 571–575. [Google Scholar] [CrossRef]
- Segato, T.; Midena, E.; Grigoletto, F.; Zucchetto, M.; Fedele, D.; Piermarocchi, S.; Crepaldi, G.; Veneto Group for Diabetic Retinopathy. The Epidemiology and Prevalence of Diabetic Retinopathy in the Veneto Region of North East Italy. Diabet. Med. J. Br. Diabet. Assoc. 1991, 8, S11–S16. [Google Scholar] [CrossRef]
- Nicolucci, A.; Scorpiglione, N.; Belfiglio, M.; Carinci, F.; Cavaliere, D.; El-Shazly, M.; Labbrozzi, D.; Mari, E.; Massi Benedetti, M.; Tognoni, G. Patterns of Care an Italian Diabetic Population. The Italian Study Group for the Implementation of the St Vincent Declaration, Società Italiana Di Diabetologia, Associazione Medici Diabetologi. Diabet. Med. J. Br. Diabet. Assoc. 1997, 14, 158–166. [Google Scholar] [CrossRef]
- Porta, M.; Maurino, M.; Severini, S.; Lamarmora, E.; Trento, M.; Sitia, E.; Coppo, E.; Raviolo, A.; Carbonari, S.; Montanaro, M.; et al. Clinical characteristics influence screening intervals for diabetic retinopathy. Diabetologia 2013, 56, 2147–2152. [Google Scholar] [CrossRef]
- The Nonmydriatic Fundus Camera in Diabetic Retinopathy Screening: A Cost-Effective Study with Evaluation for Future Large-Scale Application. Available online: https://www.hindawi.com/journals/joph/2016/4625096/ (accessed on 14 February 2021).
- Invernizzi, A.; Bevilacqua, M.T.; Cozzi, M.; Bianchi, C.; Pagani, A.; Cigada, M.; Staurenghi, G. Diabetic Retinopathy Screening: The First Telemedical Approach in an Italian Hospital. Eur. J. Ophthalmol. 2016, 26, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Huemer, J.; Wagner, S.K.; Sim, D.A. The Evolution of Diabetic Retinopathy Screening Programmes: A Chronology of Retinal Photography from 35 Mm Slides to Artificial Intelligence. Available online: https://www.dovepress.com/the-evolution-of-diabetic-retinopathy-screening-programmes-a-chronolog-peer-reviewed-fulltext-article-OPTH (accessed on 17 February 2021).
- Martín-Merino, E.; Fortuny, J.; Rivero, E.; García-Rodríguez, L.A. Validation of Diabetic Retinopathy and Maculopathy Diagnoses Recorded in a U.K. Primary Care Database. Diabetes Care 2012, 35, 762–767. [Google Scholar] [CrossRef][Green Version]
- Scanlon, P.H. The English National Screening Programme for diabetic retinopathy 2003–2016. Acta Diabetol. 2017, 54, 515–525. [Google Scholar] [CrossRef]
- NIDESP Annual Report 1617. Available online: https://www.publichealth.hscni.net/publications/diabetic-eye-screening-programme-annual-report-2016-2017Final260618.Pdf (accessed on 14 February 2021).
- Kristinsson, J.K.; Stefánsson, E.; Jonasson, F.; Gíslason, I.; Björnsson, S. Systematic screening for diabetic eye disease in insulin dependent diabetes. Acta Ophthalmol. 2009, 72, 72–78. [Google Scholar] [CrossRef]
- Carlsen, S.; Skrivarhaug, T.; Thue, G.; Cooper, J.G.; Gøransson, L.; Løvaas, K.; Sandberg, S. Glycemic control and complications in patients with type 1 diabetes - a registry-based longitudinal study of adolescents and young adults. Pediatr. Diabetes 2017, 18, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, P.; Madzharova, B.; Cekova, R.; Proynova, M. Epidemiology of Diabetic Retinopathy. Bulg. Rev. Ophthalmol. 1996, 1, 12–14. [Google Scholar]
- Zlatarova, Z.; Decheva, D.; Barbukova, A. Diabetic Retinopathy—Incidence, Risk Factors and Screening Results. Bulg. Ophthalmol. Ref. Bull. 2008, 6, 16–20. [Google Scholar]
- Zlatarova, Z.; Hristozov, K.; Dokova, K. Prevalence and Risk Factors of Diabetic Retinopathy among Patients with Low Control of Diabetes Mellitus. Bulg. Rev. Ophthalmol. 2011, 2, 21–25. [Google Scholar]
- Long-Term Comparative Effectiveness of Telemedicine in Providing Diabetic Retinopathy Screening Examinations: A Randomized Controlled Trial. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6023855/ (accessed on 2 June 2021).
- Abràmoff, M.D.; Folk, J.C.; Han, D.P.; Walker, J.D.; Williams, D.F.; Russell, S.; Massin, P.; Cochener, B.; Gain, P.; Tang, L.; et al. Automated Analysis of Retinal Images for Detection of Referable Diabetic Retinopathy. JAMA Ophthalmol. 2013, 131, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Gargeya, R.; Leng, T. Automated Identification of Diabetic Retinopathy Using Deep Learning. Ophthalmology 2017, 124, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and Validation of a Deep Learning Algorithm for Detection of Diabetic Retinopathy in Retinal Fundus Photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Sabanayagam, C. Strategies to Tackle the Global Burden of Diabetic Retinopathy: From Epidemiology to Artificial Intelligence. Int. J. Ophthalmol. 2020, 243, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Van Der Heijden, A.A.; Abramoff, M.D.; Verbraak, F.; Van Hecke, M.V.; Liem, A.; Nijpels, G. Validation of automated screening for referable diabetic retinopathy with the IDx-DR device in the Hoorn Diabetes Care System. Acta Ophthalmol. 2018, 96, 63–68. [Google Scholar] [CrossRef] [PubMed]

| Country | First Author and Year of Publication | Time Period | Covered Geographical Area | Age Group Screened (Years) | Number of Participants/DR Relative Share | Name of Register/ Type of Study |
|---|---|---|---|---|---|---|
| Finland | Laitinen et al. 2010 [10] | 2000–2001 | Whole of Finland | ≥30 | 7413/1% | Cross-sectional nationwide population survey |
| Hautala et al. 2014 [11] | 2007–2011 | 35 municipalities of the Northern Ostrobothnia Hospital District | No age limitation | 14,866/23% mild background retinopathy, 31% moderate or severe background retinopathy, 3%—PDR | Finnish Register of Visual Impairment | |
| Laatikainen et al. 2016 [12] | 1982–2010 | Whole of Finland | 18–64 | 42,626/0.09% | Finnish Register of Visual Impairment | |
| France | Massin et al. 2008 [13] | 2004–2006 | Paris region | 1–106 | 15,307/23.4% DR | Regional telemedical network/OPHDIAT/ |
| Schulze-Döbold et al. 2012 [14] | 2004–2009 | Paris region | All ages | 38,596/14.7% advanced stage retinopathy | Regional telemedical network/OPHDIAT/ | |
| Ireland | Kelliher et al. 2006 [15] | 1996–2003 | Whole of Ireland | No age limitation | 470/7% DR | The National Council for the Blind in Ireland (NCBI) |
| James et al. 2016 [16] | 2010–2012 | Mid-West of Ireland | ≥20 | 1434/20.1% background retinopathy, 8.2% sight threatening retinopathy | Mid-West Diabetic Retinopathy Screening Pro- gramme (MWDRS) | |
| Tracey et al. 2016 [17] | 2004–2013 | Whole of Ireland | 18–69 | 57,626–109,842/0.4–1.9% during 10-year period | National Council for the Blind of Ireland | |
| Denmark | Andersen et al. 2016 [18] | 2014–2015 | Whole of Denmark | ≥18 years | 77,968/18% NPDR, 4% PDR | Danish Registry of Diabetic Retinopathy (DiaBase) |
| Holm et al. 2018 [19] | 2010 and still ongoing | Copenhagen City | No age limitation | 21,000/11.3% DR | National Patient Register Danish Adult Diabetes Database | |
| Scotland | Looker et al. 2014 [20] | 2006–2010 | >99% of the Scottish population | ≥12 | 187,822/0.6–1.8% referable background retinopathy | National diabetes registry—Scottish Care Information-Diabetes Collaboration (SCI-DC) database |
| Wales | Thomas et al. 2015 [21] | 2005 and still ongoing | Whole of Wales | ≥12 | 91,393/32.4% DR | Diabetic Retinopathy Screening Service for Wales (DRSSW) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hristova, E.; Koseva, D.; Zlatarova, Z.; Dokova, K. Diabetic Retinopathy Screening and Registration in Europe—Narrative Review. Healthcare 2021, 9, 745. https://doi.org/10.3390/healthcare9060745
Hristova E, Koseva D, Zlatarova Z, Dokova K. Diabetic Retinopathy Screening and Registration in Europe—Narrative Review. Healthcare. 2021; 9(6):745. https://doi.org/10.3390/healthcare9060745
Chicago/Turabian StyleHristova, Elitsa, Darina Koseva, Zornitsa Zlatarova, and Klara Dokova. 2021. "Diabetic Retinopathy Screening and Registration in Europe—Narrative Review" Healthcare 9, no. 6: 745. https://doi.org/10.3390/healthcare9060745
APA StyleHristova, E., Koseva, D., Zlatarova, Z., & Dokova, K. (2021). Diabetic Retinopathy Screening and Registration in Europe—Narrative Review. Healthcare, 9(6), 745. https://doi.org/10.3390/healthcare9060745