Assessment of Pharmacists Prescribing Practices in Poland—A Descriptive Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Designed and Settings
2.2. Ethical Considerations
2.3. Data Collection
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zimmermann, A.; Cieplikiewicz, E.; Wąż, P.; Gaworska-Krzemińska, A.; Olczyk, P. The implementation process of nurse prescribing in Poland—A descriptive study. Int. J. Env. Res. Public Health 2020, 17, 2020–2417. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, D.; Rees, J.A.; Smith, I. Ethical awareness of community pharmacists. Int. J. Pharm. Pract. 2000, 8, 82–87. [Google Scholar] [CrossRef]
- Yuksel, N.; Eberhart, G.; Bungard, T.J. Prescribing by pharmacists in Alberta. Am. J. Health Syst. Pharm. 2008, 65, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Morecroft, C.W.; Mackridge, A.J.; Stokes, E.C.; Gray, N.J.; Wilson, S.E.; Ashcroft, D.M.; Mensah, N.; Pickup, G.B. Emergency supply of prescription-only medicines to patients by community pharmacists: A mixed methods evaluation incorporating patient, pharmacist and GP perspectives. BMJ Open 2015, 5, e006934. [Google Scholar] [CrossRef]
- Pharmaceutical Law of 6 September 2001 (JL No. 126, item 1381) Consolidated Text of 15 March 2019 (JL item 499) and Consolidated Text of 28 May 2021 (JL item 974). Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=wdu20011261381 (accessed on 7 July 2021).
- Kim, J.J.; Tian, A.H.; Pham, L.; Nakhla, N.; Houle, S.K.; Wong, W.W.; Alsabbagh, M.W. Economic evaluation of pharmacists prescribing for minor ailments in Ontario, Canada: A cost-minimization analysis. Int. J. Pharm. Prac. 2021, 29, 228–234. [Google Scholar] [CrossRef]
- Mossialos, E.; Courtin, E.; Naci, H.; Benrimoj, S.; Bouvy, M.; Farris, K.; Noyce, P.; Sketris, I. From “retailers” to health care providers: Transforming the role of community pharmacists in chronic disease management. Health Policy 2015, 119, 628–639. [Google Scholar] [CrossRef]
- Nachtigal, P.; Šimůnek, T.; Atkinson, J. Pharmacy Practice and Education in the Czech Republic. Pharmacy 2017, 9, 54. [Google Scholar] [CrossRef]
- Soares, I.B.; Imfeld-Isenegger, T.L.; Makovec, U.N.; Horvat, N.; Kos, M.; Arnet, I.; Hersberger, K.E.; Costa, F.A. A survey to assess the availability, implementation rate and remuneration of pharmacist-led cognitive services throughout Europe. Res. Soc. Adm. Pharm. 2020, 16, 41–47. [Google Scholar] [CrossRef]
- Weiss, M.C.; Sutton, J. The changing nature of prescribing: Pharmacists as prescribers and challenges to medical dominance. Sociol. Health Illness 2009, 31, 406–421. [Google Scholar] [CrossRef]
- Latter, S.; Blenkinsopp, A.; Smith, A.; Chapman, S.; Tinelli, M.; Gerard, K.; Little, P.; Celino, N.; Granby, T.; Nicholls, P.; et al. Evaluation of Nurse and Pharmacist Independent Prescribing; Keele University: Southampton, UK, 2011. [Google Scholar]
- Bhanbhro, S.; Drennan, V.M.; Grant, R.; Harris, R. Assessing the contribution of prescribing in primary care by nurses and professionals allied to medicine: A systematic review of literature. BMC Health Serv. Res. 2011, 11, 330. [Google Scholar] [CrossRef]
- Andersson, K.; Melander, A.; Svensson, C.; Lind, O.; Nilsson, J.L.G. Repeat prescriptions: Refill adherence in relation to patient and prescriber characteristics, reimbursement level and type of medication. Eur. J. Public Health 2005, 15, 621–626. [Google Scholar] [CrossRef]
- Riley, R.; Weiss, M.C.; Platt, J.; Taylor, G.; Horrocks, S.; Taylor, A. A comparison of GP, pharmacist and nurse prescriber responses to patients’ emotional cues and concerns in primary care consultations. Patient Educ. Couns. 2013, 91, 65–71. [Google Scholar] [CrossRef][Green Version]
- Famiyeh, I.M.; MacKeigan, L.; Thompson, A.; Kuluski, K.; McCarthy, L.M. Exploring pharmacy service users’ support for and willingness to use community pharmacist prescribing services. Res. Soc. Adm. Pharm. 2019, 15, 575–583. [Google Scholar] [CrossRef]
- Hoti, K.; Hughes, J.; Sunderland, B. Expanded prescribing: A comparison of the views of Australian hospital and community pharmacists. Int. J. Clin. Pharm. 2013, 35, 469–475. [Google Scholar] [CrossRef]
- George, J.; Pfleger, D.; McCaig, D.; Bond, C.; Stewart, D. Independent prescribing by pharmacists: A study of the awareness, views and attitudes of Scottish community pharmacists. Pharm. World Sci. 2006, 28, 45–53. [Google Scholar] [CrossRef]
- Auta, A.; Strickland-Hodge, B.; Maz, J. Stakeholders’ views on granting prescribing authority to pharmacists in Nigeria: A qualitative study. Int. J. Clin. Pharm. 2016, 38, 960–967. [Google Scholar] [CrossRef][Green Version]
- Smith, J.; Picton, C.; Dayan, M. Now or never: Shaping pharmacy for the future. In The Report of the Commission on Future Models of Care Delivered through Pharmacy; Royal Pharmaceutical Society of Great Britain: London, UK, 2013. [Google Scholar]
- Law, M.R.; Morgan, S.G.; Majumdar, S.R.; Lynd, L.D.; Marra, C.A. Effects of prescription adaptation by pharmacists. BMC Health Serv. Res. 2010, 10, 313. [Google Scholar] [CrossRef]
- O’Neill, R.; Rowley, E.; Smith, F. The emergency supply of prescription-only medicines: A survey of requests to community pharmacists and their views on the procedures. Int. J. Pharm. Pract. 2002, 10, 77–83. [Google Scholar] [CrossRef]
- Shepherd, M.D. Examination of why some community pharmacists do not provide 72-hour emergency prescription drugs to Medicaid patients when prior authorization is not available. J. Manag. Care Spec. Pharm. 2013, 19, 527–533. [Google Scholar] [CrossRef]
- Guillaume, L.; Cooper, R.; Avery, A.; Mitchell, S.; Ward, P.; Anderson, C.; Bissell, P.; Hutchinson, A.; James, V.; Lymn, J.; et al. Supplementary prescribing by community pharmacists: An analysis of PACT data, 2004–2006. J. Clin. Pharm. Ther. 2008, 33, 11–16. [Google Scholar] [CrossRef]
- Nazar, H.; Nazar, Z.; Simpson, J.; Yeung, A.; Whittlesea, C. Summative service and stakeholder evaluation of an NHS-funded community Pharmacy Emergency Repeat Medication Supply Service (PERMSS). BMJ Open 2016, 6, e009736. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.L.; Avery, A.J.; Howard, P.D.; Partridge, M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: Observational study. Qual. Saf. Health Care 2003, 12, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Runkle, D. Advocacy in science. In Summary of Workshop Convened by the American Association for the Advancement of Science; Frankel, M.S., Ed.; American Association for the Advancement of Science: Washington, DC, USA, 2012. [Google Scholar]

| Pharmacist Prescribing Rules | Before 31st March 2020 | After 1st April 2020 |
|---|---|---|
| Prescription type | Only the paper version was technically possible | The electronic prescription is preferred and made available. The paper version only in exceptional cases |
| Reasons for issuing a prescription | Immediate health risk | Health risk |
| Amount of medicine | The smallest, single registered packet of the given medicine | In the case of electronic prescriptions, the amount of medicine required for 180 days of therapy for the given dose |
| Types of medicines | Prescription-only medicines except narcotics and psychotropic medication | Prescription-only medicines except narcotics and psychotropic medication |
| Information needed on the prescription | The same as on the prescription issued by a physician, and the reason for dispensing the medicine | The same as on the prescription issued by a physician and the reason for dispensing the medicine |
| Reimbursement | 0% | 0%, excluding prescriptions issued for the pharmacist himself/herself or his/her family members |
| Location | Rural Area | City < 100,000 | City > 100,000 | ||
|---|---|---|---|---|---|
| Position | n | % | n | % | n |
| Neighborhood of out-patient clinic or hospital | 59 | 52.7 | 134 | 35.7 | 107 |
| Shopping center | 2 | 1.8 | 35 | 9.3 | 37 |
| Residential area | 43 | 38.4 | 85 | 22.7 | 153 |
| Town/city center | 8 | 7.1 | 121 | 32.3 | 58 |
| Total | 112 | 100.0 | 375 | 100.0 | 355 |
| Pharmacy Location | n | Mean | SD | CI −95.0% | CI +95.0% | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| Neighborhood of out-patient clinic or hospital | 300 | 1.11 | 13.421 | −0.42 | 2.63 | 0.0 | 218.0 |
| Shopping center | 74 | 1.42 | 7.503 | −0.32 | 3.16 | 0.0 | 53.0 |
| Residential area | 281 | 3.48 | 30.094 | −0.05 | 7.01 | 0.0 | 431.0 |
| Town/city center | 187 | 4.14 | 30.816 | −0.31 | 8.58 | 0.0 | 371.0 |
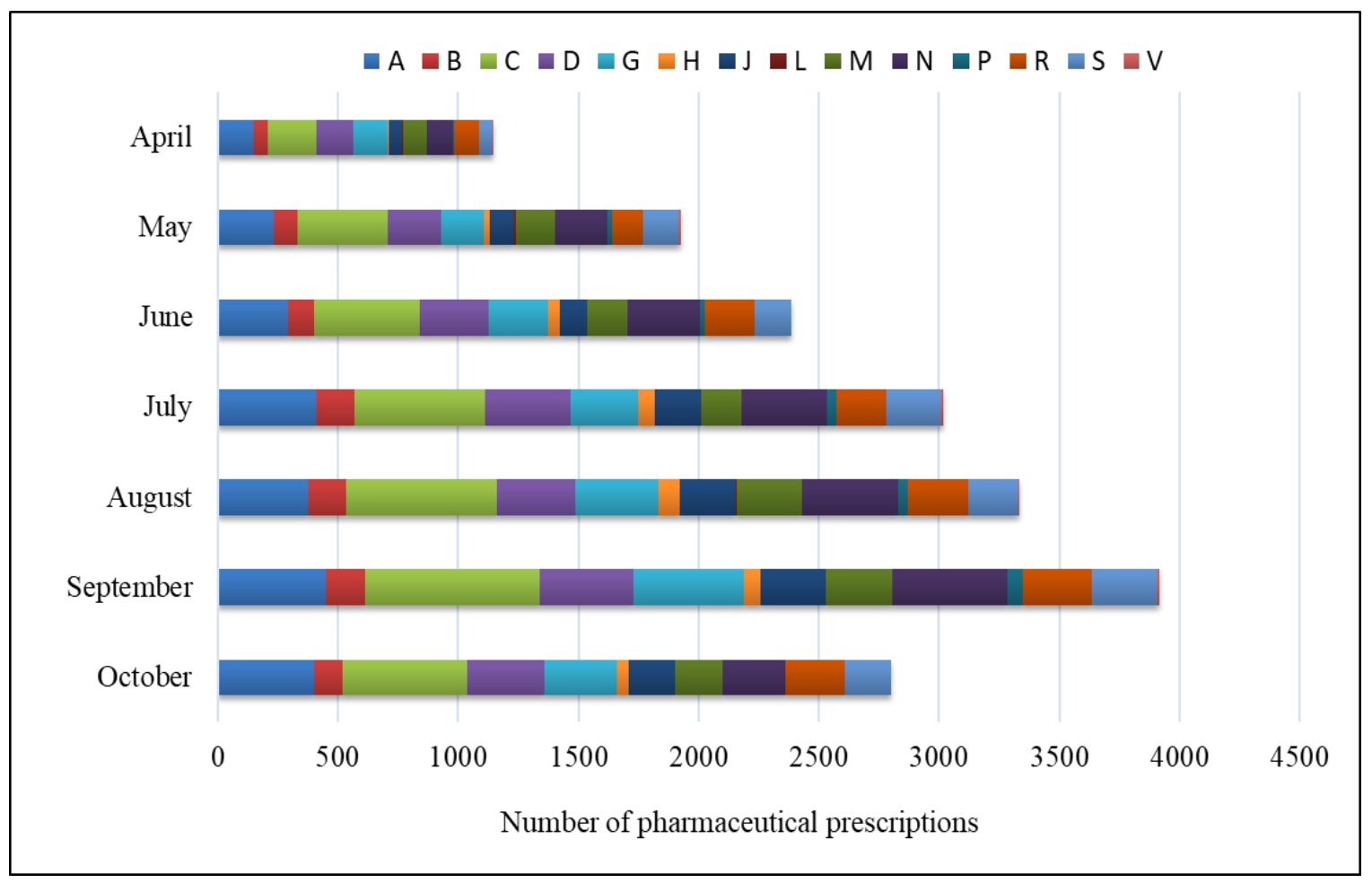
| ATC Code | Total Pharmacist Prescribing 1 April–30 October | Percentage of Pharmacist’s Prescribing in the Month | ∑ [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| April | May | June | July | August | September | October | |||
| A | 2316 | 0.80 | 1.27 | 1.59 | 2.23 | 2.03 | 2.43 | 2.16 | 12.50 |
| B | 859 | 0.32 | 0.51 | 0.58 | 0.83 | 0.86 | 0.87 | 0.65 | 4.64 |
| C | 3436 | 1.10 | 2.02 | 2.36 | 2.95 | 3.37 | 3.93 | 2.81 | 18.54 |
| D | 2057 | 0.82 | 1.21 | 1.55 | 1.93 | 1.77 | 2.09 | 1.73 | 11.10 |
| G | 1948 | 0.78 | 0.94 | 1.33 | 1.51 | 1.86 | 2.48 | 1.62 | 10.51 |
| H | 357 | 0.02 | 0.13 | 0.26 | 0.38 | 0.47 | 0.39 | 0.27 | 1.93 |
| J | 1151 | 0.30 | 0.54 | 0.62 | 1.02 | 1.25 | 1.46 | 1.03 | 6.21 |
| L | 22 | 0.02 | 0.05 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | 0.12 |
| M | 1348 | 0.52 | 0.89 | 0.91 | 0.91 | 1.47 | 1.51 | 1.08 | 7.28 |
| N | 2121 | 0.60 | 1.16 | 1.62 | 1.93 | 2.16 | 2.57 | 1.40 | 11.45 |
| P | 193 | 0.02 | 0.11 | 0.13 | 0.23 | 0.22 | 0.34 | 0.00 | 1.04 |
| R | 1427 | 0.56 | 0.70 | 1.10 | 1.10 | 1.34 | 1.55 | 1.35 | 7.70 |
| S | 1256 | 0.30 | 0.78 | 0.84 | 1.25 | 1.12 | 1.46 | 1.03 | 6.78 |
| V | 38 | 0.02 | 0.05 | 0.00 | 0.04 | 0.04 | 0.05 | 0.00 | 0.21 |
| ∑ | 18,529 | 6.20 | 10.39 | 12.89 | 16.28 | 18.00 | 21.13 | 15.11 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmermann, A.; Płaczek, J.; Wrzosek, N.; Owczarek, A. Assessment of Pharmacists Prescribing Practices in Poland—A Descriptive Study. Healthcare 2021, 9, 1505. https://doi.org/10.3390/healthcare9111505
Zimmermann A, Płaczek J, Wrzosek N, Owczarek A. Assessment of Pharmacists Prescribing Practices in Poland—A Descriptive Study. Healthcare. 2021; 9(11):1505. https://doi.org/10.3390/healthcare9111505
Chicago/Turabian StyleZimmermann, Agnieszka, Jakub Płaczek, Natalia Wrzosek, and Artur Owczarek. 2021. "Assessment of Pharmacists Prescribing Practices in Poland—A Descriptive Study" Healthcare 9, no. 11: 1505. https://doi.org/10.3390/healthcare9111505
APA StyleZimmermann, A., Płaczek, J., Wrzosek, N., & Owczarek, A. (2021). Assessment of Pharmacists Prescribing Practices in Poland—A Descriptive Study. Healthcare, 9(11), 1505. https://doi.org/10.3390/healthcare9111505






