Identification of Heavy Tobacco Smoking Predictors-Influence of Marijuana Consuming Peers and Truancy among College Students
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Heavy Smoker Status
3.2. Marijuana Consumption among Peers
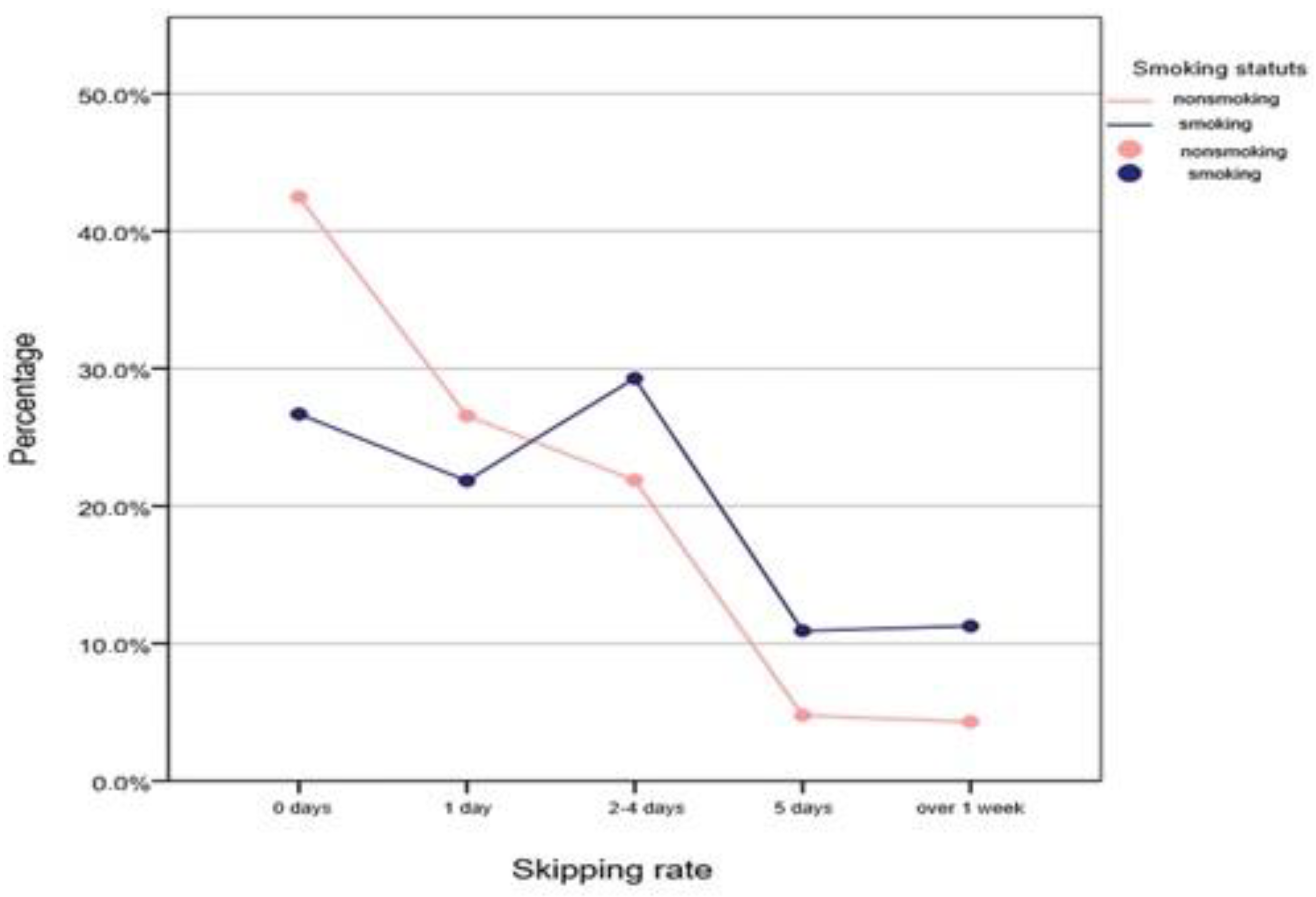
3.3. Absenteeism over A Period of 30 Days—“Truancy Percentage”
4. Discussion
5. Limitations of The Study
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kessler, D.A. Nicotine addiction in young people. N. Engl. J. Med. 1995, 333, 1018. [Google Scholar] [CrossRef] [PubMed]
- Fagerstrom, K. The epidemiology of smoking: Health consequences and benefits of cessation. Drugs 2002, 62, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Borza, C.; Oancea, C.; Mateescu, R.; Balint-Savoiu, G.; Cristescu, C.; Andoni, M.; Simu, G.; Butur, M.; Dehelea, C.; Pauncu, E.A. Evaluation of the environmental and work risk factors in building-workers. J. Food Agric. Environ. Sect. Health 2011, 9, 175–176. [Google Scholar]
- Balint, G.S.; Borza, C.; Cristescu, C.; Andoni, M.; Simu, G.M.; Malita, D.; Malita, I.; Cheveresan, A. Endogenous and exogenous antioxidant protection for endothelial dysfunction. Rev. Chim. 2011, 62, 680–683. [Google Scholar]
- Boia, S.; Stratul, Ş.I.; Boariu, M.; Ursoniu, S.; Goţia, S.L.; Boia, E.R.; Borza, C. Evaluation of antioxidant capacity and clinical assessment of patients with chronic periodontitis treated with non-surgical periodontal therapy and adjunctive systemic antibiotherapy. Rom. J. Morphol. Embryol. 2018, 59, 1107–1113. [Google Scholar] [PubMed]
- Savoiu, G.; Cristescu, C.; Borza, C. The influence of cigarette smoking on endothelial function. J. Food Agric. Environ. 2012, 10, 25–28. [Google Scholar]
- Savoiu, G.; Cristescu, C.; Serban, C.; Borza, C.; Andoni, M.; Fira-Mladinescu, O.; Chiru, D.; Simu, G.M.; Susan, L.; Mateescu, R. Association between oxidized low-density cholesterol concentration and atherosclerosis. Rev. Rom. Lab. 2009, 15, 49–54. [Google Scholar]
- Savoiu, G.; Cristescu, C.; Borza, C.; Serban, C.; Noveanu, L.; Dehelean, C.; Andoni, M.; Andor, M.; Fira-Mladinescu, O. Endothelin-1 plasma concentration in patients with essential hypertension, atherogenic dyslipidemia and coronary artery disease. Rev. Rom. Med. Lab. 2010, 18, 25–30. [Google Scholar]
- Balint, G.S.; Toth, E.; Andoni, M.; Demeter, I.; Borza, C.; Floroni, E.; Pacurar, M.; Ciobanu, V. Study on the influence of free radicals and oxidative stress on Peptic Ulcers. Rev. Chim. 2019, 70, 3254–3257. [Google Scholar] [CrossRef]
- Doll, R.; Peto, R.; Boreham, J.; Sutherland, I. Mortality in relation to smoking: 50 years’ observations on male British doctors. BMJ 2004, 328, 1519. [Google Scholar] [CrossRef] [Green Version]
- CDC. Preventing Tobacco Use Among Young People—A Report of the Surgeon General. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00030927.htm (accessed on 11 March 1994).
- Steinberg, L. Risk taking in adolescence: What changes, and why? Ann. N. Y. Acad. Sci. 2004, 1021, 51–58. [Google Scholar] [CrossRef] [PubMed]
- CDC. Reasons for Tobacco Use and Symptoms of Nicotine Withdrawal among Adolescent and Young Adult Tobacco Users—United States, 1993, Centers for Disease Control and Prevention, Morbidity and Mortality Weekly Report Morbidity and Mortality Weekly Report. 1994. Available online: https://www.cdc.gov/mmwr/PDF/wk/mm4341.pdf (accessed on 21 October 1994).
- Dalton, M.A.; Beach, M.L.; Adachi-Mejia, A.M.; Longacre, M.R.; Matzkin, A.L.; Sargent, J.D.; Heatherton, T.F.; Titus-Ernstoff, L. Early exposure to movie smoking predicts established smoking by older teens and young adults. Pediatrics 2009, 123, e551–e558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, J.; Rose, E.J.; Behm, F.M.; Westman, E.C.; Coleman, R. Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: Implications for addiction. Drug Alcohol Depend. 1999, 56, 99–107. [Google Scholar] [CrossRef]
- Crooks, P.A.; Dwoskin, L.P. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem. Pharmacol. 1997, 54, 743–753. [Google Scholar] [CrossRef]
- Dwoskin, L.P.; Teng, L.H.; Crooks, P.A. Nornicotine, a nicotine metabolite and tobacco alkaloid: Desensitization of nicotinic receptor-stimulated dopamine release from rat striatum. Eur. J. Pharmacol. 2001, 428, 69–79. [Google Scholar] [CrossRef]
- CDC. Health Effects of Exposure to Environmental Tobacco Smoke: The Report of the California Environmental Protection Agency, National Institute of Health, Bethesda. Available online: https://cancercontrol.cancer.gov/brp/tcrb/monographs/monograph-10 (accessed on 24 September 2020).
- Fischer, B. Unraveling smoke: As cigarette makers face new challenges, the pressure to understand tobacco smoke is greater than ever. Tob. Rep. 2000, 127, 24–29. [Google Scholar]
- Hecht, S.S. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat. Rev. Cancer 2003, 3, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Perez-Stable, E.J.; Fong, I.; Modin, G.; Herrera, B.; Jacob, P. Ethnic differences in N-glucuronidation of nicotine and cotinine. J. Pharmacol. Exp. Ther. 1999, 291, 1196–1203. [Google Scholar]
- Stitzer, M.; De Wit, H. Abuse liability of nicotine. In Nicotine Safety and Toxicity; Benowitz, N.L., Ed.; Oxford University Press: New York, NY, USA, 1998; pp. 119–131. [Google Scholar]
- Vlaicu, B. Comportamente cu Risc la Studenții din Județul Timiș; Editura Eurobit: Timișoara, București, 2009. [Google Scholar]
- Alesci, N.L.; Forster, J.L.; Blaine, T. Smoking visibility, perceived acceptability, and frequency in various locations among youth and adults. Prev. Med. 2003, 36, 272–281. [Google Scholar] [CrossRef]
- Ali, M.M.; Dwyer, D.S. Estimating peer effects in adolescent smoking behavior: A longitudinal analysis. J. Adolesc. Health 2009, 45, 402–408. [Google Scholar] [CrossRef]
- Arnett, J.J. The myth of peer influence in adolescent smoking initiation. Health Educ. Behav. 2006, 34, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Kobus, K. Peers and adolescent smoking. Addiction 2003, 98, 37–55. [Google Scholar] [CrossRef]
- Petraitis, J.; Flay, B.; Miller, T.Q. Reviewing theories of adolescent substance use: Organizing pieces in the puzzle. Psychol. Bull. 1995, 117, 67–86. [Google Scholar] [CrossRef] [PubMed]
- West, P.; Sweeting, H.; Young, R. Smoking in Scottish youths: Personal income, parental social class and the cost of smoking. Tob. Control. 2007, 16, 329–335. [Google Scholar] [CrossRef]
- Rosca, C.I.; Kundnani, N.R.; Tudor, A.; Rosca, M.S.; Nicoras, V.A.; Otiman, G.; Ciurariu, E.; Ionescu, A.; Stelian, M.; Sharma, A.; et al. Benefits of prescribing low-dose digoxin in atrial fibrillation. Available online: https://journals.sagepub.com/doi/full/10.1177/20587384211051955 (accessed on 1 November 2021).
- National Cancer Institute. Strategies to Control Tobacco Use in United States: A Blue-Print for Public Health Action in the 1990’s. Available online: https://cancercontrol.cancer.gov/brp/tcrb/monographs/monograph-01 (accessed on 1 December 1990).
- Dilliott, D.; Fazel, S.; Ehsan, N.; Sibbald, S.L. The attitudes and behaviors of students, staff and faculty towards smoke-free and tobacco-free campus policies in North American universities: A narrative review. Tob. Prev. Cessat. 2020, 6. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, L.; Ma, N.; Sun, G. Logic analysis of how the emergency management legal system used to deal with public emerging infectious diseases under balancing of competing interests—The case of COVID-19. Healthcare 2021, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- NHS. Smoking, Survey of Smoking, Drinking and Drug Use among Young People in England. Available online: https://natcen.ac.uk/our-research/research/survey-of-smoking,-drinking-and-drug-use-among-young-people-in-england/ (accessed on 20 December 2014).

| Variables in the Equation | B | S.E. | Wald | df | Sig. | Exp(B) | 95% C.I. for EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Sex (M) | −0.528 | 0.266 | 3.95 | 1 | 0.047 * | 0.590 | 0.350 | 0.992 |
| Last graduated school of the father | −0.116 | 0.128 | 0.83 | 1 | 0.362 | 0.890 | 0.693 | 1.143 |
| Last graduated school of the mother | −0.056 | 0.134 | 0.17 | 1 | 0.674 | 0.945 | 0.727 | 1.229 |
| Satisfaction regarding the family’s financial situation | −0.117 | 0.123 | 0.90 | 1 | 0.342 | 0.890 | 0.699 | 1.133 |
| Smoking status of the father (Yes) | −0.123 | 0.239 | 0.26 | 1 | 0.606 | 0.884 | 0.554 | 1.412 |
| Smoking status of the mother (Yes) | −0.499 | 0.248 | 4.04 | 1 | 0.044 * | 0.607 | 0.373 | 0.988 |
| Smoking status of brothers and sisters (Yes) | 0.077 | 0.236 | 0.10 | 1 | 0.743 | 1.080 | 0.681 | 1.714 |
| Number of smoking friends | 0.744 | 0.207 | 12.89 | 1 | <0.001 * | 2.105 | 1.402 | 3.159 |
| Number of friends becoming drunk | 0.313 | 0.184 | 2.88 | 1 | 0.089 | 1.367 | 0.953 | 1.961 |
| Number of marijuana-smoking friends | 0.550 | 0.273 | 4.05 | 1 | 0.044 * | 1.733 | 1.015 | 2.960 |
| Number of days since individual skipped school | 0.384 | 0.091 | 17.81 | 1 | <0.001 * | 1.468 | 1.228 | 1.754 |
| Education situation at the end of last semester | 0.177 | 0.145 | 1.47 | 1 | 0.224 | 1.193 | 0.897 | 1.586 |
| Age of first cigarette | 0.135 | 0.118 | 1.30 | 1 | 0.254 | 1.144 | 0.908 | 1.442 |
| Attempts at stopping smoking | 0.567 | 0.273 | 4.32 | 1 | 0.038 * | 1.763 | 1.033 | 3.008 |
| Number of days practicing binge-drinking | 0.137 | 0.107 | 1.63 | 1 | 0.201 | 1.147 | 0.930 | 1.414 |
| Marijuana consumption | −0.037 | 0.319 | 0.01 | 1 | 0.907 | 0.964 | 0.515 | 1.802 |
| Feelings of sadness | 0.264 | 0.274 | 0.93 | 1 | 0.334 | 1.303 | 0.762 | 2.227 |
| Suicidal thoughts | 0.781 | 0.354 | 4.87 | 1 | 0.027 * | 2.184 | 1.092 | 4.370 |
| Knowledge about smoking effects (Yes) | −0.165 | 0.282 | 0.34 | 1 | 0.557 | 0.847 | 0.488 | 1.472 |
| Constants | −3.300 | 0.835 | 15.63 | 1 | 0.000 | 0.037 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, M.-D.; Sharma, A.; Kundnani, N.R.; Gag, O.L.; Rosca, C.I.; Mocanu, V.; Tudor, A.; Popovici, R.A.; Vlaicu, B.; Borza, C. Identification of Heavy Tobacco Smoking Predictors-Influence of Marijuana Consuming Peers and Truancy among College Students. Healthcare 2021, 9, 1666. https://doi.org/10.3390/healthcare9121666
Popa M-D, Sharma A, Kundnani NR, Gag OL, Rosca CI, Mocanu V, Tudor A, Popovici RA, Vlaicu B, Borza C. Identification of Heavy Tobacco Smoking Predictors-Influence of Marijuana Consuming Peers and Truancy among College Students. Healthcare. 2021; 9(12):1666. https://doi.org/10.3390/healthcare9121666
Chicago/Turabian StylePopa, Mihaela-Daiana, Abhinav Sharma, Nilima Rajpal Kundnani, Otilia Lavinia Gag, Ciprian Ilie Rosca, Valeria Mocanu, Anca Tudor, Ramona Amina Popovici, Brigitha Vlaicu, and Claudia Borza. 2021. "Identification of Heavy Tobacco Smoking Predictors-Influence of Marijuana Consuming Peers and Truancy among College Students" Healthcare 9, no. 12: 1666. https://doi.org/10.3390/healthcare9121666
APA StylePopa, M.-D., Sharma, A., Kundnani, N. R., Gag, O. L., Rosca, C. I., Mocanu, V., Tudor, A., Popovici, R. A., Vlaicu, B., & Borza, C. (2021). Identification of Heavy Tobacco Smoking Predictors-Influence of Marijuana Consuming Peers and Truancy among College Students. Healthcare, 9(12), 1666. https://doi.org/10.3390/healthcare9121666








