Associations of Sleep Quality, Anxiety, and Depression with Cognitive and Executive Functions among Community-Dwelling Women Aged ≥ 65 Years: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
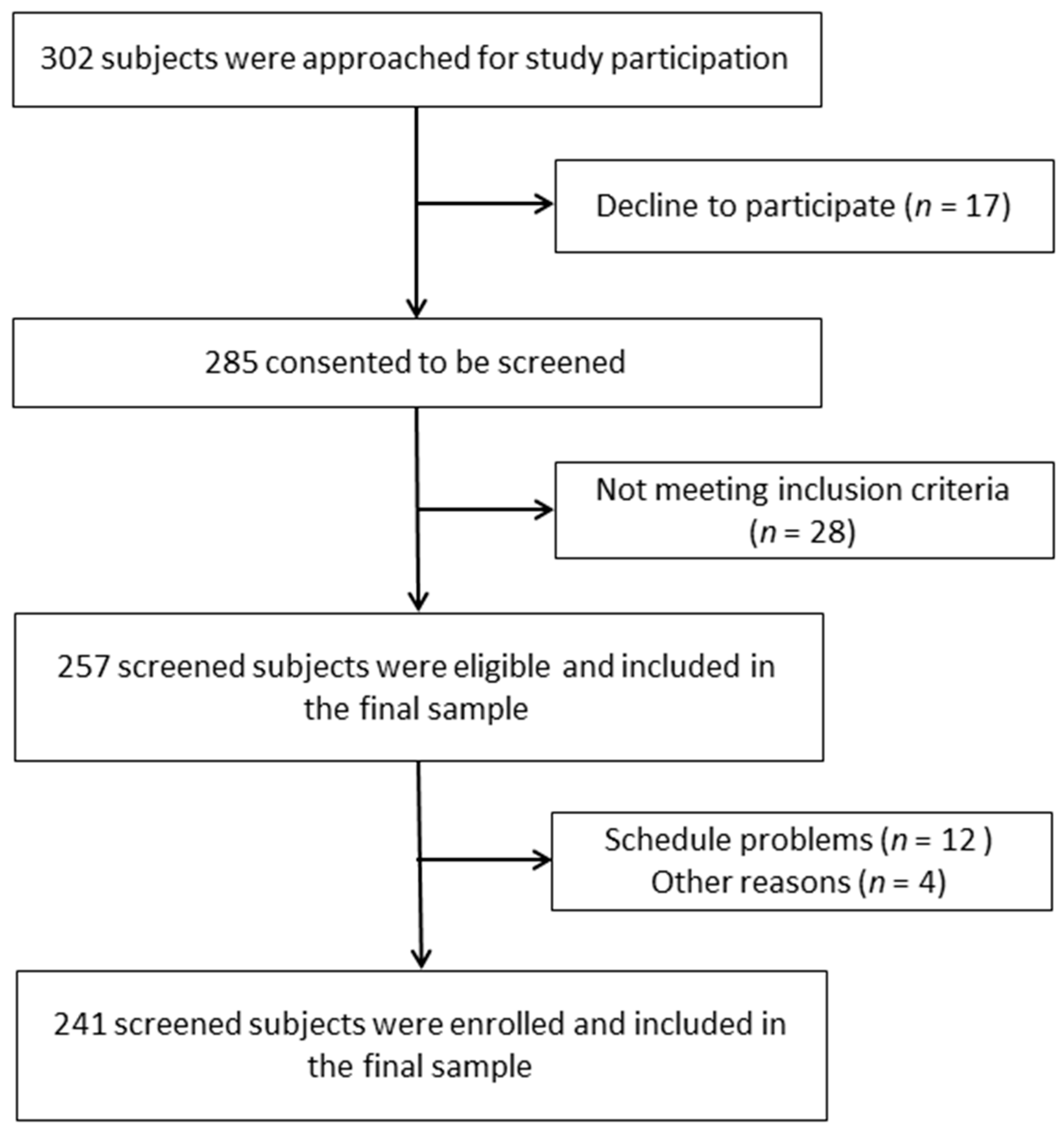
2.1. Study Participants and Design
2.2. Outcomes
2.2.1. Anxiety and Depression
2.2.2. Sleep Quality
2.2.3. Cognitive Performance
2.2.4. Cognitive Impairment
2.2.5. Verbal Fluency
2.2.6. Executive Functions
2.3. Sample Size Calculation
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alvarado, A.M.; Salazar, A.M. Aging concept analysis. Gerokomos 2014, 25, 57–62. [Google Scholar]
- Estadísticas Mundiales: Una Mina de Información Sobre Salud Pública Mundial; World Health Organization: Geneva, Switzerland, 2014.
- Luarte, C.; Poblete, F.; Flores, C.; Duarte, F. Physical Parameters, cognition and its relationship with the quality of life in elderly Talcahuano, Concepción, Valdivia y Osorno. Revista Ciencias de la Actividad Física 2016, 17, 9–17. [Google Scholar]
- Shirvani, M.; Heidari, M. Quality of Life in Postmenopausal Female Members and Non-members of the Elderly Support Association. J. Menopausal Med. 2016, 22, 154–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawlak, I.E.; Wolinska, W.; Mroczek, B. Impact of climacteric and depressive symptoms on the quality of life of postmenopausal women. Fam. Med. Prim. Care Rev. 2016, 18, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, E.K.; Berk, M.; Nelson, M.R.; Wing, L.M.; Reig, C.M. Association of depression with mortality in an elderly treated hypertensive population. Int. Spychogeriat. 2019, 31, 371–381. [Google Scholar] [CrossRef]
- Valencia, P.D. The Depression Anxiety Stress Scales (DASS-21): Do they measure anything beyond a general factor? Avances en Psicologia 2019, 27, 177–189. [Google Scholar]
- Centikol, G.; Bastug, G.; Ozel, E.T. Poor Acceptance of the Past is Related to Depressive Symptoms in Older Adults. GeroPsych 2020, 33, 246–251. [Google Scholar]
- Briant, C.; Jackson, H.; Ames, D. The prevalence of anxiety in older adults. J. Affect. Disord. 2008, 109, 233–250. [Google Scholar] [CrossRef]
- Zisberg, A. Anxiety and depression in older patients: The role of culture and acculturation. Int. J. Equity Heath 2017, 16, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Rubio, J.A.; Rodríguez, R.; Andreu, L.; Martínez, L.M.; Martínez, A.; Ramos, D.J. Effect of Sleep Quality on the Prevalence of Sarcopenia in Older Adults: A Systematic Review with Meta-Analysis. J. Clin. Med. 2019, 8, 2156. [Google Scholar] [CrossRef] [Green Version]
- Dzierzewski, J.M.; Dautovich, N.; Ravyts, S. Sleep and Cognition in Older Adults. Sleep Med Clin. 2018, 13, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.; Krauchi, K.; Grimaldi, D.; Sbarboro, J.; Attarian, H.; Malkani, R.; Matteo, M.; Cee, P.C. Effects of manipulating body temperature on sleep in postmenopausal women. Sleep Med. 2021, 81, 109–115. [Google Scholar] [CrossRef]
- Sutter, C.; Zollig, G.; Allemand, M.; Martin, M. Sleep Quality and Cognitive Function in Healthy Old Age: The Moderating Role of Subclinical Depression. Neurospsychiology 2012, 26, 768–775. [Google Scholar] [CrossRef] [PubMed]
- López, A.G.; Calero, M.D. Predictors of cognitive decline in the elderly. Rev. Esp. Geriatr. Gerontol. 2009, 44, 220–224. [Google Scholar] [CrossRef]
- Bojar, I.; Pinkas, J.; Gujski, M.; Owoc, A.; Raczkiewicz, D.; Gustaw-Rothenberg, K. Postmenopausal cognitive changes and androgen levels in the context of apolipoprotein E polymorphism. Arch. Med. Sci. 2017, 5, 1149–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheyer, O.; Rahman, A.; Hristov, H.; Bercowitz, C.; Iaacson, R.S.; Diaz, R.; Mosconi, L. Female Sex and Alzheimer’s Risk: The Menopause Connection. J. Prev. Alzheimer Dis. 2018, 5, 225–230. [Google Scholar] [CrossRef]
- Evans, H.M.; Howe, P.R.; Wong, R.H. Effects of Resveratrol on Cognitive Performance, Mood and Cerebrovascular Function in Post-MenopausalWomen; A 14-Week Randomised Placebo-Controlled Intervention Trial. Nutrients 2017, 9, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Obesity: Preventing and Management of the Global Epidemic. Report of the WHO Consultation. Technical Report Series. No. 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta. Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [Green Version]
- Herrero, M.J.; Blanch, J.; Peri, J.M.; De Pablo, J.; Pintor, L.; Bulbena, A. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen. Hosp. Psychiatr. 2003, 25, 277–283. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Hita-Contreras, F.; Martínez-López, E.; Latorre-Román, P.A.; Garrido, F.; Santos, M.A.; Martínez-Amat, A. Reliability and validity of the Spanish version of the Pittsburgh Sleep Quality Index (PSQI) in patients with fibromyalgia. Rheumatol. Int. 2014, 34, 929–936. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatr. Res. 2000, 97, 165–172. [Google Scholar] [CrossRef]
- Lobo, A.; Saz, P.; Marcos, G.; Día, J.L.; de la Cámara, C.; Ventura, T.; Morales-Asín, F.; Fernando-Pascual, L.; Montañés, J.A.; Aznar, S. Revalidation and standardization of the cognition mini-exam (first Spanish version of the Mini-Mental Status Examination) in the general geriatric population. Med. Clin. 1999, 112, 767–774. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, B.; Kennie, A.T. The Set Test as an Aid to the Detection of Dementia in Old People. Br. J. Psychiat. 1971, 23, 467–470. [Google Scholar] [CrossRef]
- Reitan, R.M. Trail Making Test: Manual for Administration, Scoring and Interpretation; Indiana University Medical Center: Indianapolis, IN, USA, 1958. [Google Scholar]
- Concato, J.; Peduzzi, P.; Holford, T.R.; Feinstein, A.R. Importance of events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. J. Clin. Epidemiol. 1995, 48, 1495–1501. [Google Scholar] [CrossRef]
- Cohen, J.A. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Strine, T.W.; Chapman, D.P. Associations of frequent sleep insufficiency with health-related quality of life and health behaviors. Sleep Med. 2005, 6, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Denison, H.J.; Jameson, K.A.; Sayer, A.A.; Patel, H.P.; Edwards, M.H.; Arora, T.; Dennison, E.M.; Cooper, C.; Baird, J. Poor sleep quality and physical performance in older adults. Sleep Health 2021, 7, 205–211. [Google Scholar] [CrossRef]
- Nebes, R.D.; Buysse, D.J.; Halligan, E.M.; Houck, P.R.; Monk, T.H. Self-reported sleep quality predicts poor cognitive performance in healthy older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 2009, 64, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.R.; Dong, C.; Elkind, M.S.; Boden, B.; Sacco, R.L.; Rundek, T.; Wrihte, C.B. Association between sleep duration and the mini-mental score: The Northern Manhattan study. J. Clin. Sleep Med. 2013, 9, 669–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackwell, T.; Yaffe, K.; Laffan, A.; Ancoli-Israel, S.; Redline, S.; Ensrud, K.E.; Song, Y.; Stone, K.L.; Osteoporotic Fractures in Men (MrOS) Study Group. Associations of objectively and subjectively measured sleep quality with subsequent cognitive decline in older community-dwelling men: The MrOS sleep study. Sleep 2014, 37, 655–663. [Google Scholar] [PubMed]
- Horne, J.A. Sleep loss and "divergent" thinking ability. Sleep 1988, 11, 528–536. [Google Scholar] [CrossRef]
- Henneghan, A.M.; Carter, P.; Stuifbergan, A.; Parmelee, B.; Kesler, S. Relationships between self-reported sleep quality components and cognitive functioning in breast cancer survivors up to 10 years following chemotherapy. Psychooncology 2018, 27, 1937–1943. [Google Scholar] [CrossRef]
- Lambiase, M.J.; Gabriel, K.P.; Kuller, L.H.; Matthews, K.A. Sleep and executive function in older women: The moderating effect of physical activity. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Vyazovskiy, V.V. Sleep, recovery, and metaregulation: Explaining the benefits of sleep. Nat. Sci. Sleep 2015, 7, 171–184. [Google Scholar] [CrossRef] [Green Version]
- Faubel, R.; López-García, E.; Guallar-Castillón, P.; Graciani, A.; Banegas, J.R.; Rodríguez-Artalejo, F. Usual sleep duration and cognitive function in older adults in Spain. J. Sleep Res. 2009, 18, 427–435. [Google Scholar] [CrossRef]
- Tangen, T.; Mykletun, A. Depression and anxiety through the climacteric period: An epidemiological study (HUNT-II). J. Psychosom. Obstet. Gynaecol. 2008, 29, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.K.; Jones, C.K.; Newhouse, P.A. The role of estrogen in brain and cognitive aging. Neurotherapeutics 2019, 16, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, M.; Birling, H.; Kieseppä, T.; Tuulio-Henriksson, A. Is cognitive performance associated with anxiety and depression in first-episode psychosis? J. Affect. Disord. 2020, 263, 221–227. [Google Scholar] [CrossRef]
- Castaneda, A.E.; Tuulio-Henriksson, A.; Marttunen, M.; Suvisaari, J.; Lönnqvist, J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J. Affect. Disord. 2008, 106, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Eysenck, M.W.; Derakshan, N.; Santos, R.; Calvo, M.G. Anxiety and cognitive performance: Attentional control theory. Emotion 2007, 7, 336–353. [Google Scholar] [CrossRef] [Green Version]
- Petkus, A.J.; Filoteo, J.V.; Schiehser, D.M.; Gomez, M.E.; Petzinger, G. Worse cognitive performance predicts increased anxiety and depressive symptoms in patients with Parkinson’s disease: A bidirectional analysis. Neuropsychology 2019, 33, 35–46. [Google Scholar] [CrossRef]
- Salthouse, T.A. How general are the effects of trait anxiety and depressive symptoms on cognitive functioning? Emotion 2012, 12, 1075–1084. [Google Scholar] [CrossRef] [Green Version]
- Miyake, A.; Friedman, N.P. The Nature and Organization of Individual Differences in Executive Functions: Four General Conclusions. Curr. Dir. Psychol. Sci. 2012, 21, 8–14. [Google Scholar] [CrossRef]
- Franz, C.E.; Lyons, M.J.; O’Brien, R.; Panizzon, M.S.; Kim, K.; Bhat, R.; Grant, M.D.; Toomey, R.; Eisen, S.; Xian, H.; et al. A 35-year longitudinal assessment of cognition and midlife depression symptoms: The Vietnam Era Twin Study of Aging. Am. J. Geriatr. Psychiatry 2011, 19, 559–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmes, A.J.; Pizzagalli, D.A. Task feedback effects on conflict monitoring and executive control: Relationship to subclinical measures of depression. Emotion 2007, 7, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Bunce, D.; Handley, R.; Gaines, S.O. Depression, anxiety, and within-person variability in adults aged 18 to 85 years. Psychol. Aging 2008, 23, 848–858. [Google Scholar] [CrossRef]
- Zebdi, R.; Goyet, L.; Pinabiaux, C.; Guellaï, B. Psychological Disorders and Ecological Factors Affect the Front Development of Executive Functions: Some Perspectives. Front. Psychiatry 2016, 7, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Values | ||
|---|---|---|---|
| Mean | SD | ||
| Age (years) | 72.52 | 3.93 | |
| Time since menopause (years) | 23.09 | 6.59 | |
| Frequency | Percentage | ||
| Education | Primary or less | 193 | 80.08 |
| Secondary or superior | 48 | 19.92 | |
| Ocupational status | Retired | 198 | 82.16 |
| Worker | 8 | 3.32 | |
| unemployed | 35 | 14.52 | |
| Mean | SD | ||
| BMI | 28.49 | 2.40 | |
| Anxiety (HADS) | 9.73 | 6.46 | |
| Depression (HADS) | 8.83 | 5.07 | |
| PSQI | Sleep quality | 0.72 | 0,86 |
| Sleep latency | 0.80 | 1.15 | |
| Sleep duration | 0.71 | 0.86 | |
| Sleep efficiency | 0.41 | 0.65 | |
| Sleep disturbances | 1.19 | 0.44 | |
| Use of sleeping medication | 1.04 | 1.18 | |
| Daytime dysfunction | 1.30 | 0.98 | |
| Total score | 6.17 | 5.00 | |
| MMSE | 27.96 | 2.26 | |
| MoCA | 27.26 | 2.34 | |
| Isaacs test | 36.93 | 3.29 | |
| TMT-A (s) | 87.33 | 43.28 | |
| TMT-B (s) | 173.34 | 79.35 | |
| Variable | MMSE | MoCA | Isaacs Test | TMT-A | TMT-B | ||
|---|---|---|---|---|---|---|---|
| HADS Anxiety | r | −0.41 | −0.35 | −0.10 | 0.24 | 0.26 | |
| p-value | <0.001 | <0.001 | 0.130 | <0.001 | <0.001 | ||
| HADS depression | r | −0.40 | −0.34 | −0.23 | 0.36 | 0.28 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| PSQI | Sleep quality | r | −0.25 | −0.26 | −0.17 | 0.68 | 0.52 |
| p-value | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 | ||
| Sleep latency | r | −0.19 | −0.24 | −0.13 | 0.83 | 0.68 | |
| p-value | 0.003 | <0.001 | 0.043 | <0.001 | <0.001 | ||
| Sleep duration | r | −0.25 | −0.27 | −0.18 | 0.68 | 0.54 | |
| p-value | <0.001 | <0.001 | 0.005 | <0.001 | <0.001 | ||
| Sleep efficiency | r | −0.20 | −0.26 | −0.19 | 0.58 | 0.43 | |
| p-value | 0.002 | <0.001 | 0.068 | <0.001 | <0.001 | ||
| Sleep disturbances | r | −0.14 | −0.20 | −0.09 | 0.68 | 0.57 | |
| p-value | 0.028 | 0.002 | 0.148 | <0.001 | <0.001 | ||
| Use of sleeping medication | r | −0.18 | −0.21 | −0.18 | 0.78 | 0.63 | |
| p-value | 0.006 | 0.001 | 0.007 | <0.001 | <0.001 | ||
| Daytime dysfunction | r | −0.40 | −0.37 | −0.29 | 0.58 | 0.46 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Total score | r | −0.29 | −0.32 | −0.21 | 0.86 | 0.68 | |
| p-value | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 | ||
| Age | r | −0.21 | −0.24 | −0.15 | 0.11 | 0.10 | |
| p-value | 0.001 | <0.001 | 0.019 | 0.083 | 0.132 | ||
| BMI | r | −0.11 | −0.06 | −0.05 | −0.02 | 0.02 | |
| p-value | 0.102 | 0.353 | 0.407 | 0.725 | 0.793 | ||
| Years since menopause onset | r | −0.04 | −0.00 | −0.03 | 0.03 | 0.01 | |
| p-value | 0.522 | 0.955 | 0.664 | 0.645 | 0.885 | ||
| Minutes of exercise/day | r | −0.01 | 0.03 | −0.03 | −0.07 | −0.01 | |
| p-value | 0.831 | 0.597 | 0.621 | 0.263 | 0.903 | ||
| Variable | MMSE | MoCA | Isaacs Test | TMT-A | TMT-B | ||
|---|---|---|---|---|---|---|---|
| HADS Anxiety | r | −0.40 | −0.34 | −0.09 | 0.23 | 0.26 | |
| p-value | <0.001 | <0.001 | 0.172 | <0.001 | <0.001 | ||
| HADS depression | r | −0.39 | −0.33 | −0.22 | 0.36 | 0.28 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| PSQI | Sleep quality | r | −0.23 | −0.24 | −0.16 | 0.67 | 0.52 |
| p-value | <0.001 | <0.001 | 0.016 | <0.001 | <0.001 | ||
| Sleep latency | r | −0.18 | −0.23 | −0.12 | 0.82 | 0.68 | |
| p-value | 0.005 | <0.001 | 0.063 | <0.001 | <0.001 | ||
| Sleep duration | r | −0.23 | −0.25 | −0.16 | 0.68 | 0.53 | |
| p-value | <0.001 | <0.001 | 0.012 | <0.001 | <0.001 | ||
| Sleep efficiency | r | −0.18 | −0.24 | −0.10 | 0.56 | 0.42 | |
| p-value | 0.005 | <0.001 | 0.119 | <0.001 | <0.001 | ||
| Sleep disturbances | r | −0.12 | −0.18 | −0.08 | 0.68 | 0.56 | |
| p-value | 0.063 | 0.005 | 0.232 | <0.001 | <0.001 | ||
| Use of sleeping medication | r | −0.16 | −0.18 | −0.16 | 0.77 | 0.63 | |
| p-value | 0.016 | 0.006 | 0.015 | <0.001 | <0.001 | ||
| Daytime dysfunction | r | −0.39 | −0.36 | −0.28 | 0.58 | 0.45 | |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| Total score | r | −0.27 | −0.30 | −0.20 | 0.85 | 0.68 | |
| p-value | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | ||
| Variable | Smokers | Education | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No (n = 213) | Yes (n = 28) | Primary or less (n = 193) | Secondary or higher (n = 48) | |||||||
| Mean | DT | Mean | DT | p-value | Mean | DT | Mean | DT | p-value | |
| MMSE | 27.94 | 2.28 | 28.11 | 2.11 | 0.712 | 27.87 | 2.33 | 28.33 | 1.93 | 0.200 |
| MoCA | 27.27 | 2.38 | 27.21 | 2.08 | 0.910 | 27.21 | 2.37 | 27.46 | 2.26 | 0.517 |
| Isaacs test | 36.99 | 3.26 | 36.50 | 3.57 | 0.464 | 36.82 | 3.36 | 37.35 | 3.02 | 0.319 |
| TMT-A | 87.49 | 44.05 | 86.11 | 37.53 | 0.874 | 88.16 | 42.50 | 84.00 | 46.59 | 0.552 |
| TMT-B | 172.95 | 79.05 | 176.36 | 83.08 | 0.831 | 175.64 | 78.99 | 164.13 | 80.98 | 0.369 |
| Variable | B | Beta | t | 95% CI | p-Value | ||
|---|---|---|---|---|---|---|---|
| MMSE | HADS anxiety | −0.13 | −0.36 | −6.62 | −0.16 | −0.09 | <0.001 |
| HADS depression | −0.15 | −0.34 | −6.28 | −0.20 | −0.10 | <0.001 | |
| Age | −0.09 | −0.16 | −2.90 | −0.15 | −0.03 | 0.004 | |
| MoCA | Daytime dysfunction | −0.74 | −0.31 | −5.45 | −1.01 | −0.48 | <0.001 |
| HADS anxiety | −0.10 | −0.29 | −5.07 | −0.15 | −0.06 | <0.001 | |
| Age | −0.11 | −0.19 | −3.28 | −0.18 | −0.04 | 0.001 | |
| Isaacs test | Daytime dysfunction | −0.95 | −0.28 | −4.55 | −1.36 | −0.54 | <0.000 |
| Age | −0.10 | −0.12 | −2.00 | −0.21 | 0.00 | 0.047 | |
| TMT-A | PSQI total score | 4.38 | 0.50 | 7.11 | 3.16 | 5.59 | <0.001 |
| Sleep latency | 10.75 | 0.29 | 4.14 | 5.63 | 15.87 | <0.001 | |
| Sleep disturbances | 13.44 | 0.14 | 3.06 | 4.79 | 22.09 | 0.002 | |
| TMT-B | PSQI total score | 4.75 | 0.30 | 2.93 | 1.55 | 7.94 | 0.004 |
| Sleep latency | 20.68 | 0.30 | 3.03 | 7.24 | 34.12 | 0.003 | |
| HADS anxiety | 1.33 | 0.11 | 2.32 | 0.20 | 2.46 | 0.021 | |
| Sleep disturbances | 25.27 | 0.14 | 2.19 | 2.58 | 47.97 | 0.029 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Díaz, A.B.; Aibar-Almazán, A.; Martínez-Amat, A.; Jiménez-García, J.D.; Álvarez-Salvago, F.; Hita-Contreras, F. Associations of Sleep Quality, Anxiety, and Depression with Cognitive and Executive Functions among Community-Dwelling Women Aged ≥ 65 Years: A Cross-Sectional Study. Healthcare 2021, 9, 1599. https://doi.org/10.3390/healthcare9111599
Parra-Díaz AB, Aibar-Almazán A, Martínez-Amat A, Jiménez-García JD, Álvarez-Salvago F, Hita-Contreras F. Associations of Sleep Quality, Anxiety, and Depression with Cognitive and Executive Functions among Community-Dwelling Women Aged ≥ 65 Years: A Cross-Sectional Study. Healthcare. 2021; 9(11):1599. https://doi.org/10.3390/healthcare9111599
Chicago/Turabian StyleParra-Díaz, Ana Belén, Agustín Aibar-Almazán, Antonio Martínez-Amat, José Daniel Jiménez-García, Francisco Álvarez-Salvago, and Fidel Hita-Contreras. 2021. "Associations of Sleep Quality, Anxiety, and Depression with Cognitive and Executive Functions among Community-Dwelling Women Aged ≥ 65 Years: A Cross-Sectional Study" Healthcare 9, no. 11: 1599. https://doi.org/10.3390/healthcare9111599
APA StyleParra-Díaz, A. B., Aibar-Almazán, A., Martínez-Amat, A., Jiménez-García, J. D., Álvarez-Salvago, F., & Hita-Contreras, F. (2021). Associations of Sleep Quality, Anxiety, and Depression with Cognitive and Executive Functions among Community-Dwelling Women Aged ≥ 65 Years: A Cross-Sectional Study. Healthcare, 9(11), 1599. https://doi.org/10.3390/healthcare9111599











