Noninvasive Portable Hemoglobin Concentration Monitoring System Using Optical Sensor for Anemia Disease
Abstract
1. Introduction
- (1)
- This paper presents the development of a novel, low-cost, portable, easy-to-use, fast and painless hemoglobin concentration monitoring device for anemia disease.
- (2)
- This system is made noninvasive by using an optical sensor and requires no other set-up for analysis. As a result, the system is safe and flexible.
- (3)
- The system provides both an LCD display and a Bluetooth connection. The analyzed results could be shown and stored on a smartphone via the Bluetooth connection.
2. Materials and Methods
2.1. Beer–Lambert Law
2.1.1. Beer–Lambert Law along Non-Linear Mean Light Pathways
2.1.2. Modified Beer–Lambert Law (MBLL)
2.2. Data Acquisition and Signal Conditioning
2.3. Mathematical Implementation and Hemoglobin Calculation Software
2.4. Calibration with Cyanmethemoglobin Method
2.4.1. Cyanmethemoglobin Method
2.4.2. Statistical Analysis
F-Test Statistic
Linear Regression and T-test Statistic
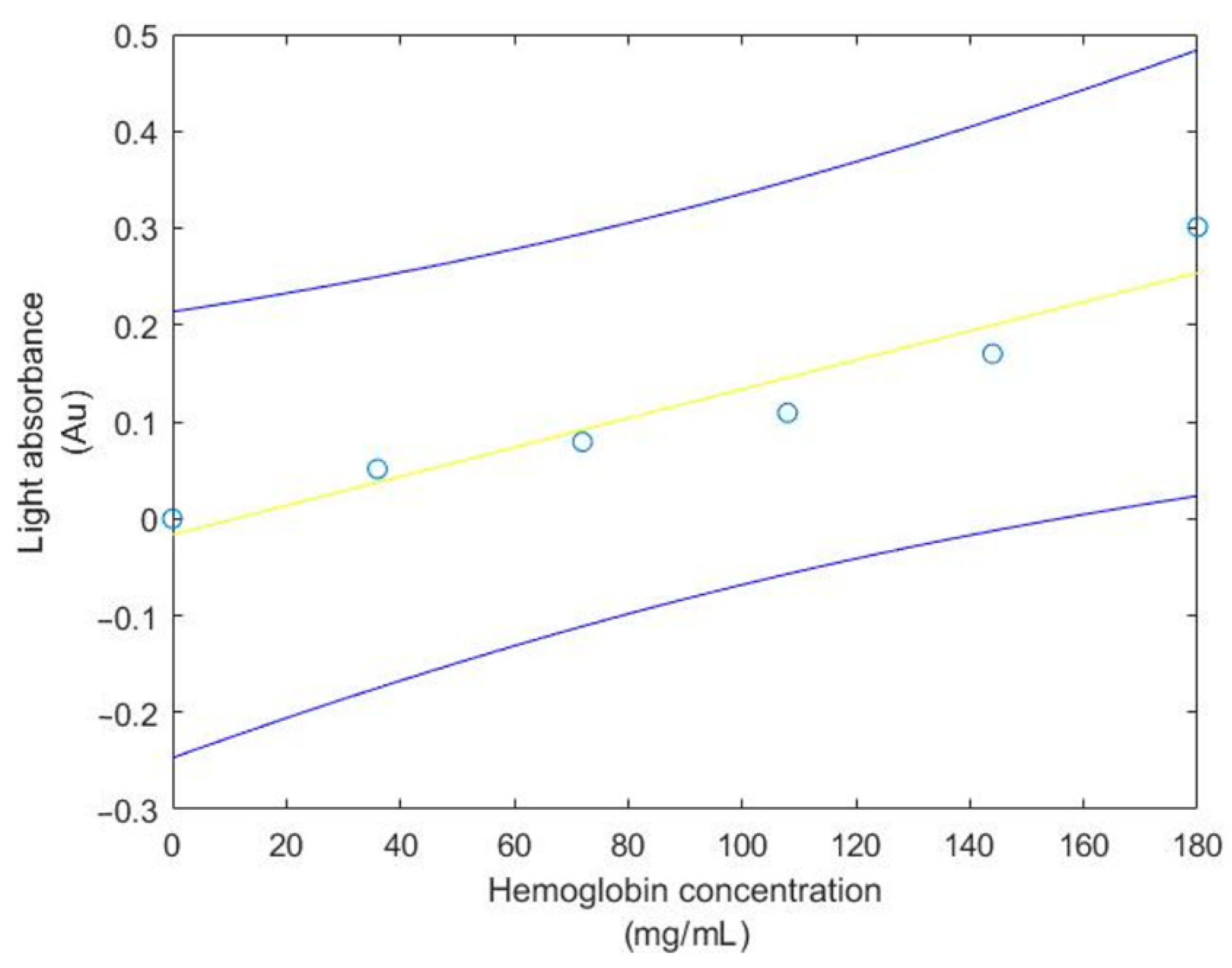
| Hemoglobin con(X)/Light ab(Y) |
| SigX = 540.0000 |
| SigY = 0.710900 |
| SigX2 = 71,280.000000 |
| SigXY = 98.046000 |
| a = −0.016695 |
| b = 0.001502 |
| N2 = 6 Stdx = 67.349833 |
| Stdy = 0.106036 |
| Syx = 0.076230 |
| Sa = 0.044171 |
| Sb = 0.000506 |
| 0.000097 < beta < 0.002907 |
| −0.169851 < alpha < 0.136460 |
| R = 0.954000 |
| r2 = 0.910117 |
| t-test = 6.364127 |
| Reject 6.364127 > 2.776000 |
2.4.3. Calibration Software
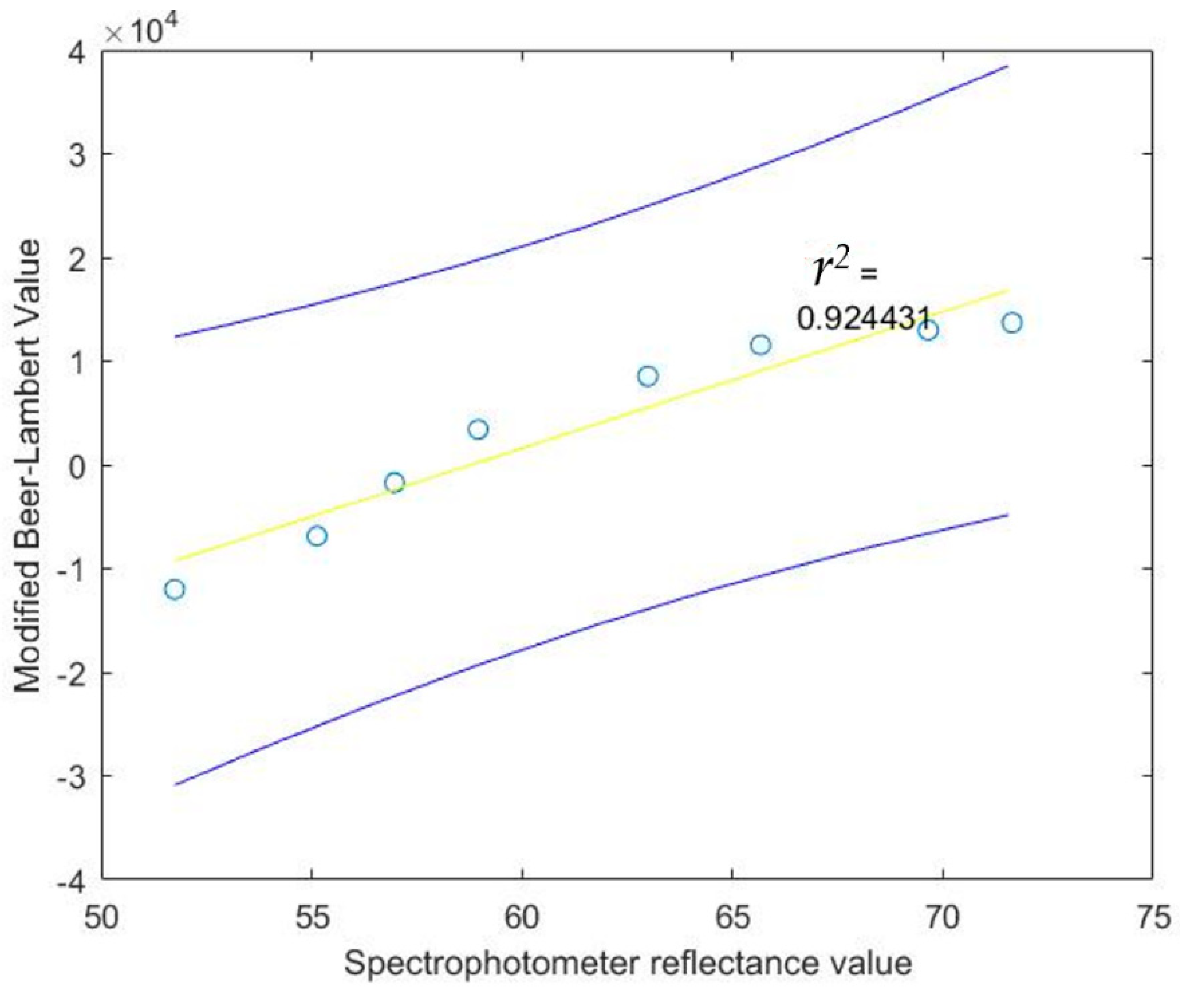
| SigX = 492.760000 |
| SigY = 29,633.000000 |
| SigX2 = 30,702.943000 |
| SigXY = 2,288,190.110000 |
| a = −77,445.200858 |
| b = 1317.466123 |
| N2 = 8 |
| Stdx = 7.085103 |
| Stdy = 9708.416900 |
| Syx = 7469.542827 |
| Sa = 24,685.608552 |
| Sb = 398.472935 |
| 342.402852 < beta < 2292.529394 |
| −137,850.884985 < alpha < −17,039.516731 |
| R = 0.961473 |
| r2 = 0.924431 |
| t-test = 8.567232 |
| Reject 8.567232 > 2.447000 |
2.5. Portable Hemoglobin Concentration Monitoring System
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wukitsch, M.W.; Petterson, M.T.; Tobler, D.R.; Prologe, J.A. Pulse oximetry: Analysis of theory, technology, and practice. J. Clin. Monit. 1988, 4, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Marengo-Rowe, A.J. Structure-function relations of human hemoglobin’s. Bayl. Univ. Med. Cent. Proc. 2006, 19, 239–245. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Iron Deficiency Anemia: Assessment, Prevention and Control a Guide for Program Managers; who reference who/nhd/01.3; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- de benoist, B.; Mclean, E.; Egli, I.; Cogswell, M.; World Health Organization. Worldwide Prevalence of Anemia 1993–2005: WHO Global Database on Anemia; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Sari, M.; Pee, S.D.; Martini, E.; Herman, S.; Bloem, M.W.; Yip, R. Estimating the prevalence of anemia: A comparison of three methods. Bull. World Health Org. 2001, 79, 506–511. [Google Scholar] [PubMed]
- Alli, N.; Vaughan, J.; Patel, M. Anemia: Approach to diagnosis. SAMJ S. Afr. Med. J. 2017, 107, 23–27. [Google Scholar] [CrossRef]
- Morris, S.S.; Ruel, M.T.; Cohen, R.J.; Dewey, K.G.; de la Briere, B.; Hassan, M.N. Precision, accuracy, and reliability of hemoglobin assessment with use of capillary blood. Am. J. Clin. Nutr. 1999, 69, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect. Med. 2013, 3, A011866. [Google Scholar] [CrossRef] [PubMed]
- Timm, U.; Lewis, E.; Leen, G.; Krait, J.; Ewald, H. Non-Invasive Continuous Online Hemoglobin Monitoring System; IEEE: New York, NY, USA, 2009. [Google Scholar]
- Al-baradie, R.S.; Bose, S.C. Portable smart non-invasive hemoglobin measurement system. In Proceedings of the 10th International Multi-Conferences on Systems, Signals & Devices 2013 (SSD13), Hammamet, Tunisia, 18–21 March 2013; pp. 1–4. [Google Scholar]
- Kraitl, J.; Ewald, H.; Timm, U. Non-invasive measurement of blood components. In Proceedings of the IEEE Fifth International Conference on Sensing Technology, Palmerston North, New Zealand, 28 November–1 December 2011. [Google Scholar]
- Aldrich, T.; Moosikasuwan, M.; Shah, S.; Deshpande, K. Length-normalized pulse photoplethysmography: A noninvasive method to measure blood hemoglobin. Ann. Biomed. Eng. 2002, 30, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Rajashree, D.; Anagha, P. Optical sensor system for hemoglobin measurement. IJCER 2013, 3, 41–45. [Google Scholar]
- Rochmanto, R.A.; Zakaria, H.; Alviana, R.D.; Shahib, N. Non-invasive hemoglobin measurement for anemia diagnosis. In Proceedings of the 2017 4th International Conference on Electrical Engineering, Computer Science and Informatics (EECSI), Yogyakarta, Indonesia, 19–21 September 2017; pp. 1–5. [Google Scholar]
- Chugh, S.; Kaur, J. Non-invasive hemoglobin monitoring device. In Proceedings of the 2015 International Conference on Control, Communication & Computing India (ICCC), Trivandrum, India, 19–21 November 2015; IEEE: New York, NY, USA; pp. 380–383. [Google Scholar]
- Rene, A.J. Principles and Applications of Fluorescence Spectroscopy; Blackwell Publishing: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kocsis, L.; Herman, P.; Eke, A. The modified beer–lambert law revisited. Phys. Med. Biol. 2006, 51, N91–N98. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Wolf, M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J. Biomed. Opt. 2013, 18, 105004. [Google Scholar] [CrossRef] [PubMed]
- Rybynok, V.; Kyriacou, P. Beer-lambert law along non-linear mean light pathways for the rational analysis of photoplethysmography. J. Phys. Conf. Ser. 2010, 238, 012061. [Google Scholar] [CrossRef]
- Jubran, A. Pulse oximetry. Crit. Care 2015, 19, 272. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.; Chan, M. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Strogonovs, R. Implementing Pulse Oximeter Using MAX30100, Morf-Coding and Engineering. 2017. Available online: https://morf.lv/implementing-pulse-oximeter-using-max30100 (accessed on 12 May 2021).
- Manish, B.; Kaylan, R.A.; Phaneendra, K.Y. Generalized Beer–Lambert model for near-infrared light propagation in thick biological tissues. J. Biomed. Opt. 2016, 21, 7. [Google Scholar] [CrossRef]
- Chatterjee, S.; Abay, T.; Phillips, J.; Kyriacou, P. Investigating optical path and differential pathlength factor in reflectance photoplethysmography for the assessment of perfusion. J. Biomed. Opt. 2018, 23, 075005. [Google Scholar] [CrossRef] [PubMed]
- Tabulated Molar Extinction Coefficient for Hemoglobin in Water. Available online: https://omlc.org/spectra/hemoglobin/summary.html (accessed on 12 May 2021).
- Bhaskaram, P.; Balakrishna, N.; Radhakrishna, K.V.; Krishnaswamy, K. Validation of hemoglobin estimation using Hemocue. Indian J. Pediatr. 2003, 70, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Jerome, E.; Groopman, J.E.; Itri, L.M. Chemotherapy-Induced Anemia in Adults: Incidence and Treatment. J. Natl. Cancer Inst. 1999, 19, 1616–1634. [Google Scholar]




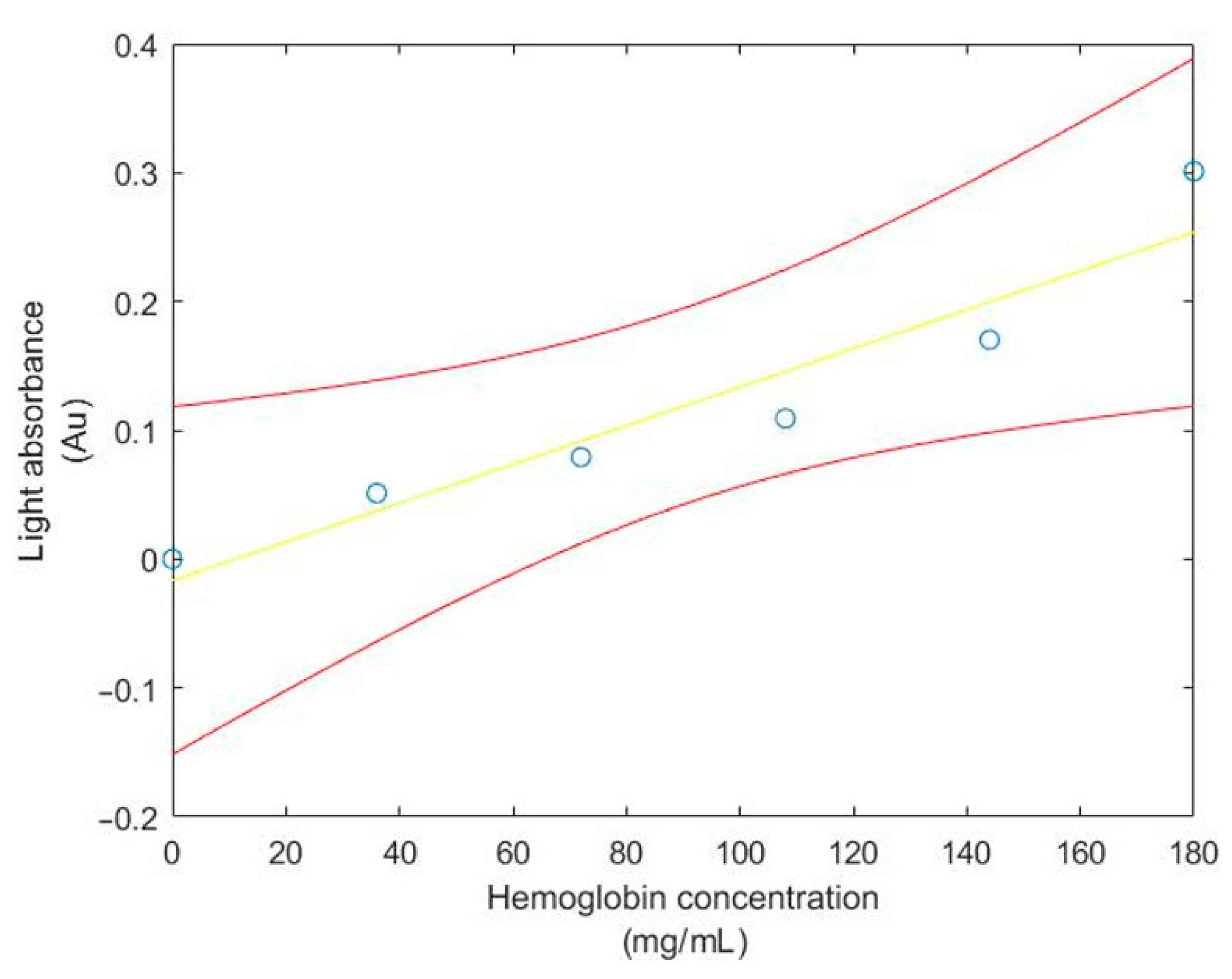


| Tube No. | Hemoglobin Concentration (mg/mL) | Drabkin’s Solution (mL) | Cyanmethemoglobin Standard Solution (mL) |
|---|---|---|---|
| 1 | 0 | 5 | 0 |
| 2 | 36 | 4 | 1 |
| 3 | 72 | 3 | 2 |
| 4 | 108 | 2 | 3 |
| 5 | 144 | 1 | 4 |
| 6 | 180 | 0 | 5 |
| Hemoglobin Concentration | MAX30100 Sensor Light Reflectance | MAX30100 Sensor Light Absorbance | MAX30100 Sensor Light Absorbance (Shifted) | Light Absorbance Spectrophotometer |
|---|---|---|---|---|
| 0 | 10,800 | 0.7830 | 0 | 00 |
| 36 | 9600 | 0.8342 | 0.0512 | 0.0740 |
| 72 | 9000 | 0.8622 | 0.0792 | 0.1517 |
| 108 | 8400 | 0.8922 | 0.1092 | 0.2313 |
| 144 | 7300 | 0.9532 | 0.1702 | 0.3103 |
| 180 | 5400 | 1.0841 | 0.3011 | 0.3920 |
| Hemoglobin Concentration | F-Test | F-Table |
|---|---|---|
| 36 | 7.05 | 7.71 |
| 72 | 7.38 | 7.71 |
| 108 | 6.74 | 7.71 |
| 144 | 7.13 | 7.71 |
| 180 | 5.63 | 7.71 |
| Cyanmethemoglobin Method | Modified Beer–Lambert Value |
|---|---|
| 176 | 4308 |
| 173.4 | 4745 |
| 175.6 | 4443 |
| 159.6 | 9358 |
| 161.5 | 8920 |
| 162.2 | 8716 |
| 107.9 | 14,111 |
| 109.1 | 13,344 |
| 107 | 14,540 |
| MAX30100 Absorbance Value | Spectrophotometer Reflectance Value | Modified Beer–Lambert Value | Calibrated Data Value |
|---|---|---|---|
| −0.0025 | 51.75 | −12,010 | 0 |
| 0.0760 | 55.13 | −6867 | 36 |
| 0.1546 | 56.97 | −1724 | 72 |
| 0.2331 | 58.96 | 3418 | 108 |
| 0.0303 | 62.99 | 7990 | 140 |
| 0.3117 | 65.67 | 8561 | 144 |
| 0.3467 | 69.65 | 10,847 | 160 |
| 0.3900 | 71.64 | 13,704 | 180 |
| Severity | Hemoglobin Concentration (g/L) |
|---|---|
| Normal | 120–160 for women 140–180 for men |
| Mild | 100 to normal limit |
| Moderate | 80–100 |
| Severe | 65–79 |
| Life-Threatening | <65 |
| SpO2 | |||||
|---|---|---|---|---|---|
| Measured Data (%) | Average (%) | Expected Result (%) | |||
| Round 1 | Round 2 | Round 3 | |||
| Subject 1 | 94.44 | 94.14 | 94.40 | 94.33 | >95 |
| Subject 2 | 94.03 | 94.63 | 94.45 | 94.37 | >95 |
| Subject 3 | 93.64 | 93.54 | 93.58 | 93.59 | >95 |
| Subject 4 | 94.26 | 94.66 | 93.98 | 94.29 | >95 |
| Subject 5 | 94.54 | 93.60 | 94.24 | 94.13 | >95 |
| Subject 6 | 94.51 | 94.50 | 94.21 | 94.41 | >95 |
| Subject 7 | 94.59 | 93.87 | 94.66 | 94.37 | >95 |
| Subject 8 | 94.50 | 94.51 | 94.91 | 94.64 | >95 |
| Subject 9 | 93.96 | 93.87 | 93.86 | 93.90 | >95 |
| Subject 10 | 94.90 | 94.60 | 95.02 | 94.84 | >95 |
| Subject 11 | 89.31 | 85.40 | 86.12 | 86.94 | >95 |
| Heartbeat | |||||
|---|---|---|---|---|---|
| Measured Data (BPM) | Average (BPM) | Expected Result (BPM) | |||
| Round 1 | Round 2 | Round 3 | |||
| Subject 1 | 82 (6.82%) | 92 (4.55%) | 88 (0.00%) | 87 (1.14%) | 88 |
| Subject 2 | 65 (99%) | 66 (1.49%) | 70 (4.47%) | 67 (0.00%) | 67 |
| Subject 3 | 84 (1.18%) | 86 (1.18%) | 88 (3.53%) | 86 (1.18%) | 85 |
| Subject 4 | 88 (0.00%) | 90 (27%) | 85 (3.41%) | 88 (0.00%) | 88 |
| Subject 5 | 72 (1.41%) | 72 (1.41%) | 75 (5.63%) | 73 (82%) | 71 |
| Subject 6 | 98 (4.56%) | 90 (4.56%) | 93 (1.06%) | 94 (0.00%) | 94 |
| Subject 7 | 85 (0.00%) | 88 (3.53%) | 85 (0.00%) | 87 (35%) | 85 |
| Subject 8 | 79 (3.66%) | 82(0.00%) | 83 (1.22%) | 81 (1.22%) | 82 |
| Subject 9 | 90 (3.22%) | 92 (1.01%) | 96 (3.22%) | 93 (0.00%) | 93 |
| Subject 10 | 85 (0.00%) | 80 (5.88%) | 91 (7.06%) | 85 (0.00%) | 85 |
| Subject 11 | 96 (13%) | 94 (0.00%) | 94 (0.00%) | 95 (1.06%) | 94 |
| Hemoglobin Concentration | |||||||
|---|---|---|---|---|---|---|---|
| Measured Data (g/L) | Average (g/L) | Actual Blood Test (g/L) | Expected Result (g/L) | Status | |||
| Round 1 | Round 2 | Round 3 | |||||
| Subject 1 | 200.48 (14.56%) | 169.55 (3.21%) | 153.25 (12.43%) | 174.43 (0.33%) | 175.00 | 140–180 | Normal |
| Subject 2 | 168.03 (3.09%) | 144.98 (11.05%) | 151.89 (6.82%) | 152.79 (6.26%) | 163.00 | 140–180 | Normal |
| Subject 3 | 164.63 (1.42%) | 171.28 (56%) | 179.98 (7.77%) | 171.96 (97%) | 167.00 | 140–180 | Normal |
| Subject 4 | 149.32 (41%) | 168.78 (10.31%) | 146.33 (4.36%) | 154.81 (1.18%) | 153.00 | 140–180 | Normal |
| Subject 5 | 165.27 (1.63%) | 169.61 (0.95%) | 163.86 (46%) | 166.25 (1.04%) | 168.00 | 140–180 | Normal |
| Subject 6 | 130.52 (16.87%) | 133.09 (15.23%) | 136.12 (13.30%) | 133.24 (15.13%) | 157.00 | 140–180 | Normal |
| Subject 7 | 166.26 (0.16%) | 167.16 (0.70%) | 167.38 (0.83%) | 166.92 (0.55%) | 166.00 | 140–180 | Normal |
| Subject 8 | 144.19 (10.44%) | 149.52 (7.13%) | 140.74 (12.58%) | 144.82 (10.05%) | 161.00 | 140–180 | Normal |
| Subject 9 | 140.74 (10.36%) | 132.38 (15.68%) | 131.54 (16.22%) | 134.89 (14.08%) | 157.00 | 140–180 | Normal |
| Subject 10 | 143.55 (4.93%) | 141.40 (6.36%) | 147.24 (55%) | 144.06 (4.60%) | 151.00 | 120–160 | Normal |
| Subject 11 | 115.77 (7.49%) | 113.18 (5.78%) | 120.07 (12.21%) | 116.34 (8.73%) | 107.00 | <110 | Mild |
| Subject Information | ||||
|---|---|---|---|---|
| Sex | Age | Race | Hemoglobin-Related Disorder | |
| Subject 1 | Male | 22 | Asian (Thai) | - |
| Subject 2 | Male | 22 | Asian (Thai) | - |
| Subject 3 | Male | 26 | Asian (Thai) | - |
| Subject 4 | Male | 26 | Asian (Thai) | - |
| Subject 5 | Male | 24 | Asian (Thai) | - |
| Subject 6 | Male | 26 | Asian (Thai) | - |
| Subject 7 | Male | 24 | Asian (Thai) | - |
| Subject 8 | Male | 25 | Asian (Thai) | - |
| Subject 9 | Male | 25 | Asian (Thai) | - |
| Subject 10 | Female | 26 | Asian (Thai) | - |
| Subject 11 | Female | 25 | Asian (Thai) | Mild Anemia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintavirooj, C.; Ni, B.; Chatkobkool, C.; Pinijkij, K. Noninvasive Portable Hemoglobin Concentration Monitoring System Using Optical Sensor for Anemia Disease. Healthcare 2021, 9, 647. https://doi.org/10.3390/healthcare9060647
Pintavirooj C, Ni B, Chatkobkool C, Pinijkij K. Noninvasive Portable Hemoglobin Concentration Monitoring System Using Optical Sensor for Anemia Disease. Healthcare. 2021; 9(6):647. https://doi.org/10.3390/healthcare9060647
Chicago/Turabian StylePintavirooj, Chuchart, Baorong Ni, Chaiwat Chatkobkool, and Kittitorn Pinijkij. 2021. "Noninvasive Portable Hemoglobin Concentration Monitoring System Using Optical Sensor for Anemia Disease" Healthcare 9, no. 6: 647. https://doi.org/10.3390/healthcare9060647
APA StylePintavirooj, C., Ni, B., Chatkobkool, C., & Pinijkij, K. (2021). Noninvasive Portable Hemoglobin Concentration Monitoring System Using Optical Sensor for Anemia Disease. Healthcare, 9(6), 647. https://doi.org/10.3390/healthcare9060647






