Chronotropic Incompetence Limits Aerobic Exercise Capacity in Patients Taking Beta-Blockers: Real-Life Observation of Consecutive Patients
Abstract
1. Introduction
2. Methods
2.1. Study Population and Inclusion Criteria
2.2. Echocardiography
2.3. Cardiopulmonary Exercise Test
2.4. Statistical Methods
3. Ethics
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | chronotropic index |
| EC | exercise capacity |
| HR | heart rate |
| VO2 | oxygen uptake |
References
- Lauer, M.S.; Francis, G.S.; Okin, P.M.; Pashkow, F.J.; Snader, C.E.; Marwick, T.H. Impaired Chronotropic Response to Exercise Stress Testing as a Predictor of Mortality. JAMA 1999, 281, 524–529. [Google Scholar] [CrossRef]
- Azarbal, B.; Hayes, S.W.; Lewin, H.C.; Hachamovitch, R.; Cohen, I.; Berman, D.S. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality. J. Am. Coll. Cardiol. 2004, 44, 423–430. [Google Scholar] [CrossRef]
- Khan, M.N.; Pothier, C.E.; Lauer, M.S. Chronotropic Incompetence as a Predictor of Death Among Patients with Normal Electrograms Taking Beta Blockers (Metoprolol or Atenolol). Am. J. Cardiol. 2005, 96, 1328–1333. [Google Scholar] [CrossRef]
- Myers, J.; Tan, S.Y.; Abella, J.; Aleti, V.; Froelicher, V.F. Comparison of the chronotropic response to exercise and heart rate recovery in predicting cardiovascular mortality. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Dobre, D.; Zannad, F.; Keteyian, S.J.; Stevens, S.R.; Rossignol, P.; Kitzman, D.W.; Landzberg, J.; Howlett, J.; Kraus, W.E.; Ellis, S.J. Association between resting heart rate, chronotropic index, and long-term outcomes in patients with heart failure receiving β-blocker therapy: Data from the HF-ACTION trial. Eur. Heart J. 2013, 34, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Engeseth, K.; Hodnesdal, C.; Grundvold, I.; Liestøl, K.; Gjesdal, K.; Kjeldsen, S.E.; Erikssen, J.E.; Bodegard, J.; Skretteberg, P.T. Temporal Reduction in Chronotropic Index Predicts Risk of Cardiovascular Death Among Healthy Middle-Aged Men: A 28-Year Follow-Up Study. J. Am. Heart Assoc. 2016, 5, e004555. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, P.H.; Kitzman, D.W. Chronotropic Incompetence. Circulation 2011, 123, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Laforgia, P.; Bandera, F.; Alfonzetti, E.; Guazzi, M. Exercise chronotropic incompetence phenotypes the level of cardiovascular risk and exercise gas exchange impairment in the general population. An analysis of the Euro-EX prevention trial. Eur. J. Prev. Cardiol. 2020, 27, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Vallebona, A.; Gigli, G.; Orlandi, S.; Reggiardo, G. Heart rate response to graded exercise correlates with aerobic and ventilatory capacity in patients with heart failure. Clin. Cardiol. 2005, 28, 25–29. [Google Scholar] [CrossRef]
- Jorde, U.P.; Vittorio, T.J.; Kasper, M.E.; Arezzi, E.; Colombo, P.C.; Goldsmith, R.L.; Ahuja, K.; Tseng, C.-H.; Haas, F.; Hirsh, D.S. Chronotropic incompetence, beta-blockers, and functional capacity in advanced congestive heart failure: Time to pace? Eur. J. Heart Fail. 2008, 10, 96–101. [Google Scholar] [CrossRef]
- Magrí, D.; Palermo, P.; Cauti, F.M.; Contini, M.; Farina, S.; Cattadori, G.; Apostolo, A.; Salvioni, E.; Magini, A.; Vignati, C.; et al. Chronotropic Incompentence and Functional Capacity in Chronic Heart Failure: No Role of β-Blockers and β-Blocker Dose. Cardiovasc. Ther. 2010, 30, 100–108. [Google Scholar] [CrossRef]
- Al-Najjar, Y.; Witte, K.K.; Clark, A.L. Chronotropic incompetence and survival in chronic heart failure. Int. J. Cardiol. 2012, 157, 48–52. [Google Scholar] [CrossRef]
- Takano, N.; Takano, H.; Fukuda, T.; Kikuchi, H.; Oguri, G.; Fukumura, K.; Iwasawa, K.; Nakajima, T. Relationship between chronotropic incompetence and β-blockers based on changes in chronotropic response during cardiopulmonary exercise testing. IJC Heart Vasc. 2015, 6, 12–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arena, R.; Myers, J.; Williams, M.A.; Gulati, M.; Kligfield, P.; Balady, G.J.; Collins, E.; Fletcher, G. Assessment of Functional Capacity in Clinical and Research Settings. Circulation 2007, 116, 329–343. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.M.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Hossack, K. Implications of Cardio-Respiratory Fitness on the Performance of Exercise Tests. Heart Lung Circ. 2019, 28, e64–e66. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise Standards for Testing and Training. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef]
- Domínguez, E.; Palau, P.; Núñez, E.; Ramón, J.M.; López, L.; Melero, J.; Bellver, A.; Santas, E.; Chorro, F.J.; Núñez, J. Heart rate response and functional capacity in patients with chronic heart failure with preserved ejection fraction. ESC Heart Fail. 2018, 5, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for Chamber Quantification: A Report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Committee Members Gibbons, R.J.; Balady, G.J.; Bricker, J.T.; Chaitman, B.R.; Fletcher, G.F.; Froelicher, V.F.; Mark, D.B.; McCallister, B.D.; Mooss, A.N. ACC/AHA 2002 Guideline Update for Exercise Testing: Summary Article. Circulation 2002, 106, 1883–1892. [Google Scholar] [CrossRef]
- Society, A.T. American College of Chest Physicians ATS/ACCP Statement on Cardiopulmonary Exercise Testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. [Google Scholar] [CrossRef]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Stringer, W.W.; Sietsema, K.E.; Sun, X.; Whipp, B.J. Normal Values. In Principles of Exercise Testing and Interpretation Including Pathophysiology and Clinical Applications, 5th ed.; Wolters Kluwer/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 154–180. [Google Scholar]
- Astrand, I. Aerobic work capacity in men and women with special reference to age. Acta Physiol. Scand. Suppl. 1960, 49, 1–92. [Google Scholar]
- Palau, P.; Seller, J.; Dominguez, E.; Gomez, I.; Ramon, J.M.; Sastre, C.; de la Espriella, R.; Santas, E.; Minana, G.; Chorro, F.J.; et al. Beta-blockers withdrawal in patients with heart failure with preserved ejection fraction and chronotropic incompetence: Effect on functional capacity rationale and study design of a prospective, randomized, controlled trial (The Preserve-HR trial). Clin. Cardiol. 2020, 43, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, J.F.; Doherty, P. Evaluation of determinants of walking fitness in patients attending cardiac rehabilitation. BMJ Open Sport Exerc. Med. 2017, 2, e000203. [Google Scholar] [CrossRef] [PubMed]
- Jamil, H.A.; Gierula, J.; Paton, M.F.; Byrom, R.; Lowry, J.E.; Cubbon, R.M.; Cairns, D.A.; Kearney, M.T.; Witte, K.K. Chronotropic Incompetence Does Not Limit Exercise Capacity in Chronic Heart Failure. J. Am. Coll. Cardiol. 2016, 67, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Gauri, A.J.; Raxwal, V.K.; Roux, L.; Fearon, W.F.; Froelicher, V.F. Effects of chronotropic incompetence and β-blocker use on the exercise treadmill test in men. Am. Heart J. 2001, 142, 136–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Witte, K.K.; Cleland, J.G.F.; Clark, A.L. Chronic heart failure, chronotropic incompetence, and the effects of blockade. Heart 2005, 92, 481–486. [Google Scholar] [CrossRef]
- Vittorio, T.J.; Lanier, G.; Zolty, R.; Sarswat, N.; Tseng, C.-H.; Colombo, P.C.; Jorde, U.P. Association between Endothelial Function and Chronotropic Incompetence in Subjects with Chronic Heart Failure Receiving Optimal Medical Therapy. Echocardiography 2010, 27, 294–299. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, M.; Van Heuverswyn, F.; Duytschaever, M.; De Buyzere, M. Chronotropic incompetence: Real life observations of a theoretical concept. Acta Cardiol. 2013, 68, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
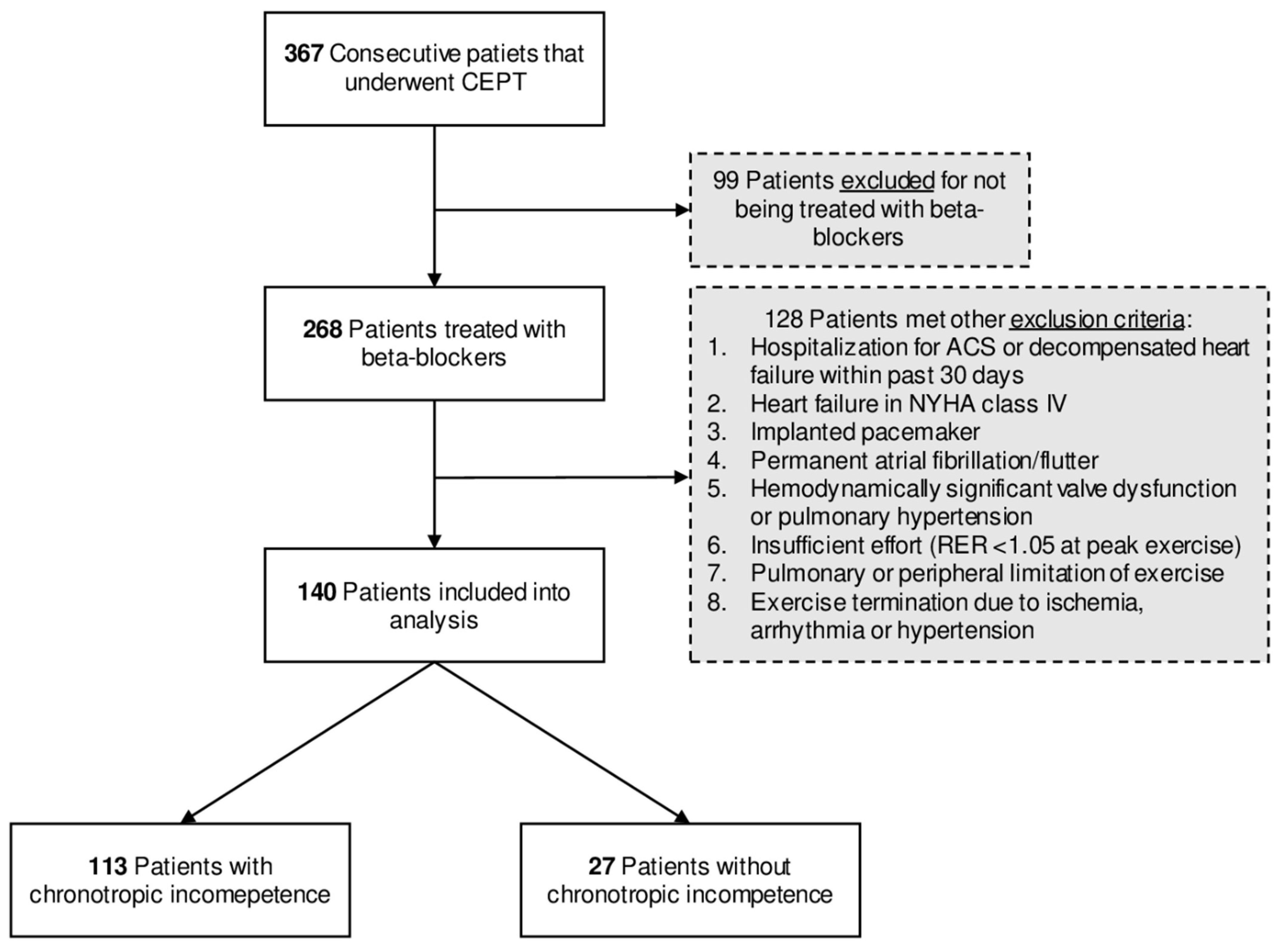
| All Patients (n = 140) | Chronotropic Incompetence a | p-Value | ||
|---|---|---|---|---|
| Yes (n = 113) | No (n = 27) | |||
| Demographics | ||||
| Age, years | 61.0 ± 9.7 | 60.7 ± 10.0 | 62.1 ± 8.5 | 0.525 |
| Male sex, n (%) | 102 (73) | 78 (69) | 24 (89) | 0.065 |
| BMI, kg/m2 | 27.8 ± 4.1 | 27.8 ± 4.0 | 27.7 ± 4.5 | 0.953 |
| Comorbidity, n (%) | ||||
| Chronic heart failure | 89 (64) | 74 (65) | 15 (56) | 0.514 |
| NYHA functional class | ||||
| I | 71 (51) | 59 (52) | 12 (44) | 0.609 |
| II | 8 (6) | 7 (6) | 1 (4) | 0.968 |
| III | 10 (7) | 8 (7) | 2 (7) | 0.968 |
| CAD | 115 (82) | 94 (83) | 21 (78) | 0.704 |
| MI | 110 (79) | 90 (80) | 20 (74) | 0.709 |
| Coronary angiography | 118 (84) | 96 (85) | 22 (81) | 0.880 |
| PCI | 107 (76) | 87 (77) | 20 (74) | 0.945 |
| CABG | 5 (4) | 4 (4) | 1 (4) | 1 |
| DM/IFG | 29 (20) | 28 (24) | 1 (4) | 0.033 |
| Hypertension | 88 (63) | 74 (65) | 14 (52) | 0.273 |
| Current smoker | 42 (30) | 35 (31) | 7 (26) | 0.779 |
| Paroxysmal atrial fibrillation | 14 (10) | 12 (11) | 2 (7) | 0.886 |
| Biochemistry | ||||
| Hemoglobin, g/L | 13.8 ± 1.5 | 13.8 ± 1.5 | 13.8 ± 1.5 | 0.934 |
| Serum creatinine (IQR), mg/dL | 0.9 (0.8–1.1) | 0.9 (0.8–1.1) | 1.1 (0.9–1.3) | 0.005 |
| Creatinine clearance, mL/min/1.73 m2 | 95 ± 30 | 97 ± 31 | 88 ± 29 | 0.188 |
| Medication, n (%) | ||||
| Bisoprolol | 77 (55) | 67 (59) | 10 (37) | 0.061 |
| Metoprolol | 49 (35) | 35 (31) | 14 (52) | 0.069 |
| Carvedilol | 9 (6) | 7 (6) | 2 (7) | 1 |
| Sotalol | 2 (1) | 2 (2) | 0 (0) | NA |
| Nebivolol | 2 (1) | 1 (1) | 1 (4) | 0.837 |
| Other heart rate lowering drugs b | 8 (6) | 6 (5) | 2 (7) | 0.950 |
| Dihydropyridine CCB | 18 (13) | 16 (14) | 2 (7) | 0.554 |
| ACEI/ARB | 111 (79) | 92 (81) | 19 (70) | 0.313 |
| Diuretics | 35 (25) | 30 (27) | 5 (19) | 0.536 |
| BB dose, bisoprolol equivalent (IQR), mg | 2.5 (2.5–5) | 2.5 (2.5–5.0) | 2.5 (1.2–2.5) | 0.033 |
| Echocardiography | ||||
| LV end-diastolic dimension, mm | 47 (44–50) | 46.0 (44–50) | 47 (44–50) | 0.523 |
| Left atrium dimension, mm | 40 ± 5 | 40 ± 5 | 39 ± 5 | 0.558 |
| LVEF, % | 53.3 ± 11.6 | 53.4 ± 11.9 | 52.8 ± 10.5 | 0.816 |
| WMSI (IQR) | 1.4 (1.1–1.7) | 1.4 (1.1–1.8) | 1.5 (1.0–1.6) | 0.940 |
| LV diastolic dysfunction, n (%) | 0.702 | |||
| Grade 1 | 114 (81) | 90 (80) | 24 (89) | 0.404 |
| Grade 2 | 8 (6) | 7 (6) | 1 (4) | 0.968 |
| Grade 3 | 2 (1) | 2 (2) | 0 (0) | NA |
| MR moderate, n (%) | 33 (24) | 25 (22) | 8 (30) | 0.567 |
| RV end-diastolic dimension (IQR), mm | 34 (30–36) | 34 (29–36) | 35 (32–38) | 0.096 |
| Right atrium dimension, mm | 35 ± 6 | 36 ± 6 | 34 ± 5 | 0.267 |
| RV systolic dysfunction, n (%) | 38 (27) | 33 (29) | 5 (19) | 0.378 |
| TR moderate, n (%) | 11 (8) | 9 (8) | 2 (7) | 1 |
| All Patients (n = 140) | Chronotropic Incompetence a | p-Value | ||
|---|---|---|---|---|
| Yes (n = 113) | No (n = 27) | |||
| Treadmill exercise test, n (%) | 109 (78) | 86 (76) | 23 (85) | 0.446 |
| Cycle ergometer exercise test, n (%) | 31 (22) | 27 (24) | 4 (15) | 0.446 |
| VO2 at anaerobic threshold, mL/kg/min | 13.7 ± 3.7 | 13.2 ± 3.7 | 16.0 ± 2.9 | <0.001 |
| VO2 at peak, mL/kg/min | 19.4 ± 6.1 | 18.3 ± 5.7 | 24.0 ± 5.3 | <0.001 |
| VO2 at peak, mL/kg/min % predicted | 73 ± 19 | 69 ± 17 | 89 ± 18 | <0.001 |
| CO2 at peak, L/min | 1.8 ± 0.8 | 1.6 ± 0.7 | 2.4 ± 0.9 | <0.001 |
| METs at peak | 5.5 ± 1.7 | 5.2 ± 1.6 | 6.9 ± 1.5 | <0.001 |
| HR at rest (IQR), bpm | 72 (64–83) | 72 (64–82) | 77 (67–86) | 0.091 |
| HR at anaerobic threshold, bpm | 97 ± 13 | 95 ± 13 | 106 ± 10 | <0.001 |
| HR at peak, bpm | 115 ± 17 | 110 ± 15 | 136 ± 10 | <0.001 |
| HR at peak, % predicted | 72 ± 10 | 69 ± 8 | 86 ± 4 | <0.001 |
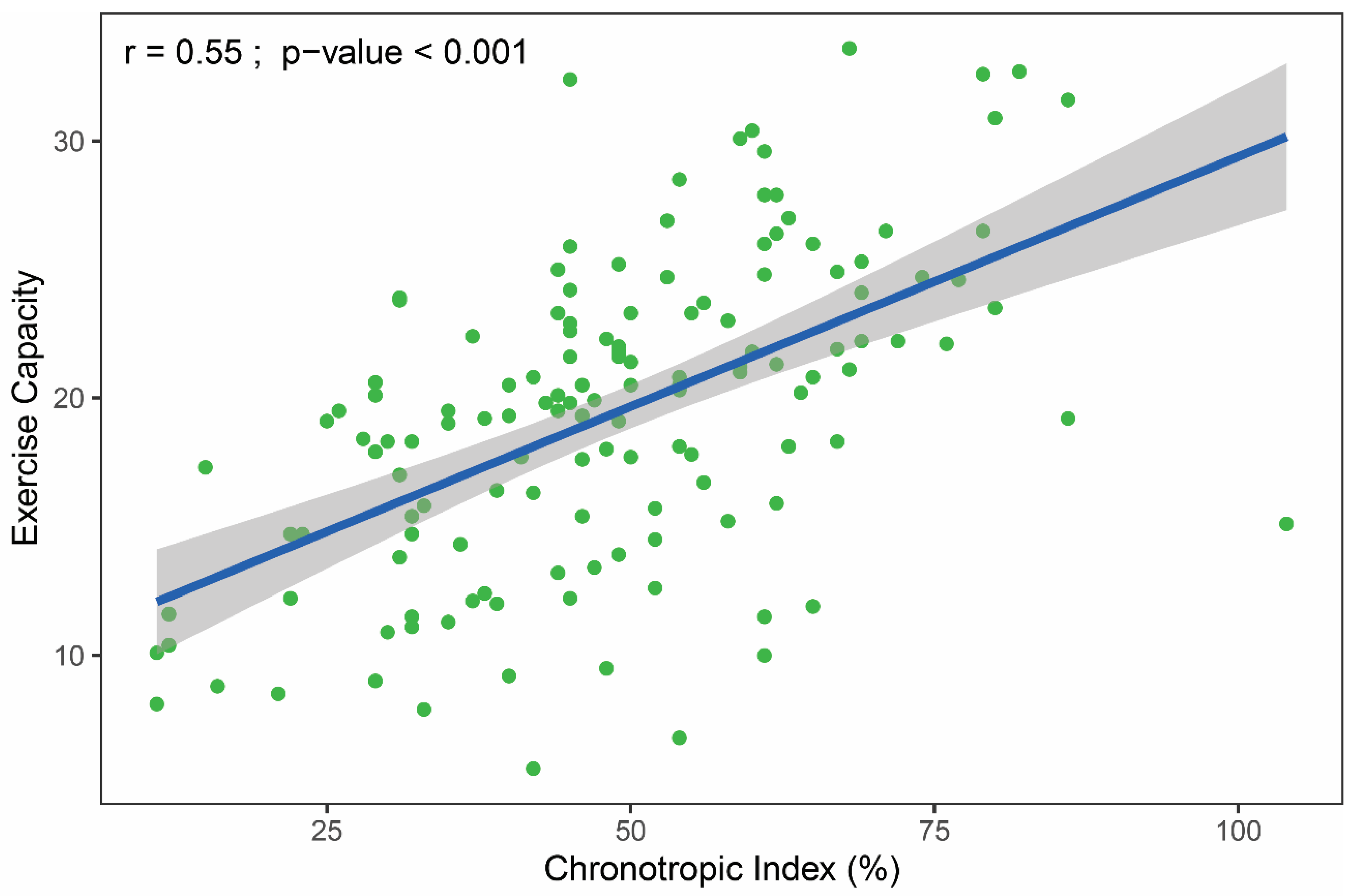
| Chronotropic index, % | 48.6 ± 17.3 | 42.7 ± 13.0 | 73.1 ± 9.3 | <0.001 |
| SBP at rest, mmHg | 127 ± 13 | 127 ± 13 | 130 ± 13 | 0.289 |
| SBP at peak (IQR), mmHg | 170 (155–180) | 170 (150–180) | 180 (160–190) | 0.075 |
| RER at peak (IQR) | 1.10 (1.05–1.16) | 1.09 (1.05–1.16) | 1.14 (1.06–1.21) | 0.072 |
| Min. ventilation vs. CO2 slope (IQR) | 24 (22–28) | 25 (23–28) | 23.6 (21–25) | 0.095 |
| Breathing reserve at peak (IQR), % | 45 (25–57) | 46 (44–50) | 47 (44–50) | 0.523 |
| FEV 1/IVC, % predicted | 93 ± 21 | 93 ± 20 | 94 ± 23 | 0.841 |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| β Regression Coefficient a | 95% CI | p-Value | β regression Coefficient a | 95% CI | p-Value | Explained Variance (%) b | |
| Chronotropic index, % | 0.20 | 0.15 to 0.24 | <0.001 | 0.14 | 0.09 to 0.18 | <0.001 | 24.7 |
| Male gender | 7.01 | 5.05 to 8.97 | <0.001 | 5.12 | 2.86 to 7.38 | <0.001 | 14.0 |
| Age, years | −0.22 | −0.32 to −0.13 | <0.001 | −0.17 | −0.26 to −0.09 | <0.001 | 12.9 |
| Heart failure | −4.55 | −6.52 to −2.60 | <0.001 | −3.35 | −4.97 to −1.72 | <0.001 | 11.8 |
| WMSI | 0.483 | 0.050 | 3.1 | ||||
| Treadmill vs. cycle ergometer | 3.92 | 1.56 to 6.28 | 0.001 | 0.066 | 2.7 | ||
| BB daily dose, bisoprolol equivalent, mg | 0.743 | 0.140 | 1.8 | ||||
| LVEF, % | 0.147 | 0.224 | 1.2 | ||||
| Hemoglobin, g/L | 0.81 | 0.15 to 1.48 | 0.017 | 0.298 | 0.9 | ||
| Serum creatinine, mg/dL | 0.295 | 0.343 | 0.7 | ||||
| LV diastolic dysfunction, grade 2 and 3 | 0.108 | 0.413 | 0.5 | ||||
| Height, cm | 0.430 | 0.430 | 0.5 | ||||
| DM/IFG | −3.25 | −5.70 to −0.80 | 0.010 | 0.560 | 0.3 | ||
| Current smoker | 0.670 | 0.586 | 0.2 | ||||
| RV systolic dysfunction | 0.931 | 0.948 | 0.0 | ||||
| SBP at peak exercise, mmHg | 0.05 | 0.00 to 0.09 | 0.034 | ||||
| Hypertension | 0.137 | ||||||
| CAD | 0.133 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smarz, K.; Tysarowski, M.; Zaborska, B.; Pilichowska-Paszkiet, E.; Sikora-Frac, M.; Budaj, A.; Jaxa-Chamiec, T. Chronotropic Incompetence Limits Aerobic Exercise Capacity in Patients Taking Beta-Blockers: Real-Life Observation of Consecutive Patients. Healthcare 2021, 9, 212. https://doi.org/10.3390/healthcare9020212
Smarz K, Tysarowski M, Zaborska B, Pilichowska-Paszkiet E, Sikora-Frac M, Budaj A, Jaxa-Chamiec T. Chronotropic Incompetence Limits Aerobic Exercise Capacity in Patients Taking Beta-Blockers: Real-Life Observation of Consecutive Patients. Healthcare. 2021; 9(2):212. https://doi.org/10.3390/healthcare9020212
Chicago/Turabian StyleSmarz, Krzysztof, Maciej Tysarowski, Beata Zaborska, Ewa Pilichowska-Paszkiet, Małgorzata Sikora-Frac, Andrzej Budaj, and Tomasz Jaxa-Chamiec. 2021. "Chronotropic Incompetence Limits Aerobic Exercise Capacity in Patients Taking Beta-Blockers: Real-Life Observation of Consecutive Patients" Healthcare 9, no. 2: 212. https://doi.org/10.3390/healthcare9020212
APA StyleSmarz, K., Tysarowski, M., Zaborska, B., Pilichowska-Paszkiet, E., Sikora-Frac, M., Budaj, A., & Jaxa-Chamiec, T. (2021). Chronotropic Incompetence Limits Aerobic Exercise Capacity in Patients Taking Beta-Blockers: Real-Life Observation of Consecutive Patients. Healthcare, 9(2), 212. https://doi.org/10.3390/healthcare9020212






