Association of Mortality-Related Risk Factors in Patients with COVID-19: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
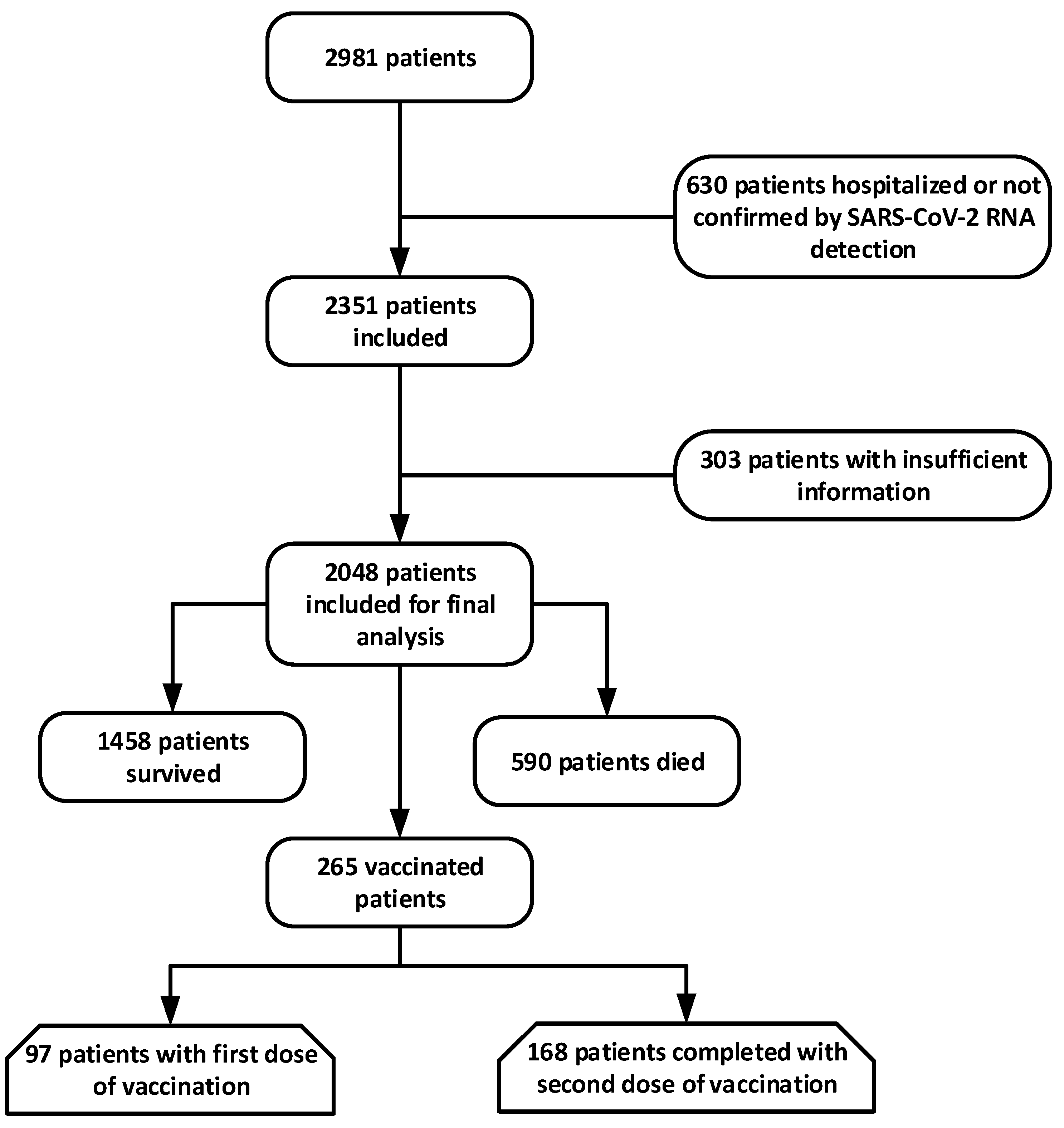
2.1. Data Source and Study Population
2.2. Statistical Analysis
3. Results
3.1. Epidemiologic, Demographic, and Clinical Characteristics
3.2. Laboratory Indicators and Imaging Features
3.3. Complications and Treatment during Hospitalization
3.4. Potential Risk Factors Associated with In-Hospital Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Chan, J.W.M.; Ng, C.K.; Chan, Y.H.; Mok, T.Y.W.; Lee, S.; Chu, S.Y.Y.; Law, W.L.; Lee, M.P.; Li, P.C.K. Short term outcome and risk factors for adverse clinical outcomes in adults with the severe acute respiratory syndrome (SARS). Thorax 2003, 58, 686–689. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Lei, Q.; Li, W.; Wang, X.; Liu, W.; Fan, X.; Li, W. Clinical Characteristics, Associated Factors, and Predicting COVID-19 Mortality Risk: A Retrospective Study in Wuhan, China. Am. J. Prev. Med. 2020, 59, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Analysis of Epidemiological characteristics of new coronavirus pneumonia. Chin. J. Epidemiol. 2020, 41, 1–7. [Google Scholar]
- Sohrabi, C.; Alsafi, Z.; O’neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y.; Ren, R.; Leung, K.S.; Lau, E.H.; Wong, J.Y.; et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, A.; Rezaei, N. Immune-epidemiological parameters of the novel coronavirus–a perspective. Expert Rev. Clin. Immunol. 2020, 16, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; He, W.; Yu, X.; Hu, D.; Bao, M.; Liu, H.; Zhou, J.; Jiang, H. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020, 90, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.; Alashwal, H.; Ibrahim, S. Association of hypertension, diabetes, stroke, cancer, kidney disease, and high-cholesterol with COVID-19 disease severity and fatality: A systematic review. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 1133–1142. [Google Scholar] [CrossRef]
- Xia, W.; Shao, J.; Guo, Y.; Peng, X.; Li, Z.; Hu, D. Clinical and CT features in pediatric patients with COVID-19 infection: Different points from adults. Pediatr. Pulmonol. 2020, 55, 1169–1174. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.-J.; Cheng, X.; Zhou, F.; Lei, F.; Akolkar, G.; Cai, J.; Zhang, X.-J.; Blet, A.; Xie, J.; Zhang, P.; et al. Redefining Cardiac Biomarkers in Predicting Mortality of Inpatients With COVID-19. Hypertension 2020, 76, 1104–1112. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, X.; Fan, Q.; Liu, H.; Liu, X.; Liu, Z.; Zhang, Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020, 18, 1324–1329. [Google Scholar] [CrossRef]
- Shi, H.; Han, X.; Jiang, N.; Cao, Y.; Alwalid, O.; Gu, J.; Fan, Y.; Zheng, C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: A descriptive study. Lancet Infect. Dis. 2020, 20, 425–434. [Google Scholar] [CrossRef]
- Paterson, R.W.; Brown, R.L.; Benjamin, L.; Nortley, R.; Wiethoff, S.; Bharucha, T.; Jayaseelan, D.L.; Kumar, G.; Raftopoulos, R.E.; Zambreanu, L.; et al. The emerging spectrum of COVID-19 neurology: Clinical, radiological and laboratory findings. Brain 2020, 143, 3104–3120. [Google Scholar] [CrossRef]
- Yang, Y.; Islam, M.S.; Wang, J.; Li, Y.; Chen, X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): A review and perspective. Int. J. Biol. Sci. 2020, 16, 1708. [Google Scholar] [CrossRef]
- Ñamendys-Silva, S.A. Respiratory support for patients with COVID-19 infection. Lancet Respir. Med. 2020, 8, e18. [Google Scholar] [CrossRef]
- Metlay, J.P.; Waterer, G.W.; Long, A.C.; Anzueto, A.; Brozek, J.; Crothers, K.; Cooley, L.A.; Dean, N.C.; Fine, M.J.; Flanders, S.A.; et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019, 200, e45–e67. [Google Scholar] [CrossRef] [PubMed]
- Asghar, M.S.; Kazmi, S.J.H.; Khan, N.A.; Akram, M.; Khan, S.A.; Rasheed, U.; Hassan, M.; Memon, G.M. Clinical profiles, characteristics, and outcomes of the first 100 admitted COVID-19 patients in Pakistan: A single-center retrospective study in a tertiary care hospital of Karachi. Cureus 2020, 12, c34. [Google Scholar] [CrossRef]
- Wu, J.; Liu, J.; Zhao, X.; Liu, C.; Wang, W.; Wang, D.; Xu, W.; Zhang, C.; Yu, J.; Jiang, B.; et al. Clinical Characteristics of Imported Cases of Coronavirus Disease 2019 (COVID-19) in Jiangsu Province: A Multicenter Descriptive Study. Clin. Infect. Dis. 2020, 71, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Galdamez, D.H.; González-Block, M.; Romo-Dueñas, D.K.; Lima-Morales, R.; Hernández-Vicente, I.A.; Lumbreras-Guzmán, M.; Méndez-Hernández, P. Increased Risk of Hospitalization and Death in Patients with COVID-19 and Pre-existing Noncommunicable Diseases and Modifiable Risk Factors. Arch. Med. Res. 2020, 51, 683–689. [Google Scholar] [CrossRef]
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and cardiovascular disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [Green Version]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus–induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef]
- Assiri, A.; Al-Tawfiq, J.A.; Al-Rabeeah, A.A.; Al-Rabiah, F.A.; Al-Hajjar, S.; Al-Barrak, A.; Flemban, H.; Al-Nassir, W.N.; Balkhy, H.H.; Al-Hakeem, R.F.; et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: A descriptive study. Lancet Infect. Dis. 2013, 13, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; Wunderink, R.G. MERS, SARS and other coronaviruses as causes of pneumonia. Respirology 2018, 23, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Corrales-Medina, V.F.; Musher, D.M.; Shachkina, S.; Chirinos, J.A. Acute pneumonia and the cardiovascular system. Lancet 2013, 381, 496–505. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omland, T.; Pfeffer, M.A.; Solomon, S.D.; de Lemos, J.A.; Røsjø, H.; Šaltytė Benth, J.; Maggioni, A.; Domanski, M.J.; Rouleau, J.L.; Sabatine, M.S.; et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J. Am. Coll. Cardiol. 2013, 61, 1240–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kociol, R.D.; Pang, P.S.; Gheorghiade, M.; Fonarow, G.C.; O’Connor, C.M.; Felker, G.M. Troponin elevation in heart failure: Prevalence, mechanisms, and clinical implications. J. Am. Coll. Cardiol. 2010, 56, 1071–1078. [Google Scholar] [CrossRef] [Green Version]
| Frequency n (%) | Discharged n (%) | Deceased n (%) | p-Value | |
|---|---|---|---|---|
| Age (years) | 56 (18–88) | 54 (46–63) | 65 (58–88) | 0.000 |
| ~50 | 125 (6.1) | 77 (5.3) | 48 (8.2) | |
| 51–60 | 242 (11.8) | 135 (9.3) | 107 (18.0) | |
| 61–70 | 734 (35.8) | 570 (39.1) | 164 (27.9) | |
| 71~ | 947 (46.2) | 676 (46.4) | 271 (45.9) | |
| Sex | 0.102 | |||
| Female | 831 (40.6) | 656 (45.0) | 175 (29.7) | |
| Male | 1217 (59.4) | 802 (55.0) | 415 (70.3) | |
| Obese (BMI ≥ 30) | 1468 (71.7) | 1265 (86.8) | 203 (34.4) | 0.026 |
| Smoker | 522 (25.5) | 416 (28.5) | 106 (18.0) | 0.050 |
| Exposure history | 1613 (78.8) | 1420 (97.4) | 193 (32.8) | 0.861 |
| Time from illness onset to outpatient visit, (d) | 2 (1–5) | 2 (1–4) | 3 (1–6) | 0.011 |
| Time from illness onset to hospitalization, (d) | 12 (8–15) | 11 (8–14) | 12 (9–16) | 0.024 |
| Symptoms at the time of admission, n (%) | ||||
| Fever (temp ≥ 37 °C) | 1941 (94.8) | 1439 (98.7) | 502 (85.1) | 0.001 |
| Cough | 1486 (72.6) | 1061 (72.8) | 425 (72.0) | 0.659 |
| Fatigue | 1177 (57.5) | 781 (53.6) | 396 (67.1) | 0.316 |
| Sore throat | 1167 (57.0) | 781 (53.6) | 386 (65.4) | 0.681 |
| Chest pain | 1120 (54.7) | 850 (58.3) | 270 (45.8) | 0.121 |
| Nasal congestion/rhinorrhea | 1034 (50.5) | 858 (58.9) | 176 (29.8) | 0.551 |
| Dyspnea | 946 (46.2) | 637 (43.7) | 309 (52.4) | 0.332 |
| Chill | 946 (46.2) | 570 (39.1) | 376 (63.7) | 0.835 |
| Dizziness and headache | 686 (33.5) | 396 (27.2) | 290 (49.2) | 0.054 |
| Urinary incontinency | 626 (30.6) | 521 (35.8) | 105 (17.8) | 0.724 |
| Chest distress | 598 (29.2) | 482 (33.1) | 116 (19.7) | 0.011 |
| Abdominal pain | 409 (20.0) | 319 (21.9) | 90 (15.3) | 0.933 |
| Myalgia or arthralgia | 309 (15.1) | 202 (13.9) | 107 (18.1) | 0.021 |
| Diarrhea | 202 (9.9) | 87 (6.0) | 115 (19.5) | 0.431 |
| Nausea and vomiting | 125 (6.1) | 48 (3.3) | 77 (13.1) | 0.657 |
| Comorbidities, n (%) | ||||
| CVDs | 1177 (57.5) | 975 (66.9) | 202 (34.2) | 0.000 |
| Hypertension | 975 (47.6) | 531 (36.4) | 444 (75.3) | 0.000 |
| Cerebrovascular diseases | 629 (30.7) | 319 (21.9) | 310 (52.5) | 0.423 |
| DM | 608 (29.7) | 213 (14.6) | 395 (66.9) | 0.001 |
| COPD | 154 (7.5) | 88 (6.0) | 66 (11.2) | 0.132 |
| Asthma | 135 (6.6) | 58 (4.0) | 77 (13.1) | 0.010 |
| Tuberculosis | 96 (4.7) | 48 (3.3) | 48 (8.1) | 0.067 |
| Chronic liver disease | 79 (3.8) | 38 (2.6) | 41 (6.9) | 0.042 |
| Chronic renal disease | 68 (3.3) | 48 (3.3) | 20 (3.4) | 0.044 |
| Cancer | 50 (2.4) | 29 (2.0) | 21 (3.6) | 0.452 |
| HIV | 29 (1.4) | 19 (1.3) | 10 (1.7) | 0.547 |
| Severity assessment on admission | 0.002 | |||
| Non-severe | 637 (31.1) | 483 (33.1) | 154 (26.1) | |
| Severe | 1411 (68.9) | 1138 (78.1) | 273 (46.3) |
| Frequency n (%) | Discharged n (%) | Deceased n (%) | p-Value | |
|---|---|---|---|---|
| Laboratory indicators, n (%) | 655 (32.0) | 424 (29.1) | 231 (39.2) | 0.005 |
| Higher neutrophil count (×109/L) | 1169 (57.1) | 627 (43.0) | 542 (91.9) | 0.000 |
| Lower lymphocyte count (×109/L) | 590 (28.8) | 280 (19.2) | 310 (52.5) | 0.863 |
| Higher leukocyte count (×109/L) | 696 (34.0) | 338 (23.2) | 358 (60.7) | 0.471 |
| Lower platelet count (×10⁹/L) | 1081 (52.8) | 531 (36.4) | 550 (93.2) | 0.000 |
| Elevated cardiac troponin I, ng/mL | 320 (15.6) | 155 (10.6) | 165 (27.9) | 0.002 |
| Increased CK (>185 U/L) | 754 (36.8) | 357 (24.5) | 397 (67.3) | 0.004 |
| High LDH (>250 U/L) | 686 (33.5) | 397 (27.2) | 289 (49.1) | 0.093 |
| Elevated ALT (>45 IU/L) | 629 (30.7) | 319 (21.9) | 310 (52.5) | 0.631 |
| Elevated AST (>45 IU/L) | 522 (25.5) | 376 (25.8) | 146 (24.7) | 0.841 |
| Higher creatinine (>1.3 mg/dL) | 975 (47.6) | 579 (39.7) | 396 (67.1) | 0.334 |
| Albumin ≤ 35 (g/L) | 860 (42.0) | 608 (41.7) | 252 (42.7) | 0.451 |
| Globulin > 85 (g/L) | 590 (28.8) | 280 (19.2) | 310 (52.5) | 0.001 |
| Increased PCT | 1806 (88.2) | 1304 (89.4) | 502 (85.1) | 0.663 |
| Increased CRP | 821 (40.1) | 328 (22.5) | 493 (83.6) | 0.821 |
| ESR (>20 mm/h) | 445 (21.7) | 193 (13.2) | 252 (42.7) | 0.000 |
| High serum ferritin level (>300 µg/L) | 1785 (87.2) | 1264 (86.7) | 521 (88.3) | 0.000 |
| IL-6 > 7 ng/L | 502 (24.5) | 232 (15.9) | 270 (45.8) | 0.237 |
| IL-8 > 62 ng/L | 899 (43.9) | 435 (29.8) | 464 (78.6) | 0.065 |
| TNF-α > 8.1 ng/L | 1575 (76.9) | 1245 (85.4) | 330 (55.9) | 0.289 |
| Proteinuria | 975 (47.6) | 416 (28.5) | 559 (94.7) | 0.000 |
| Increased D-dimer > 1µg/L | 145 (7.1) | 77 (5.3) | 68 (11.5) | 0.303 |
| Prolonged thrombin time | 0.005 | |||
| Imaging features | 1169 (57.1) | 695 (47.7) | 474 (80.3) | |
| Bilateral distribution, n (%) | 522 (25.5) | 125 (8.6) | 397 (67.2) | |
| Pleural effusion, n (%) | 655 (32.0) | 424 (29.1) | 231 (39.2) | 0.005 |
| Frequency n (%) | Discharged n (%) | Deceased n (%) | p-Value | |
|---|---|---|---|---|
| Complications | ||||
| Septicemia | 1062 (51.9) | 502 (34.4) | 560 (94.9) | 0.000 |
| ARDS | 975 (47.6) | 397 (27.2) | 578 (98.0) | 0.000 |
| Respiratory failure | 850 (41.5) | 280 (19.2) | 570 (96.6) | 0.000 |
| Acute kidney injury | 367 (17.9) | 29 (2.0) | 338 (57.3) | 0.225 |
| Acute cardiac injury | 395 (19.3) | 15 (1.0) | 380 (64.4) | 0.000 |
| Liver dysfunction | 522 (25.5) | 222 (15.2) | 300 (50.8) | 0.872 |
| Coagulopathy | 406 (19.8) | 106 (7.3) | 300 (50.8) | 0.423 |
| Bacteremia | 358 (17.5) | 184 (12.6) | 174 (29.5) | 0.060 |
| Hyperglycemia | 1331 (30.7) | 808 (55.4) | 523 (88.6) | 0.001 |
| Acidosis | 205 (10.0) | 87 (6.0) | 118 (20.0) | 0.478 |
| Treatment | ||||
| Antibiotics | 1970 (96.2) | 1381 (94.7) | 589 (99.8) | 0.022 |
| Antiviral drugs | 502 (24.5) | 233 (16.0) | 269 (45.6) | 0.032 |
| Intravenous corticosteroid | 627 (30.6) | 328 (22.5) | 299 (50.7) | 0.475 |
| Intravenous immunoglobulin | 530 (25.9) | 165 (11.3) | 365 (61.9) | 0.012 |
| Oxygen therapy | 0.000 | |||
| High flow oxygen therapy | 395 (19.3) | 96 (6.6) | 299 (50.7) | |
| Nasal cannula or mask | 377 (18.4) | 106 (7.3) | 271 (45.9) | |
| Noninvasive mechanical ventilation | 291 (14.2) | 29 (2.0) | 262 (44.4) | |
| Invasive mechanical ventilation | 737 (36.0) | 165 (11.3) | 572 (96.9) | |
| ECMO | 68 (3.3) | 15 (1.0) | 53 (9.0) | 0.020 |
| Continuous Renal replacement therapy | 174 (8.5) | 15 (1.0) | 159 (26.9) | 0.000 |
| Regression Coefficient (95% CI) | p-Value | |
|---|---|---|
| Age | 1.121 (0.974–2.131) | 0.000 |
| Fever | 1.302 (1.126–2.032) | 0.124 |
| Hypertension | 2.634 (2.132–3.131) | 0.001 |
| Diabetes | 2.851 (1.565–3.325) | 0.055 |
| CVD | 2.043 (1.965–2.456) | 0.001 |
| Higher neutrophil count (×109/L) | 0.788 (0.374–1.212) | 0.131 |
| Lower lymphocyte count (×109/L) | −0.620 (−0.830–0.004) | 0.052 |
| Elevated cardiac troponin I, ng/mL | 2.133 (1.067–2.541) | 0.000 |
| High LDH | 0.861 (0.473–1.033) | 0.051 |
| Increased CK (>185 U/L) | 0.412 (0.101–1.001) | 0.330 |
| Increased PCT | 0.023 (0.003–0.063) | 0.228 |
| High serum ferritin level (>300 µg/L) | 1.006 (0.937–1.073) | 0.144 |
| Increased D-dimer (>1 µg/L) | 1.411 (1.226–2.161) | 0.001 |
| IL-6 > 7 ng/L | 0.056 (0.009–0.125) | 0.236 |
| Septicemia | 0.119 (0.106–0.328) | 0.002 |
| ARDS | 1.236 (1.110–1.409) | 0.001 |
| Respiratory failure | 1.070 (0.968–1.114) | 0.165 |
| Acute cardiac injury | 1.339 (0.997–1.581) | 0.065 |
| Hyperglycemia | 1.12 (1.074–1.241) | 0.078 |
| Bilateral distribution, n (%) | −0.050 (−0.089–0.456) | 0.543 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, S.; Rehman, N.; Mumtaz, A.; Jiang, J. Association of Mortality-Related Risk Factors in Patients with COVID-19: A Retrospective Cohort Study. Healthcare 2021, 9, 1468. https://doi.org/10.3390/healthcare9111468
Rehman S, Rehman N, Mumtaz A, Jiang J. Association of Mortality-Related Risk Factors in Patients with COVID-19: A Retrospective Cohort Study. Healthcare. 2021; 9(11):1468. https://doi.org/10.3390/healthcare9111468
Chicago/Turabian StyleRehman, Shazia, Nadia Rehman, Ayesha Mumtaz, and Jindong Jiang. 2021. "Association of Mortality-Related Risk Factors in Patients with COVID-19: A Retrospective Cohort Study" Healthcare 9, no. 11: 1468. https://doi.org/10.3390/healthcare9111468
APA StyleRehman, S., Rehman, N., Mumtaz, A., & Jiang, J. (2021). Association of Mortality-Related Risk Factors in Patients with COVID-19: A Retrospective Cohort Study. Healthcare, 9(11), 1468. https://doi.org/10.3390/healthcare9111468






