Probiotics for the Prevention of Acute Respiratory-Tract Infections in Older People: Systematic Review
Abstract
1. Introduction
2. Materials and Methods
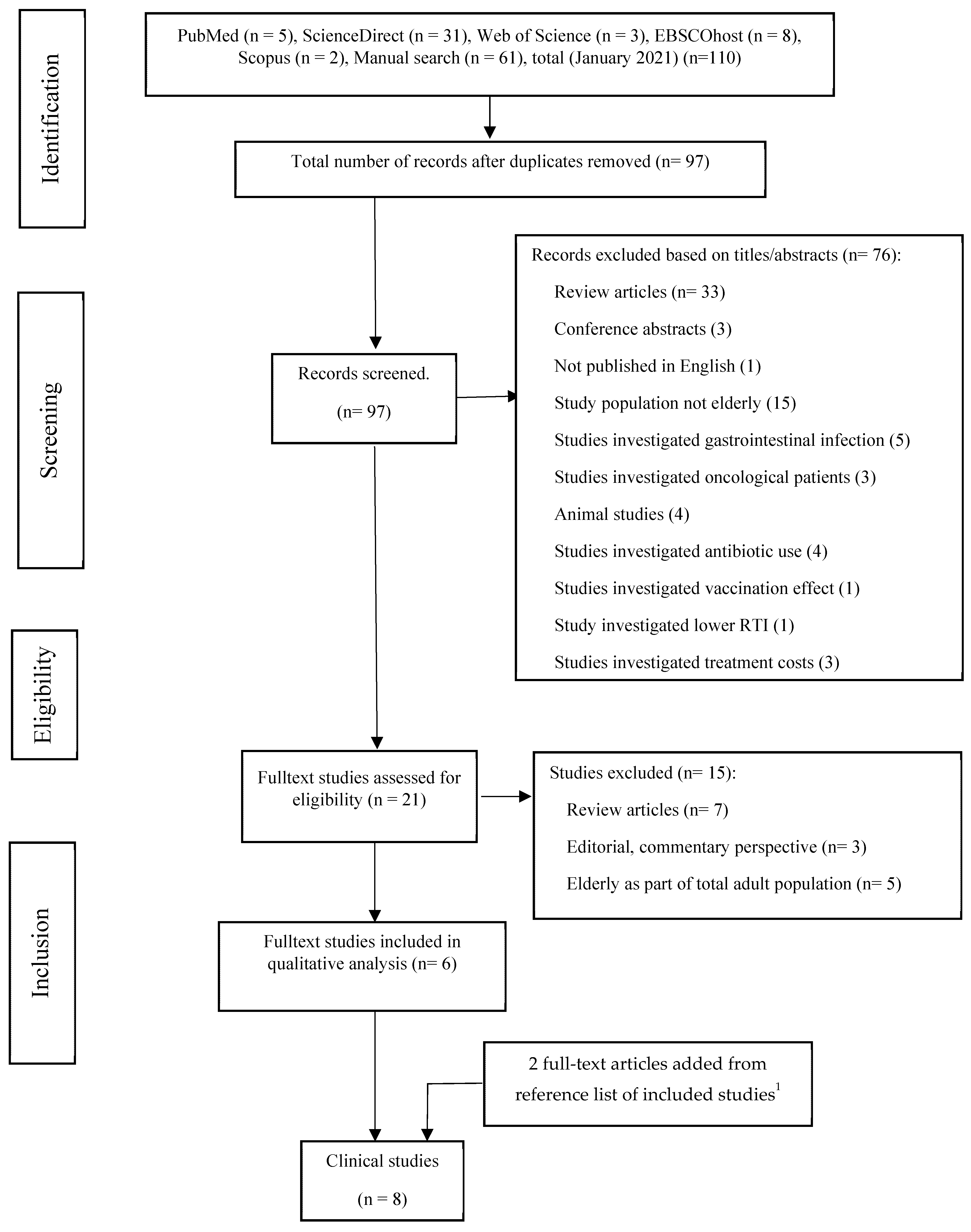
2.1. The Literature Selection Process
2.2. Data Extraction and Assessment of Selected Clinical Trials
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Berni Canani, R.; Flint, H.J.; Salminnen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; Food and Agriculture Organization of the United Nations, World Health Organization: Cordoba, Argentina, 2001. [Google Scholar]
- FAO/WHO. Guidelines for the Evaluation of Probiotics in Food; Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations, World Health Organization: London, ON, Canada, 2002. [Google Scholar]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literature. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Bagga, D.; Reichert, J.L.; Koschutnig, K.; Aigner, C.S.; Holzer, P.; Koskinen, K.; Moissl-Eichinger, C.; Schöpf, V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial effects of probiotic consumption on the immune system. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Alookaran, J.; Rhoads, J. Probiotics in Autoimmune and Inflammatory Disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef]
- Lau, C.S.; Chamberlain, R.S. Probiotics are effective at preventing Clostridium difficile-associated diarrhea: A systematic review and meta-analysis. Int. J. Gen. Med. 2016, 9, 27. [Google Scholar] [PubMed]
- Fijan, S.; Frauwallner, A.; Langerholc, T.; Krebs, B.; Ter Haar, J.A.; Heschl, A.; Mičetić Turk, D.; Rogelj, I. Efficacy of Using Probiotics with Antagonistic Activity against Pathogens of Wound Infections: An Integrative Review of Literature. Biomed Res. Int. 2019, 2019, 1–21. [Google Scholar] [CrossRef]
- Marco, M.L.; Sanders, M.E.; Gänzle, M.; Arrieta, M.C.; Cotter, P.D.; De Vuyst, L.; Hill, C.; Holzapfel, W.; Lebeer, S.; Merenstein, D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Lavefve, L.; Marasini, D.; Carbonero, F. Microbial Ecology of Fermented Vegetables and Non-Alcoholic Drinks and Current Knowledge on Their Impact on Human Health. Adv. Food Nutr. Res. 2019, 87, 147–185. [Google Scholar] [PubMed]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health benefits of lactic acid bacteria (Lab) fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Ranadheera, C.; Vidanarachchi, J.; Rocha, R.; Cruz, A.; Ajlouni, S. Probiotic Delivery through Fermentation: Dairy vs. Non-Dairy Beverages. Fermentation 2017, 3, 67. [Google Scholar] [CrossRef]
- Hao, Q.; Dong, B.R.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Meneghetti, A. Upper Respiratory Tract Infection Medication. Medscape 2015, 11, 1–18. [Google Scholar]
- Dasaraju, P.V.; Liu, C. Infections of the Respiratory System. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; p. 65. [Google Scholar]
- Bourke, S.J.; Burns, G.P. Respiratory Medicine, 9th ed.; Wiley-Blackwell Publishing: Hoboken, NJ, USA, 2015; p. 264. [Google Scholar]
- Kompanikova, J.; Zumdick, A.; Neuschlova, M.; Sadlonova, V.; Novakova, E. Microbiologic methods in the diagnostics of upper respiratory tract pathogens. Clin. Res. Pract. 2017, 1020, 25–31. [Google Scholar]
- Boncristiani, H.F.; Criado, M.F.; Arruda, E. Respiratory Viruses. In Encyclopedia of Microbiology, 3rd ed.; Schaechter, M., Ed.; Elsevier Inc: Amsterdam, The Netherlans, 2009; pp. 500–518. [Google Scholar]
- Jama-Kmiecik, A.; Frej-Mądrzak, M.; Sarowska, J.; Choroszy-Król, I. Pathogens causing upper respiratory tract infections in outpatients. Adv. Exp. Med. Biol. 2016, 28, 934–989. [Google Scholar]
- Haskins, R. Acute illness in day care: How much does it cost? J. Urban Health 1989, 65, 319–343. [Google Scholar]
- Hojsak, I.; Fabiano, V.; Pop, T.L.; Goulet, O.; Zuccotti, G.V.; Çokuğraş, F.C.; Pettoello-Mantovani, M.; Kolaček, S. Guidance on the use of probiotics in clinical practice in children with selected clinical conditions and in specific vulnerable groups. Acta Paediatr. 2018, 107, 927–937. [Google Scholar] [CrossRef]
- King, S.; Tancredi, D.; Lenoir-Wijnkoop, I.; Gould, K.; Vann, H.; Connors, G.; Sanders, M.E.; Linder, J.A.; Shane, A.L.; Merenstein, D. Does probiotic consumption reduce antibiotic utilization for common acute infections? A systematic review and meta-analysis. Eur. J. Public Health 2019, 29, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Poon, T.; Juana, J.; Noori, D.; Jeansen, S.; Pierucci-Lagha, A.; Musa-Veloso, K. Effects of a fermented dairy drink containing lacticaseibacillus paracasei subsp. Paracasei cncm i-1518 (lactobacillus casei cncm i-1518) and the standard yogurt cultures on the incidence, duration, and severity of common infectious diseases: A systematic. Nutrients 2020, 12, 3443. [Google Scholar] [CrossRef]
- Hao, Q.; Lu, Z.; Dong, B.R.; Huang, C.Q.; Wu, T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2011, 7, CD006895. [Google Scholar] [CrossRef]
- Quick, M. Cochrane commentary: Probiotics for prevention of acute upper respiratory infection. Explore 2015, 1, 418–420. [Google Scholar] [CrossRef]
- Williams, K.; Tang, M. Probiotics may prevent upper respiratory tract infections, but should we recommend them? J. Paediatr. Child Health 2012, 48, 942–943. [Google Scholar] [CrossRef] [PubMed]
- Ozen, M.; Sandal, G.K.; Dinleyici, E.C. Probiotics for the prevention of pediatric upper respiratory tract infections: A systematic review. Expert Opin. Biol. Ther. 2015, 15, 9–20. [Google Scholar] [CrossRef]
- Baindara, P.; Chakraborty, R.; Holliday, Z.M.; Mandal, S.M.; Schrum, A.G. Oral probiotics in coronavirus disease 2019: Connecting the gut-lung axis to viral pathogenesis, inflammation, secondary infection and clinical trials. New Microbes New Infect 2021, 40, 1–7. [Google Scholar] [CrossRef]
- Long, J.D.; Morris, A. Probiotics in Preventing Acute Upper Respiratory Tract Infections. Am. J. Nurs. 2017, 117, 69. [Google Scholar] [CrossRef]
- King, S.; Glanville, J.; Sanders, M.E.; Fitzgerald, A.; Varley, D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: A systematic review and meta-analysis. Br. J. Nutr. 2014, 112, 41–54. [Google Scholar] [CrossRef]
- Pu, F.; Guo, Y.; Li, M.; Zhu, H.; Wang, S.; Shen, X.; He, M.; Huang, C.; He, F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin. Interv. Aging 2017, 12, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- De Vrese, M.; Winkler, P.; Rautenberg, P.; Harder, T.; Noah, C.; Laue, C.; Ott, S.; Hampe, J.; Schreiber, S.; Heller, K.; et al. Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: A double blind, randomized, controlled trial. Clin. Nutr. 2005, 24, 481–491. [Google Scholar] [CrossRef]
- Hor, Y.Y.; Lew, L.C.; Lau, A.S.Y.; Ong, J.S.; Chuah, L.O.; Lee, Y.Y.; Choi, S.B.; Rashid, F.; Wahid, N.; Sun, Z.; et al. Probiotic Lactobacillus casei Zhang (LCZ) alleviates respiratory, gastrointestinal & RBC abnormality via immuno-modulatory, anti-inflammatory & anti-oxidative actions. J. Funct. Foods 2018, 44, 235–245. [Google Scholar]
- Guillemard, E.; Tondu, F.; Lacoin, F.; Schrezenmeir, J. Consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114001 reduces the duration of respiratory infections in the elderly in a randomised controlled trial. Br. J. Nutr. 2010, 103, 58–68. [Google Scholar] [CrossRef]
- Makino, S.; Ikegami, S.; Kume, A.; Horiuchi, H.; Sasaki, H.; Orii, N. Reducing the risk of infection in the elderly by dietary intake of yoghurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Br. J. Nutr. 2010, 104, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Urtamo, A.; Jyväkorpi, S.K.; Strandberg, T.E. Definitions of successful ageing: A brief review of a multidimensional concept. Acta Biomed. 2019, 90, 359–363. [Google Scholar]
- Saint-Criq, V.; Lugo-Villarino, G.; Thomas, M. Dysbiosis, malnutrition and enhanced gut-lung axis contribute to age-related respiratory diseases. Ageing Res. Rev. 2021, 66, 1–18. [Google Scholar] [CrossRef]
- Suetens, C. Healthcare-associated infections in European long-term care facilities: How big is the challenge? Eurosurveillance 2012, 30, 1–5. [Google Scholar]
- Uršič, T.; Miksić, N.G.; Lusa, L.; Strle, F.; Petrovec, M. Viral respiratory infections in a nursing home: A six-month prospective study. BMC Infect. Dis. 2016, 16, 1–9. [Google Scholar] [CrossRef]
- Orimo, H.; Ito, H.; Suzuki, T.; Araki, A.; Hosoi, T.; Sawabe, M. Reviewing the definition of “elderly”. Geriatr. Gerontol. Int. 2006, 6, 149–158. [Google Scholar] [CrossRef]
- Singh, S.; Bajorek, B. Defining “elderly” in clinical practice guidelines for pharmacotherapy. Pharm. Pract. 2014, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- UN. UNHCR Policy on Age, Gender and Diversity. 2018. Available online: https://emergency.unhcr.org/entry/43935/older-persons (accessed on 24 April 2021).
- WHO. Men Ageing And Health; World Health Organisation: Geneva, Switzerland, 2001. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, 1–6. [Google Scholar] [CrossRef]
- Joanna Briggs Institute Critical Appraisal Tool. Available online: https://jbi.global/critical-appraisal-tools (accessed on 1 May 2021).
- Fonollá, J.; Gracián, C.; Maldonado-Lobón, J.A.; Romero, C.; Bédmar, A.; Carrillo, J.C.; Martín-Castro, C.; Cabrera, A.L.; García-Curiel, J.M.; Rodríguez, C.; et al. Effects of Lactobacillus coryniformis K8 CECT5711 on the immune response to influenza vaccination and the assessment of common respiratory symptoms in elderly subjects: A randomized controlled trial. Eur. J. Nutr. 2019, 58, 83–90. [Google Scholar] [CrossRef]
- Fujita, R.; Iimuro, S.; Shinozaki, T.; Sakamaki, K.; Uemura, Y.; Takeuchi, A.; Matsuyama, Y.; Ohashi, Y. Decreased duration of acute upper respiratory tract infections with daily intake of fermented milk: A multicenter, double-blinded, randomized comparative study in users of day care facilities for the elderly population. Am. J. Infect. Control 2013, 41, 1231–1235. [Google Scholar] [CrossRef]
- Lefevre, M.; Racedo, S.M.; Ripert, G.; Housez, B.; Cazaubiel, M.; Maudet, C.; Jüsten, P.; Marteau, P.; Urdaci, M.C. Probiotic strain Bacillus subtilis CU1 stimulates immune system of elderly during common infectious disease period: A randomized, double-blind placebo-controlled study. Immun. Ageing 2015, 12, 1–11. [Google Scholar] [CrossRef]
- Van Puyenbroeck, K.; Hens, N.; Coenen, S.; Michiels, B.; Beunckens, C.; Molenberghs, G.; Van Royen, P.; Verhoeven, V. Efficacy of daily intake of Lactobacillus casei Shirota on respiratory symptoms and influenza vaccination immune response: A randomized, double-blind, placebo-controlled trial in healthy elderly nursing home residents. Am. J. Clin. Nutr. 2012, 95, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Hylwka, T.; Smieja, M.; Surrette, M.; Bowdish, D.M.E.; Loeb, M. Probiotics to Prevent Respiratory Infections in Nursing Homes: A Pilot Randomized Controlled Trial. J. Am. Geriatr. Soc. 2018, 66, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Turchet, P.; Laurenzano, M.; Auboiron, S.; Antoine, J. Effect of fermented milk containing the probiotic Lactobacillus casei DN-114001 on winter infections in free-living elderly subjects: A randomised, controlled pilot study. J. Nutr. Health 2003, 7, 5–7. [Google Scholar]
- De Vrese, M.; Winkler, P.; Rautenberg, P.; Harder, T.; Noah, C.; Laue, C.; Ott, S.; Hampe, J.; Schreiber, S.; Heller, K.; et al. Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine 2006, 24, 6670–6674. [Google Scholar] [CrossRef] [PubMed]
- Wolvers, D.; Antoine, J.M.; Myllyluoma, E.; Schrezenmeir, J.; Szajewska, H.; Rijkers, G.T. Guidance for substantiating the evidence for beneficial effects of probiotics: Prevention and management of infections by probiotics. J. Nutr. 2010, 14, 1–49. [Google Scholar] [CrossRef]
- Boge, T.; Rémigy, M.; Vaudaine, S.; Tanguy, J.; Bourdet-Sicard, R.; van der Werf, S. A probiotic fermented dairy drink improves antibody response to influenza vaccination in the elderly in two randomised controlled trials. Vaccine 2009, 27, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Guillemard, E.; Tanguy, J.; Flavigny, A.L.; De la Motte, S.; Schrezenmeir, J. Effects of consumption of a fermented dairy product containing the probiotic lactobacillus casei DN-114 001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J. Am. Coll. Nutr. 2010, 29, 455–468. [Google Scholar] [CrossRef]
- Merenstein, D.; Murphy, M.; Fokar, A.; Hernandez, R.K.; Park, H.; Nsouli, H.; Sanders, M.E.; Davis, B.A.; Niborski, V.; Tondu, F.; et al. Use of a fermented dairy probiotic drink containing Lactobacillus casei (DN-114 001) to decrease the rate of illness in kids: The DRINK study A patient-oriented, double-blind, cluster-randomized, placebo-controlled, clinical trial. Eur. J. Clin. Nutr. 2010, 4, 669–677. [Google Scholar] [CrossRef]
- Pedone, C.A.; Bernabeu, A.O.; Postaire, E.R.; Bouley, C.F.; Reinert, P. The effect of supplementation with milk fermented by Lactobacillus casei (strain DN-114 001) on acute diarrhoea in children attending day care centres. Int. J. Clin. Pract. 1999, 179–184. [Google Scholar]
- Nagata, S.; Asahara, T.; Wang, C.; Suyama, Y.; Chonan, O.; Takano, K.; Daibou, M.; Takahashi, T.; Nomoto, K.; Yamashiro, Y. The Effectiveness of Lactobacillus Beverages in Controlling Infections among the Residents of an Aged Care Facility: A Randomized Placebo-Controlled Double-Blind Trial. Ann. Nutr. Metab. 2016, 68, 51–59. [Google Scholar] [CrossRef]
- Dong, H.; Rowland, I.; Thomas, L.V.; Yaqoob, P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur. J. Nutr. 2013, 52, 1853–1863. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, S.; Wang, Y.; Yu, D.; Hua, L.; Guo, C.; Wang, D.; Lei, M. Oral administration of Lactobacillus casei Shirota improves recovery of hand functions after distal radius fracture among elder patients: A placebo-controlled, double-blind, and randomized trial. J. Orthop. Surg. Res. 2019, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cassani, E.; Privitera, G.; Pezzoli, G.; Pusani, C.; Madio, C.; Iorio, L.; Barichella, M. Use of probiotics for the treatment of constipation in Parkinson’s disease patients. Minerva Gastroenterol. Dietol. 2011, 57, 117–121. [Google Scholar] [PubMed]
- Nagata, S.; Asahara, T.; Ohta, T.; Yamada, T.; Kondo, S.; Bian, L.; Wang, C.; Yamashiro, Y.; Nomoto, K. Effect of the continuous intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on fever in a mass outbreak of norovirus gastroenteritis and the faecal microflora in a health service facility for the aged. Br. J. Nutr. 2011, 106, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kanazawa, A.; Azuma, K.; Ikeda, F.; Goto, H.; Komiya, K.; Kanno, R.; Tamura, Y.; Asahara, T.; Takahashi, T.; et al. Probiotic reduces bacterial translocation in type 2 diabetes mellitus: A randomised controlled study. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Saruta, J.; Takahashi, T.; To, M.; Shimizu, T.; Hayashi, T.; Morozumi, T.; Kubota, N.; Kamata, Y.; Makino, S.; et al. Effect of ingesting yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on influenza virus-bound salivary IgA in elderly residents of nursing homes: A randomized controlled trial. Acta Odontol. Scand. 2019, 77, 517–524. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujino, K.; Saruta, J.; Takahashi, T.; To, M.; Fuchida, S.; Shimizu, T.; Kamata, Y.; Misawa, K.; Tsukinoki, K. Effects of yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 on the IgA flow rate of saliva in elderly persons residing in a nursing home: A before-after non-randomised intervention study. Gerodontology 2017, 34, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Maruyama, K.; Suyama, K.; Nishijima, M.; Akamatsu, K.; Jogamoto, A.; Katakami, K.; Saito, I. The effects of OLL1073R-1 yogurt intake on influenza incidence and immunological markers among women healthcare workers: A randomized controlled trial. Food Funct. 2019, 10, 8129–8136. [Google Scholar] [CrossRef]
- Binda, S.; Hill, C.; Johansen, E.; Obis, D.; Pot, B.; Sanders, M.E.; Tremblay, A.; Ouwehand, A.C. Criteria to Qualify Microorganisms as “Probiotic” in Foods and Dietary Supplements. Front. Microbiol. 2020, 11, 1662. [Google Scholar] [CrossRef]
- Sanders, M.E.; Benson, A.; Lebeer, S.; Merenstein, D.J.; Klaenhammer, T.R. Shared mechanisms among probiotic taxa: Implications for general probiotic claims. Curr. Opin. Biotechnol. 2018, 49, 207–216. [Google Scholar] [CrossRef]
- Khuong Huu, M.; Fioramonti, J.; Urdaci, M. Probiotics strains for treating and/or preventing diarrhea. Pat. Appl. Publ. 2020, 10736925, 1–15. [Google Scholar]
- Lefevre, M.; Racedo, S.M.; Denayrolles, M.; Ripert, G.; Desfougères, T.; Lobach, A.R.; Simon, R.; Pélerin, F.; Jüsten, P.; Urdaci, M.C. Safety assessment of Bacillus subtilis CU1 for use as a probiotic in humans. Regul. Toxicol. Pharmacol. 2017, 83, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Díaz-Ropero, M.P.; Gómez, N.; Lara-Villoslada, F.; Sierra, S.; Maldonado, J.A.; Martín, R.; Rodríguez, J.M.; Xaus, J. The consumption of two new probiotic strains, Lactobacillus gasseri CECT 5714 and Lactobacillus coryniformis CECT 5711, boosts the immune system of healthy humans. Int. Microbiol. 2006, 9, 47–52. [Google Scholar] [PubMed]
- Lara-Villoslada, F.; Sierra, S.; Boza, J.; Xaus, J.; Olivares, M. Efectos beneficiosos en niños sanos del consumo de un producto lácteo que contiene dos cepas probióticas. Lactobacillus coryniformis CECT5711 y Lactobacillus gasseri CECT5714. Nutr. Hosp. 2007, 22, 496–502. [Google Scholar]
- Redondo, N.; Nova, E.; Gheorghe, A.; Díaz, L.E.; Hernández, A.; Marcos, A. Evaluation of Lactobacillus coryniformis CECT5711 strain as a coadjuvant in a vaccination process: A randomised clinical trial in healthy adults. Nutr. Metab. 2017, 14, 2. [Google Scholar] [CrossRef][Green Version]
- Vandenplas, Y.; Huys, G.; Daube, G. Probiotics: An update. J. Pediatr. 2015, 91, 6–21. [Google Scholar] [CrossRef]
- Capurso, L. Thirty Years of Lactobacillus rhamnosus GG A Review. J. Clin. Gastroenterol. 2019, 53, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Hibberd, P.L.; Kleimola, L.; Fiorino, A.M.; Botelho, C.; Haverkamp, M.; Andreyeva, I.; Poutsiaka, D.; Fraser, C.; Solano-Aguilar, G.; Snydman, D.R. No evidence of harms of probiotic Lactobacillus rhamnosus GG ATCC 53103 in healthy elderly—A phase i open label study to assess safety, tolerability and cytokine responses. PLoS ONE 2014, 9, e113456. [Google Scholar] [CrossRef] [PubMed]
- Solano-Aguilar, G.; Molokin, A.; Botelho, C.; Fiorino, A.M.; Vinyard, B.; Li, R.; Chen, C.; Urban, J.; Dawson, H.; Andreyeva, I.; et al. Transcriptomic profile of whole blood cells from elderly subjects fed probiotic bacteria lactobacillus rhamnosus gg atcc 53103 (lgg) in a phase i open label study. PLoS ONE 2016, 11, e0147426. [Google Scholar] [CrossRef] [PubMed]
- Eloe-Fadrosh, E.A.; Brady, A.; Crabtree, J.; Drabek, E.F.; Ma, B.; Mahurkar, A.; Ravel, J.; Haverkamp, M.; Fiorino, A.M.; Botelho, C.; et al. Functional dynamics of the gut microbiome in elderly people during probiotic consumption. MBio 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.C.; Lau, M.; Gillespie, D.; Owen-Jones, E.; Lown, M.; Wootton, M.; Calder, P.C.; Bayer, A.J.; Moore, M.; Little, P.; et al. Effect of Probiotic Use on Antibiotic Administration among Care Home Residents: A Randomized Clinical Trial. JAMA J. Am. Med. Assoc. 2020, 324, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Hibberd, P.L.; Goldin, B.; Thorpe, C.; McDermott, L.; Snydman, D.R. Effect of Lactobacillus rhamnosus GG administration on vancomycin-resistant Enterococcus colonization in adults with comorbidities. Antimicrob. Agents Chemother. 2015, 59, 4593–4599. [Google Scholar] [CrossRef] [PubMed]
- Hatakka, K.; Martio, J.; Korpela, M.; Herranen, M.; Poussa, T.; Laasanen, T.; Saxelin, M.; Vapaatalo, H.; Moilanen, E.; Korpela, R. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis—A pilot study. Scand. J. Rheumatol. 2003, 32, 211–215. [Google Scholar] [CrossRef]
- Akour, A. Probiotics and COVID-19: Is there any link? Lett. Appl. Microbiol. 2020, 71, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bottari, B.; Castellone, V.; Neviani, E. Probiotics and Covid-19. Int. J. Food Sci. Nutr. 2020, 72, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.; Samson, R.; Dastager, S.; Dharne, M. Probiotics in the prophylaxis of COVID-19: Something is better than nothing. Biotech 2021, 11, 1–10. [Google Scholar]
- Walton, G.E.; Gibson, G.R.; Hunter, K.A. Mechanisms linking the human gut microbiome to prophylactic and treatment strategies for COVID-19. Br. J. Nutr. 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; de Salis, L.V.V.; Cardoso, C.R.d.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Din, A.U.; Mazhar, M.; Wasim, M.; Ahmad, W.; Bibi, A.; Hassan, A.; Ali, N.; Gang, W.; Qian, G.; Ullah, R.; et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed Pharm. 2021, 133, 1–10. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and majore depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 1–13. [Google Scholar] [CrossRef]
- Sharpton, S.R.; Maraj, B.; Harding-Theobald, E.; Vittinghoff, E.; Terrault, N.A. Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: A systematic review, meta-analysis, and meta-regression. Am. J. Clin. Nutr. 2019, 110, 139–149. [Google Scholar] [CrossRef]
- Ardeshirlarijani, E.; Tabatabaei-Malazy, O.; Mohseni, S.; Qorbani, M.; Larijani, B.; Baradar Jalili, R. Effect of probiotics supplementation on glucose and oxidative stress in type 2 diabetes mellitus: A meta-analysis of randomized trials. J. Pharm. Sci. 2019, 27, 827–837. [Google Scholar] [CrossRef]
- Asha, M.Z.; Khalil, S.F.H. Efficacy and safety of probiotics, prebiotics and synbiotics in the treatment of irritable bowel syndrome a systematic review and meta-analysis. Sultan Qaboos Univ. Med J. 2020, 20, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mullish, B.H.; Marchesi, J.R.; McDonald, J.A.K.; Pass, D.A.; Masetti, G.; Michael, D.R.; Plummer, S.; Jack, A.A.; Davies, T.S.; Hughes, T.R.; et al. Probiotics reduce self-reported symptoms of upper respiratory tract infection in overweight and obese adults: Should we be considering probiotics during viral pandemics? Gut Microbes 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Ahrén, I.L.; Hillman, M.; Nordström, E.A.; Larsson, N.; Niskanen, T.M. Fewer Community-Acquired Colds with Daily Consumption of Lactiplantibacillus plantarum HEAL9 and Lacticaseibacillus paracasei 8700:2. A Randomized, Placebo-Controlled Clinical Trial. J. Nutr. 2021, 151, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.; Geiger, D.; Schauer, M.; Gostner, J.M.; Gatterer, H.; Burtscher, M.; Fuchs, D. Probiotic supplements beneficially affect tryptophan–kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: A randomized, double-blinded, placebo-controlled trial. Nutrients 2016, 23, 752. [Google Scholar] [CrossRef]
- Michalickova, D.; Minic, R.; Dikic, N.; Andjelkovic, M.; Kostic-Vucicevic, M.; Stojmenovic, T.; Nikolic, I.; Djordjevic, B. Lactobacillus helveticus Lafti L10 supplementation reduces respiratory infection duration in a cohort of elite athletes: A randomized, double-blind, placebo-controlled trial. Appl. Physiol. Nutr. Metab. 2016, 41, 782–789. [Google Scholar] [CrossRef]
- Meng, H.; Lee, Y.; Ba, Z.; Peng, J.; Lin, J.; Boyer, A.S.; Fleming, J.A.; Furumoto, E.J.; Roberts, R.F.; Kris-Etherton, P.M.; et al. Consumption of Bifidobacterium animalis subsp. lactis BB-12 impacts upper respiratory tract infection and the function of NK and T cells in healthy adults. Mol. Nutr. Food Res. 2016, 60, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Manti, S.; Parisi, G.F.; Papale, M.; Licari, A.; Salpietro, C.; Miraglia Del Giudice, M.; Marseglia, G.L.; Leonardi, S. Bacteriotherapy with Streptococcus salivarius 24SMB and Streptococcus oralis 89a nasal spray for treatment of upper respiratory tract infections in children: A pilot study on short-term efficacy. Ital. J. Pediatr. 2020, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Loyola, M.A.; Enciso-Moreno, J.A.; López-Ramos, J.E.; García-Marín, G.; Orozco Álvarez, M.Y.; Vega-García, A.M.; Mosqueda, J.; García-Gutiérrez, D.G.; Keller, D.; Pérez-Ramírez, I.F. Bacillus coagulans GBI-30, 6068 decreases upper respiratory and gastrointestinal tract symptoms in healthy Mexican scholar-aged children by modulating immune-related proteins. Food Res. Int. 2019, 125, 1–9. [Google Scholar] [CrossRef]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P.; et al. Preventive effect of cow’s milk fermented with lactobacillus paracasei CBA L74 on common infectious diseases in children: A multicenter randomized controlled trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef]
- Fijan, S.; Frauwallner, A.; Varga, L.; Langerholc, T.; Rogelj, I.; Lorber, M.; Lewis, P.; Povalej Bržan, P. Health Professionals’ Knowledge of Probiotics: An International Survey. Int. J. Environ. Res. Public Health 2019, 16, 3128. [Google Scholar] [CrossRef] [PubMed]
- Saxelin, M. Probiotic formulations and applications, the current probiotics market, and changes in the marketplace: A European perspective. Clin. Infect. Dis. 2008, 46, 76–79. [Google Scholar] [CrossRef]
- Sefidani Forough, A.; Lau, E.T.L.; Steadman, K.J.; Kyle, G.J.; Cichero, J.A.Y.; Serrano Santos, J.M.; Nissan, L.M. Appropriateness of oral dosage form modification for aged care residents: A video-recorded observational study. Int. J. Clin. Pharm. 2020, 42, 938–947. [Google Scholar] [CrossRef]
- Ranadheera, R.D.C.S.; Baines, S.K.; Adams, M.C. Importance of food in probiotic efficacy. Food Res. Int. 2010, 43, 1–7. [Google Scholar] [CrossRef]
- Shane, A.L.; Cabana, M.D.; Vidry, S.; Merenstein, D.; Hummelen, R.; Ellis, C.L.; Heimbach, J.T.; Hempel, S.; Lynch, S.V.; Sanders, M.E.; et al. Guide to designing, conducting, publishing and communicating results of clinical studies involving probiotic applications in human participants. Gut Microbes 2010, 1, 243–253. [Google Scholar] [CrossRef]
| Frame | Inclusion Criteria | Exclusion Criteria | Search Terms 1 |
|---|---|---|---|
| Population | Older people, over 60 years | Children, adults younger than 60 years or older people as undetermined part of adults | elder* OR “older people” OR “older adult” OR senior |
| Intervention | Probiotic fermented foods/drinks or probiotic food supplements | Heat-killed “probiotic” supplements, fermented foods without added probiotics | probiotic* OR “probiotic fermented” |
| Comparison | Control group (can be placebo, prebiotic or non) | Another probiotic | / |
| Outcome | Incidence and/or duration of upper respiratory tract infections (URTI) | Upper respiratory tract infections not reported or only reported as undetermined part of common infectious disease that can also include gastrointestinal infections, | “respiratory tract infections” OR RTI, |
| Study type | Randomised, placebo controlled clinical trials | Non-randomised, non-controlled clinical trials. Reviews and meta-analyses. Conference proceedings, editorial letters, only abstract available. | 2 |
| First Author, Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Turchet 2003 [55] | unclear | no | yes | no | no | no | yes | yes | yes | yes | yes | yes | no |
| Makino 2010 [39] | unclear | yes | yes | unclear | unclear | yes | yes | yes | yes | yes | yes | yes | unclear |
| Guillemard 2010a [38] | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Fujita 2013 [51] | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Puyenbroeck 2012 [53] | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Fonolla 2019 [50] | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes | yes |
| Lefevre 2015 [52] | yes | yes | yes | yes | yes | yes | yes | yes | yes | no | yes | yes | no |
| Wang 2018 [54] | yes | no | yes | no | no | yes | yes | yes | yes | yes | yes | yes | yes |
| Study | Manufacturing Information | Name of Probiotic Strain, Mentioned in Study | Name according to New Taxonomic Note 1 or Otherwise Updated Name | Abbreviation |
|---|---|---|---|---|
| Turchet 2003 [55] | Danone (France) | Lactobacillus casei DN-114 001 | Lacticaseibacillus paracasei subsp. paracasei CNCM I-1518 | LpCNCM |
| Makino 2010 [39] | Meiji Dairies cooperation (Japan) | Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1 | unchanged | LbR-1 |
| Guillemard 2010a [38] | Danone (France) | Lactobacillus casei DN-114 001 | Lacticaseibacillus paracasei subsp. paracasei CNCM I-1518 | LpCNCM |
| Fujita 2013 [51] | Yakult Honsha (Japan) | Lactobacilluscasei Shirota (Lactobacillus casei YIT 9029) | Lacticaseibacillusparacasei Shirota | LcS |
| Puyenbroeck 2012 [53] | Yakult Honsha (Japan) | Lactobacilluscasei Shirota (Lactobacillus casei YIT 9029) | Lacticaseibacillusparacasei Shirota | LcS |
| Fonolla 2019 [50] | Biosearch life (Spain) | Lactobacillus coryniformis K8 CECT5711 | Loigolactobacillus coryniformis K8 CECT5711 | LK8 |
| Lefevre 2015 [52] | Lesaffre (France) | Bacillus subtilis CU1 (Bacillus subtilis CNCM I-2745) | N/A | BsCU |
| Wang 2018 [54] | Culturelle (Denmark) | Lactobacillus rhamnosus GG (L. rhamnosus ATCC 53103) | Lacticaseibacillus rhamnosus GG | LGG |
| Reference | Study Design | Setting/Timeline | Enrolments and Allocation | Intervention | ||
|---|---|---|---|---|---|---|
| Active | Control | Duration | ||||
| Supplementation with Fermented Milk or Yoghurt with Probiotic Strains | ||||||
| Turchet 2003 [55] | randomized, open label, placebo- controlled pilot study. | Referential medical centers, Cordenons (Italy). Winter season. Time unspecified. | 360 healthy free-living individuals over 60 years of age. 180 in treatment group (mean age: 67.1 ± 6.0 years), 180 in control group (mean age: 69.3 ± 5.6 years). | 100 mL bottle of fermented dairy drink with Lacticaseibacillus paracasei subsp. paracasei CNCM I-1518 (108 cfu/mL) and yoghurt cultures. | None taken. | 1 bottle per day for 3 weeks |
| Makino 2010 [39] | randomized, double blind, placebo- controlled two-arm study. | Funagata (Japan): 13 March 2005–7 May 2005. | 57 healthy elderly individuals.29 in treatment group, 28 in control group (mean age: 74.5 years). | 90 g yoghurt fermented with Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1 (2.0–3.5 × 108 cfu/g) and yoghurt culture Streptococcus thermophilus OLS3059 (6.3–8.8 × 108 cfu/g). Extracellular polysaccharides 36.5–68.0 mg/kg | 100 mL milk. | 1 portion daily for 8 weeks. |
| Arita (Japan): 14 November 2006–5 February 2007. | 85 healthy elderly individuals. 42 in treatment group, 43 in control group (mean age: 67.7 years). | |||||
| Guillemard 2010 [38] | multicenter, randomized, double blind, placebo-controlled parallel group study. | 125 general practitioners in 25 centers (France). November 2006 to May 2007, including follow-up period. | 1072 free-living elderly individuals. 537 in treatment group (mean age: 76 years), 535 in control group (mean age: 76 years). | Fermented dairy drink (Actimel) containing Lacticaseibacillus paracasei subsp. paracasei CNCM I-1518 (1010 cfu/100 g) and yoghurt cultures: Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus (1010 cfu g). | Non- fermented, acidified, sweetened, flavored dairy drink | 2 drinks daily for 3 months (84 days) and 1-month follow-up phase. |
| Fujita 2013 [51] | Multicenter, randomized, double blind, placebo-controlled study. | Four day-care facilities in Tokyo (Japan). December to June 2009, including observation period. | 154 users of day-care facilities. (mean age: 83.2 ± 9.1 years). 78 in treatment group, 76 in control group. | 80 mL fermented milk containing lactic acid bacteria, high-fructose corn syrup, sugar, skimmed milk powder and 4 × 1010 cfu Lacticaseibacillus casei Shirota (LcS). | Fermented drink containing the same as above except probiotic (LcS). | 1 drink daily for 4 months and 3-months observation period. |
| Puyenbroeck 2012 [53] | Multicenter, randomized, double blind, placebo-controlled study. | 53 nursing homes in Antwerp region (Belgium). October 2007 to April 2008. | 554 nursing home residents (mean age: 84.17 years). 282 in treatment group, 272 in control group. | Fermented milk with Lacticaseibacillus casei Shirota (LcS) (6.5 × 109 cfu). | Similar drink without probiotic. | Twice daily for 176 days. |
| Supplementation with food supplements containing probiotics | ||||||
| Fonolla 2019 [50] | Multicenter, randomized, double blind, placebo-controlled trial. | Five nursing homes in Granada (Spain). October/November to April 2016, including observation period. | 84 nursing home residents, older than 65 years. (mean age: 81.76 ± 7.2 years). 38 in treatment group, 46 in control group. | Capsule with 3 × 107 Loigolactobacillus coryniformis K8 CECT5711 cfu in matrix of maltodextrin | Capsule with 300 mg maltodextrin. | 1 capsule daily 2 weeks before influenza vaccination. 5-month follow-up period. |
| Lefevre 2015 [52] | randomized, double blind, placebo-controlled, parallel arms study. | Nantes area (France). Winter season 2010–2011, including observation period | 100 free-living subjects, aged 60–74 years, 50 in treatment group (mean age: 63.3 (2.8) years). 50 in control group (mean age: 63.0 (2.4) years) | Capsule with Bacillus subtilis CU1 (2 × 109) and excipients: maltodextrin DE14, dicalcic phosphate, magnesium stearate, colloidal silica. | Capsule with excipient mix. | 10 days intermittently, alternating 18-day break, repeated 4 times. |
| Wang 2018 [54] | multicenter, pilot, double-blind, randomized, placebo-controlled study. | 14 nursing homes in Ontario (Canada). March 2013 to July 20, including observation period. | 196 nursing home residents aged 65 and older. 100 in treatment group (mean age: 85.2 ± 7.1 years). 96 in control group, (mean age: 85.9 ± 7.0 years) | Capsule with Lacticaseibacillus rhamnosus GG (109) | Capsule with calcium carbonate. | 2 capsules daily for 6 months. |
| Reference | Probiotic | Incidence of Respiratory Tract Disease or Winter Infections | Duration of Respiratory Tract Disease | Immunological Parameters | Other Reported Outcomes |
|---|---|---|---|---|---|
| Turchet 2003 | LpCNCM | No difference in incidence of winter infections (including influenza, gastrointestinal disease, ear-nose-throat pathology, bacterial broncho-pneumopathy) between groups | Significantly lower duration of all pathologies in treatment group (7.0 ± 3.2 days; n = 180) vs. control (8.7 ± 3.7 days; n = 180) (p = 0.024) | Not reported | Significantly lower maximal temperature (38.3 ± 0.5 °C) in treatment group vs. control (38.5 ± 0.6 °C) (p = 0.01). |
| Makino 2010 | LbR-1 | Significantly lower risk of catching the common cold (OR 0.39; p = 0.019) in treatment group vs. control. The risk was about 2.6 times lower. | Duration of URTI not reported | Significantly higher increase of natural killer cell activity in treatment group vs. control (p = 0.028). | / |
| Guillemard 2010 | LpCNCM | Significantly lower incidence of URTI in treatment group vs. control (p = 0.004). | Significantly lower cumulative duration of URTI in treatment group vs. control (p = 0.004). The median episode duration was 1–1.5 days shorter for treatment group vs. control. | Immunological parameters were comparable between the two groups. | Significantly lower duration of CID episodes and the cumulative duration of CID in treatment group vs. control (p = 0.008 and 0.009, respectively). No statistically significant difference between groups regarding cumulative number of CID. |
| Fujita 2013 | LcS | Total number of acute URTI events/total days of observation was lower in treatment group (0.0066) vs. control (0.0372), but not statistically significant (p = 0.64). | Statistically significant lower mean duration of infection per infection event was shorter in treatment group (3.71 ± 2.18 days) vs. control (5.40 ± 3.86 days), (p = 0.037). | Not reported. | Total symptom score/total days of observation (LcS: 0.0412, placebo: 0.0372, p = 0.89) was higher in treatment group vs. control, but not statistically significant. |
| Puyenbroeck 2012 | LcS | No significant difference between treatment group vs. control for number of participants with respiratory symptoms (p = 0.325). | No significant difference between treatment group vs. control for the number of days with respiratory symptoms (p = 0.342). | Not reported. | No significant differences between groups regarding influenza vaccination response. |
| Fonolla 2019 | LcK8 | Incidences of symptoms usually associated with respiratory infections were lower in the treatment group vs. control, although the differences reached significance only for sore throat. Incidence of local respiratory symptoms (sore throat, cough and/or nasal congestion) was approximately 48% lower in treatment group vs. control (p = 0.007). | Duration of URTI not reported | No significant differences in immunological parameters 1 were found in both groups. | The odds of seroconversion for at least one of the antigens of the vaccine was 4.94 times higher in the treatment group vs. control (p = 0.036). The odds of analgesic consumption were significantly lower in the treatment group (more than 6 times) vs. control (OR = 0.151; 95% CI 0.022–0.641; p = 0.021). |
| Lefevre 2015 | BsCU1 | In the subset of 44 subjects, the frequency of respiratory infections was significantly lower in the treatment group vs. control (p = 0.0323): a mean number of 0.6 (0.7) respiratory infections was observed in the probiotic group vs. 1.1 (0.9) in the placebo group | Duration of URTI not reported | In a subset of 44 subjects, IFN-γ concentrations significantly increased in the treatment group (p = 0.0090). No significant differences in concentrations of cytokines 2. | No statistically significant difference between treatment group vs. control in mean duration, intensity and frequency of CID (p = 0.2361, p = 0.7400, p = 0.3290, respectively). |
| Wang 2018 | LGG | No statistically significant differences in confirmed viral respiratory infections, influenza-like illness, hospitalization over pneumonia or other measured outcome between groups. | Duration of URTI not reported | Not reported | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strauss, M.; Mičetić-Turk, D.; Pogačar, M.Š.; Fijan, S. Probiotics for the Prevention of Acute Respiratory-Tract Infections in Older People: Systematic Review. Healthcare 2021, 9, 690. https://doi.org/10.3390/healthcare9060690
Strauss M, Mičetić-Turk D, Pogačar MŠ, Fijan S. Probiotics for the Prevention of Acute Respiratory-Tract Infections in Older People: Systematic Review. Healthcare. 2021; 9(6):690. https://doi.org/10.3390/healthcare9060690
Chicago/Turabian StyleStrauss, Maja, Dušanka Mičetić-Turk, Maja Šikić Pogačar, and Sabina Fijan. 2021. "Probiotics for the Prevention of Acute Respiratory-Tract Infections in Older People: Systematic Review" Healthcare 9, no. 6: 690. https://doi.org/10.3390/healthcare9060690
APA StyleStrauss, M., Mičetić-Turk, D., Pogačar, M. Š., & Fijan, S. (2021). Probiotics for the Prevention of Acute Respiratory-Tract Infections in Older People: Systematic Review. Healthcare, 9(6), 690. https://doi.org/10.3390/healthcare9060690







