Discrimination and Leukocyte Telomere Length by Depressive Symptomatology: The Jackson Heart Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Leukocyte Telomere Length
2.3. Measures of Perceived Discrimination
2.4. Coping with Discrimination
2.5. Depressive Symptoms
2.6. Covariates
2.7. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Discrimination and LTL by Sex and Depressive Symptoms
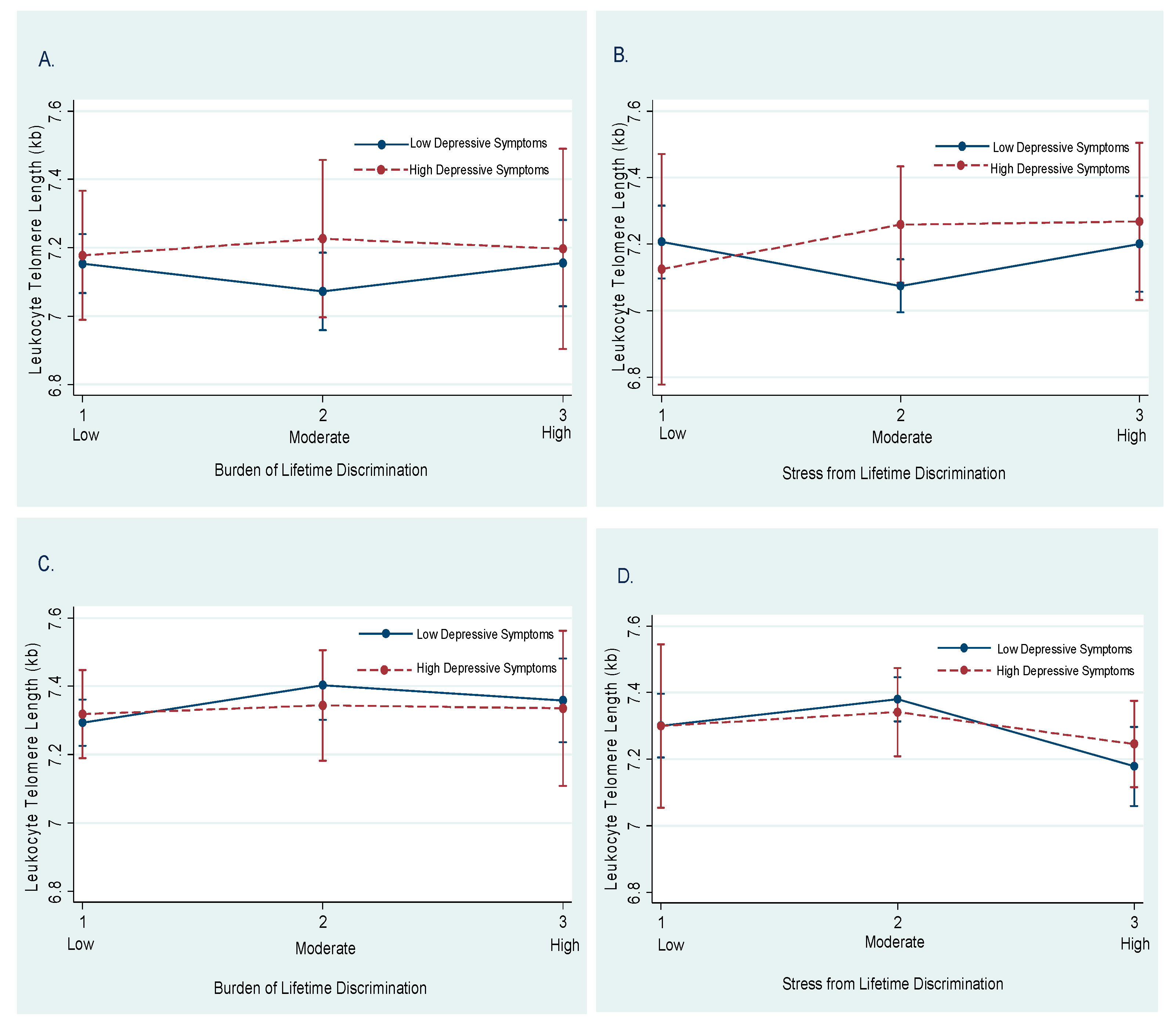
3.3. Moderation by Depressive Symptoms
3.4. Coping with Discrimination and LTL by Sex and Depressive Symptoms
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
JHS Disclaimer
References
- Yeh, J.-K.; Wang, C.-Y. Telomeres and Telomerase in Cardiovascular Diseases. Genes 2016, 7, 58. [Google Scholar] [CrossRef]
- Needham, B.L.; Rehkopf, D.; Adler, N.; Gregorich, S.; Lin, J.; Blackburn, E.H.; Epel, E.S. Leukocyte Telomere Length and Mortality in the National Health and Nutrition Examination Survey, 1999–2002. Epidemiology 2015, 26, 528–535. [Google Scholar] [CrossRef]
- Aviv, A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat. Res. Mol. Mech. Mutagen. 2012, 730, 68–74. [Google Scholar] [CrossRef]
- Mathur, M.B.; Epel, E.; Kind, S.; Desai, M.; Parks, C.G.; Sandler, D.P.; Khazeni, N. Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav. Immun. 2016, 54, 158–169. [Google Scholar] [CrossRef]
- Coimbra, B.M.; Carvalho, C.M.; Moretti, P.N.; De Mello, M.F.; Belangero, S.I.N. Stress-related telomere length in children: A systematic review. J. Psychiatr. Res. 2017, 92, 47–54. [Google Scholar] [CrossRef]
- Epel, E.S.; Blackburn, E.H.; Lin, J.; Dhabhar, F.S.; Adler, N.E.; Morrow, J.D.; Cawthon, R.M. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. USA 2004, 101, 17312–17315. [Google Scholar] [CrossRef]
- Shalev, I.; Entringer, S.; Wadhwa, P.D.; Wolkowitz, O.M.; Puterman, E.; Lin, J.; Epel, E.S. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology 2013, 38, 1835–1842. [Google Scholar] [CrossRef]
- Chae, D.H.; Epel, E.S.; Nuru-Jeter, A.M.; Lincoln, K.D.; Taylor, R.J.; Lin, J.; Blackburn, E.H.; Thomas, S.B. Discrimination, mental health, and leukocyte telomere length among African American men. Psychoneuroendocrinology 2016, 63, 10–16. [Google Scholar] [CrossRef]
- Moody, D.L.B.; Leibel, D.K.; Darden, T.M.; Ashe, J.J.; Waldstein, S.R.; Katzel, L.I.; Liu, H.B.; Weng, N.-P.; Evans, M.K.; Zonderman, A.B. Interpersonal-level discrimination indices, sociodemographic factors, and telomere length in African-Americans and Whites. Biol. Psychol. 2019, 141, 1–9. [Google Scholar] [CrossRef]
- Chae, D.H.; Nuru-Jeter, A.M.; Adler, N.E.; Brody, G.H.; Lin, J.; Blackburn, E.H.; Epel, E.S. Discrimination, Racial Bias, and Telomere Length in African-American Men. Am. J. Prev. Med. 2014, 46, 103–111. [Google Scholar] [CrossRef]
- Sims, M.; Glover, L.S.M.; Gebreab, S.Y.; Spruill, T.M. Cumulative psychosocial factors are associated with cardiovascular disease risk factors and management among African Americans in the Jackson Heart Study. BMC Public Health 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Burroughs Peña, M.S.; Mbassa, R.S.; Slopen, N.B.; Williams, D.R.; Buring, J.E.; Albert, M.A. Cumulative Psychosocial Stress and Ideal Cardiovascular Health in Older Women: Data by Race/Ethnicity. Circulation 2019, 139, 2012–2021. [Google Scholar] [CrossRef]
- Sternthal, M.J.; Slopen, N.; Williams, D.R. RACIAL DISPARITIES IN HEALTH: How Much Does Stress Really Matter? Du Bois Rev. Soc. Sci. Res. Race 2011, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Bailey, Z.D.; Krieger, N.; Agénor, M.; Graves, J.; Linos, N.; Bassett, M.T. Structural racism and health inequities in the USA: Evidence and interventions. Lancet 2017, 389, 1453–1463. [Google Scholar] [CrossRef]
- Adler, N.; Pantell, M.S.; O’Donovan, A.; Blackburn, E.; Cawthon, R.; Koster, A.; Opresko, P.; Newman, A.B.; Harris, T.B.; Epel, E. Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain Behav. Immun. 2013, 27, 15–21. [Google Scholar] [CrossRef]
- Lu, D.; Palmer, J.R.; Rosenberg, L.; Shields, A.E.; Orr, E.H.; DeVivo, I.; Cozier, Y.C. Perceived racism in relation to telomere length among African American women in the Black Women’s Health Study. Ann. Epidemiol. 2019, 36, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Schrock, J.M.; Adler, N.E.; Epel, E.S.; Nuru-Jeter, A.M.; Lin, J.; Blackburn, E.H.; Taylor, R.J.; Chae, D.H. Socioeconomic Status, Financial Strain, and Leukocyte Telomere Length in a Sample of African American Midlife Men. J. Racial Ethn. Health Disparities 2017, 5, 459–467. [Google Scholar] [CrossRef]
- Lynch, S.M.; Peek, M.K.; Mitra, N.; Ravichandran, K.; Branas, C.; Spangler, E.; Zhou, W.; Paskett, E.D.; Gehlert, S.; DeGraffinreid, C.; et al. Race, Ethnicity, Psychosocial Factors, and Telomere Length in a Multicenter Setting. PLoS ONE 2016, 11, e0146723. [Google Scholar] [CrossRef]
- Roux, A.V.D.; Ranjit, N.; Jenny, N.S.; Shea, S.; Cushman, M.; Fitzpatrick, A.; Seeman, T. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell 2009, 8, 251–257. [Google Scholar] [CrossRef]
- Rewak, M.; Buka, S.; Prescott, J.; De Vivo, I.; Loucks, E.B.; Kawachi, I.; Non, A.L.; Kubzansky, L.D. Race-related health disparities and biological aging: Does rate of telomere shortening differ across blacks and whites? Biol. Psychol. 2014, 99, 92–99. [Google Scholar] [CrossRef]
- Jordan, C.D.; Glover, L.; Gao, Y.; Musani, S.K.; Mwasongwe, S.; Wilson, J.G.; Reiner, A.; Diez-Roux, A.; Sims, M. Association of psychosocial factors with leukocyte telomere length among African Americans in the Jackson Heart Study. Stress Health 2018, 35, 138–145. [Google Scholar] [CrossRef]
- Schutte, N.S.; Malouff, J.M. The association between depression and leukocyte telomere length: A meta-analysis. Depress. Anxiety 2015, 32, 229–238. [Google Scholar] [CrossRef]
- Sims, M.; Redmond, N.; Khodneva, Y.; Durant, R.W.; Halanych, J.; Safford, M.M. Depressive symptoms are associated with incident coronary heart disease or revascularization among blacks but not among whites in the Reasons for Geographical and Racial Differences in Stroke study. Ann. Epidemiol. 2015, 25, 426–432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Williams, D.R.; González, H.M.; Neighbors, H.; Nesse, R.; Abelson, J.M.; Sweetman, J.; Jackson, J.S. Prevalence and Distribution of Major Depressive Disorder in African Americans, Caribbean Blacks, and Non-Hispanic Whites. Arch. Gen. Psychiatry 2007, 64, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, S.R.; Wyatt, S.B.; Andrew, M.E.; Sarpong, D.F.; Henderson, F.R.; Cunningham, M.F.; Taylor, H.A. Recruiting African-American research participation in the Jackson Heart Study: Methods, response rates, and sample description. Ethn. Dis. 2005, 15, S6–18. [Google Scholar] [PubMed]
- Carpenter, M.A.; Crow, R.; Steffes, M.; Rock, W.; Skelton, T.; Heilbraun, J.; Evans, G.; Jensen, R.; Sarpong, D. Laboratory, Reading Center, and Coordinating Center Data Management Methods in the Jackson Heart Study. Am. J. Med. Sci. 2004, 328, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Stone, R.C.; Hunt, S.C.; Skurnick, J.; Lu, X.; Cao, X.; Harley, C.B.; Aviv, A. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat. Protoc. 2010, 5, 1596–1607. [Google Scholar] [CrossRef]
- Williams, D.R.; Yu, Y.; Jackson, J.S.; Anderson, N.B. Racial differences in physical and mental health: Socio-economic status, stress and discrimination. J. Health Psychol. 1997, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Krieger, N.; Sidney, S. Racial discrimination and blood pressure: The CARDIA Study of young black and white adults. Am. J. Public Health 1996, 86, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Okhomina, V.I.; Glover, L.; Taylor, H.; Sims, M. Dimensions of and Responses to Perceived Discrimination and Subclinical Disease Among African-Americans in the Jackson Heart Study. J. Racial Ethn. Health Disparities 2018, 5, 1084–1092. [Google Scholar] [CrossRef]
- Vilagut, G.; Forero, C.G.; Barbaglia, M.G.; Alonso, J. Screening for Depression in the General Population with the Center for Epidemiologic Studies Depression (CES-D): A Systematic Review with Meta-Analysis. PLoS ONE 2016, 11, e0155431. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Kao, T.-W.; Chang, Y.-W.; Wu, C.-J.; Peng, T.-C.; Wu, L.-W.; Yang, H.-F.; Liaw, F.-Y.; Chen, W.-L. Examining the gender difference in the association between metabolic syndrome and the mean leukocyte telomere length. PLoS ONE 2017, 12, e0180687. [Google Scholar] [CrossRef]
- Khan, R.J.; Gebreab, S.Y.; Gaye, A.; Crespo, P.R.; Xu, R.; Davis, S.K. Associations of smoking indicators and cotinine levels with telomere length: National Health and Nutrition Examination Survey. Prev. Med. Rep. 2019, 15, 100895. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, Z.; Su, X.; Da, M.; Yang, Z.; Duan, W.; Mo, X. Association between leucocyte telomere length and cardiovascular disease in a large general population in the United States. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Ward, J.B.; Gartner, D.R.; Keyes, K.M.; Fliss, M.D.; McClure, E.S.; Robinson, W.R. How do we assess a racial disparity in health? Distribution, interaction, and interpretation in epidemiological studies. Ann. Epidemiol. 2019, 29, 1–7. [Google Scholar] [CrossRef]
- Liu, S.Y.; Kawachi, I. Discrimination and Telomere Length Among Older Adults in the United States. Public Health Rep. 2017, 132, 220–230. [Google Scholar] [CrossRef]
- Needham, B.L.; Mezuk, B.; Bareis, N.; Lin, J.; Blackburn, E.H.; Epel, E.S. Depression, anxiety and telomere length in young adults: Evidence from the National Health and Nutrition Examination Survey. Mol. Psychiatry 2015, 20, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Berglund, K.; Reynolds, C.A.; Ploner, A.; Gerritsen, L.; Hovatta, I.; Pedersen, N.L.; Hägg, S. Longitudinal decline of leukocyte telomere length in old age and the association with sex and genetic risk. Aging 2016, 8, 1398–1415. [Google Scholar] [CrossRef] [PubMed]
- Mwasongwe, S.; Gao, Y.; Griswold, M.; Wilson, J.G.; Aviv, A.; Reiner, A.P.; Raffield, L.M. Leukocyte telomere length and cardiovascular disease in African Americans: The Jackson Heart Study. Atherosclerosis 2017, 266, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Shi, L.; Prescott, J.; Chiuve, S.E.; Hu, F.B.; De Vivo, I.; Stampfer, M.J.; Franks, P.W.; Manson, J.E.; Rexrode, K.M. Healthy Lifestyle and Leukocyte Telomere Length in U.S. Women. PLoS ONE 2012, 7, e38374. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.L.; Fitzpatrick, A.L.; Boudreau, R.; Arnold, A.M.; Aviv, A.; Kimura, M.; Fried, L.F.; Harris, T.B.; Newman, A.B. Leukocyte Telomere Length Is Associated With Noninvasively Measured Age-Related Disease: The Cardiovascular Health Study. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2011, 67, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Hunt, S.C.; Chen, W.; Gardner, J.P.; Kimura, M.; Srinivasan, S.R.; Eckfeldt, J.H.; Berenson, G.S.; Aviv, A. Leukocyte telomeres are longer in AfricanAmericans than in whites: The National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell 2008, 7, 451–458. [Google Scholar] [CrossRef]
- Ellis, K.R.; Griffith, D.M.; Allen, J.O.; Thorpe, R.J.; Bruce, M.A. “If you do nothing about stress, the next thing you know, you’re shattered”: Perspectives on African American men’s stress, coping and health from African American men and key women in their lives. Soc. Sci. Med. 2015, 139, 107–114. [Google Scholar] [CrossRef]
- Brenner, A.B.; Diez-Roux, A.V.; Gebreab, S.Y.; Schulz, A.J.; Sims, M. The Epidemiology of Coping in African American Adults in the Jackson Heart Study (JHS). J. Racial Ethn. Health Disparities 2017, 5, 978–994. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, J.R.; Watkins, D.C.; Johnson, N.C.; Allen, J.O. An exploratory study of stress and coping among Black college men. Am. J. Orthopsychiatry 2018, 88, 538–549. [Google Scholar] [CrossRef]
- James, S.A.; Hartnett, S.A.; Kalsbeek, W.D. John Henryism and blood pressure differences among black men. J. Behav. Med. 1983, 6, 259–278. [Google Scholar] [CrossRef] [PubMed]
- Bonham, V.L.; Sellers, S.L.; Neighbors, H.W. John Henryism and Self-Reported Physical Health Among High–Socioeconomic Status African American Men. Am. J. Public Health 2004, 94, 737–738. [Google Scholar] [CrossRef]
- Light, K.C.; Brownley, K.A.; Turner, J.R.; Hinderliter, A.L.; Girdler, S.S.; Sherwood, A.; Anderson, N.B. Job Status and High-Effort Coping Influence Work Blood Pressure in Women and Blacks. Hypertension 1995, 25, 554–559. [Google Scholar] [CrossRef]
| Characteristics | Men (n = 580) | Women (n = 910) | p-Value |
|---|---|---|---|
| Age (mean ± SD) | 52.46 ± 12.34 | 53.51 ± 11.73 | 0.101 |
| Education (%) | 0.480 | ||
| <HS | 12.59 | 14.30 | |
| HS–Some College | 40.52 | 41.58 | |
| College Degree or More | 46.90 | 44.11 | |
| Waist circumference (mean ± SD) | 101.39 ± 15.61 | 100.89 ± 16.79 | 0.564 |
| Smoking (%) | 0.007 | ||
| Poor Smoking Health | 17.93 | 12.86 | |
| Ideal Smoking Health | 82.07 | 87.14 | |
| Mean LTL (mean ± SD) | 7.14 ± 0.70 | 7.32 ± 0.67 | <0.001 |
| Mean CES-D score (mean ± SD) | 10.08 ± 7.38 | 11.98 ± 8.55 | <0.001 |
| Everyday Discrimination (%) | 0.415 | ||
| Low | 46.82 | 50.48 | |
| Moderate | 26.97 | 24.94 | |
| High | 26.22 | 24.58 | |
| Lifetime Discrimination (%) | 0.079 | ||
| Low | 39.92 | 46.07 | |
| Moderate | 33.52 | 29.52 | |
| High | 26.55 | 24.40 | |
| Burden of Lifetime Discrimination (%) | 0.035 | ||
| Low | 49.22 | 55.67 | |
| Moderate | 28.68 | 27.15 | |
| High | 22.09 | 17.19 | |
| Stress from Lifetime Discrimination (%) | 0.081 | ||
| Low | 23.03 | 26.01 | |
| Moderate | 52.86 | 54.80 | |
| High | 24.11 | 19.19 | |
| Coping with Everyday Discrimination (%) | 0.001 | ||
| Passive | 45.06 | 54.35 | |
| Active | 43.80 | 52.47 | |
| Coping with Lifetime Discrimination (%) | 0.173 | ||
| Passive | 14.57 | 17.46 | |
| Active | 6.19 | 5.01 |
| MEN | WOMEN | |||||||
|---|---|---|---|---|---|---|---|---|
| CES-D Score < 16 (n = 220) | CES-D Score ≥ 16 (n = 99) | CES-D Score < 16 (n = 690) | CES-D Score ≥16 (n = 220) | |||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Everyday | ||||||||
| Low (referent) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Moderate | 0.02 (0.07) | 0.02 (0.07) | 0.07 (0.16) | 0.07 (0.17) | −0.02 (0.06) | −0.02 (0.06) | 0.15 (0.10) | 0.16 (0.10) |
| High | −0.08 (0.08) | −0.07 (0.08) | 0.05 (0.05) | 0.08 (0.15) | −0.02 (0.06) | −0.01 (0.06) | −0.10 (0.11) | −0.10 (0.12) |
| p for trend | 0.424 | 0.500 | 0.896 | 0.840 | 0.985 | 0.940 | 0.136 | 0.108 |
| SD units | −0.02 (0.03) | −0.01 (0.03) | −0.01 (0.05) | −0.004 (0.05) | −0.006 (0.03) | −0.01 (0.03) | −0.001 (0.04) | −0.004 (0.05) |
| Lifetime | ||||||||
| Low (referent) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Moderate | −0.06 (0.07) | −0.05 (0.07) | −0.07 (0.14) | −0.08 (0.14) | −0.08 (0.06) | −0.08 (0.06) | 0.05 (0.10) | 0.06 (0.11) |
| High | 0.004 (0.08) | 0.02 (0.08) | −0.05 (0.16) | −0.05 (0.17) | 0.04 (0.07) | 0.04 (0.07) | 0.09 (0.10) | 0.10 (0.11) |
| p for trend | 0.702 | 0.686 | 0.883 | 0.858 | 0.177 | 0.252 | 0.731 | 0.637 |
| SD units | −0.01 (0.03) | −0.01 (0.03) | −0.04 (0.07) | −0.03 (0.07) | 0.01 (0.03) | 0.01 (0.03) | 0.02 (0.04) | 0.02 (0.04) |
| Burden of Lifetime | ||||||||
| Low (referent) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Moderate | −0.10 (0.07) | −0.07 (0.07) | 0.05 (0.14) | 0.06 (0.15) | 0.11 (0.06) ‡ | 0.11 (0.06) ‡ | 0.01 (0.10) | 0.03 (0.10) |
| High | −0.02 (0.08) | 0.006 (0.08) | 0.02 (0.17) | 0.01 (0.17) | 0.07 (0.07) | 0.06 (0.07) | 0.02 (0.13) | 0.12 (0.13) |
| p for trend | 0.464 | 0.538 | 0.850 | 0.929 | 0.128 | 0.200 | 0.994 | 0.965 |
| SD units | 0.01 (0.03) | 0.01 (0.03) | 0.06 (0.06) | 0.06 (0.06) | 0.05 (0.03) ‡ | 0.04 (0.03) | −0.001 (0.05) | 0.01 (0.05) |
| Stress from Lifetime | ||||||||
| Low (referent) | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Moderate | −0.14 (0.07) ‡ | −0.14 (0.07) ‡ | 0.16 (0.19) | 0.23 (0.19) | 0.07 (0.06) | 0.08 (0.06) | 0.04 (0.13) | 0.04 (0.13) |
| High | 0.01 (0.09) | −0.01 (0.09) | 0.07 (0.20) | 0.14 (0.20) | −0.12 (0.08) | −0.12 (0.08) | −0.06 (0.13) | −0.06 (0.13) |
| p for trend | 0.076 | 0.092 | 0.698 | 0.454 | 0.027 * | 0.015 * | 0.615 | 0.557 |
| SD units | −0.04 (0.03) | −0.03 (0.03) | −0.02 (0.05) | −0.02 (0.05) | 0.01 (0.03) | 0.01 (0.03) | 0.001 (0.04) | 0.001 (0.04) |
| MEN | WOMEN | |||||||
|---|---|---|---|---|---|---|---|---|
| CES-D Score < 16 (n = 220) | CES-D Score ≥ 16 (n = 99) | CES-D Score < 16 (n = 690) | CES-D Score ≥ 16 (n = 220) | |||||
| Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | Model 1 | Model 2 | |
| Everyday | ||||||||
| Passive vs. Active & Other | 0.05 (0.07) | 0.05 (0.07) | 0.10 (0.12) | 0.03 (0.13) | 0.003 (0.05) | −0.02 (0.05) | −0.11 (0.09) | −0.12 (0.09) |
| Active vs. Passive & Other | −0.09 (0.06) | −0.10 (0.06) | −0.14 (0.12) | −0.07 (0.13) | −0.01 (0.05) | −0.001 (0.05) | 0.12 (0.09) | 0.13 (0.09) |
| Lifetime | ||||||||
| Passive vs. Active & Other | 0.17 (0.09) ‡ | 0.18 (0.09) * | 0.08 (0.18) | 0.03 (0.18) | −0.005 (0.07) | −0.01 (0.07) | −0.01 (0.10) | −0.02 (0.10) |
| Active Vs. Passive & Other | −0.14 (0.13) | −0.13 (0.13) | 0.95 (0.33) * | 1.18 (0.35) * | −0.02 (0.12) | −0.05 (0.12) | −0.04 (0.23) | −0.04 (0.23) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glover, L.M.; Cené, C.W.; Reiner, A.; Gebreab, S.; Williams, D.R.; North, K.E.; Sims, M. Discrimination and Leukocyte Telomere Length by Depressive Symptomatology: The Jackson Heart Study. Healthcare 2021, 9, 639. https://doi.org/10.3390/healthcare9060639
Glover LM, Cené CW, Reiner A, Gebreab S, Williams DR, North KE, Sims M. Discrimination and Leukocyte Telomere Length by Depressive Symptomatology: The Jackson Heart Study. Healthcare. 2021; 9(6):639. https://doi.org/10.3390/healthcare9060639
Chicago/Turabian StyleGlover, LáShauntá M., Crystal W. Cené, Alexander Reiner, Samson Gebreab, David R. Williams, Kari E. North, and Mario Sims. 2021. "Discrimination and Leukocyte Telomere Length by Depressive Symptomatology: The Jackson Heart Study" Healthcare 9, no. 6: 639. https://doi.org/10.3390/healthcare9060639
APA StyleGlover, L. M., Cené, C. W., Reiner, A., Gebreab, S., Williams, D. R., North, K. E., & Sims, M. (2021). Discrimination and Leukocyte Telomere Length by Depressive Symptomatology: The Jackson Heart Study. Healthcare, 9(6), 639. https://doi.org/10.3390/healthcare9060639






