Incidence and Risk Factors for Notifiable Typhoid and Paratyphoid in Taiwan during the Period 2011–2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Policy
2.2. Data Source
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basnyat, B.; Qamar, F.N.; Rupali, P.; Ahmed, T.; Parry, C.M. Department of Paediatrics and Child Healt h Division of Woman and Child Health. BMJ 2021, 372, n437. [Google Scholar] [CrossRef]
- Taiwan Centers for Disease Control. Diseases & Conditions—Important Diseases. Available online: https://www.cdc.gov.tw/Disease/SubIndex/wp-kiiztSq_5cFd6H96f9w (accessed on 23 September 2021).
- Keller, A.; Frey, M.; Schmid, H.; Steffen, R.; Walker, T.; Schlagenhauf, P. Imported typhoid fever in Switzerland, 1993 to 2004. J. Travel Med. 2008, 15, 248–251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crump, J.A.; Luby, S.P.; Mintz, E.D. The global burden of typhoid fever. Bull. World Health Organ. 2004, 82, 346–353. [Google Scholar] [PubMed]
- Date, K.A.; Bentsi-Enchill, A.D.; Fox, K.K.; Abeysinghe, N.; Mintz, E.D.; Khan, M.I.; Sahastrabuddhe, S.; Hyde, T.B. Typhoid fever surveillance and vaccine use—South-East Asia and Western Pacific regions, 2009–2013. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 855. [Google Scholar] [PubMed]
- Mogasale, V.; Maskery, B.; Ochiai, R.L.; Lee, J.S.; Mogasale, V.V.; Ramani, E.; Kim, Y.E.; Park, J.K.; Wierzba, T.F. Burden of typhoid fever in low-income and middle-income countries: A systematic, literature-based update with risk-factor adjustment. Lancet Glob. Health 2014, 2, e570–e580. [Google Scholar] [CrossRef]
- Buckle, G.C.; Walker, C.L.F.; Black, R.E. Typhoid fever and paratyphoid fever: Systematic review to estimate global morbidity and mortality for 2010. J. Glob. Health 2012, 2, 010401. [Google Scholar] [CrossRef]
- Syed, K.A.; Saluja, T.; Cho, H.; Hsiao, A.; Shaikh, H.; Wartel, T.A.; Mogasale, V.; Lynch, J.; Kim, J.H.; Excler, J.L.; et al. Review on the Recent Advances on Typhoid Vaccine Development and Challenges Ahead. Clin. Infect. Dis. 2020, 71, S141–S150. [Google Scholar] [CrossRef]
- Sasikumar, C.; Kannan, V.; Senthilkumar, B. Asymptomatic typhoid carriers in Namakkal District, Tamilnadu. J. Environ. Biol. 2005, 26, 113–115. [Google Scholar]
- Verma, R.; Bairwa, M.; Chawla, S.; Prinja, S.; Rajput, M. New generation typhoid vaccines: An effective preventive strategy to control typhoid fever in developing countries. Hum. Vaccines 2011, 7, 883–885. [Google Scholar] [CrossRef][Green Version]
- Parry, C.M.; Hien, T.T.; Dougan, G.; White, N.J.; Farrar, J.J. Typhoid fever. N. Engl. J. Med. 2002, 347, 1770–1782. [Google Scholar] [CrossRef]
- Connor, B.A.; Schwartz, E. Typhoid and paratyphoid fever in travellers. Lancet Infect. Dis. 2005, 5, 623–628. [Google Scholar] [CrossRef]
- Cho, J.H. Successful endoscopic hemoclipping and conservative management for typhoid fever complicated by massive intestinal bleeding and acute pancreatitis: Case report. Medicine 2019, 98, e16521. [Google Scholar] [CrossRef] [PubMed]
- Gilman, R.H.; Islam, S.; Rabbani, H.; Ghosh, H. Identification of gallbladder typhoid carriers by a string device. Lancet 1979, 1, 795–796. [Google Scholar] [CrossRef]
- Taiwan Centers for Disease Control. Typhoid and Paratyphoid Fever. Available online: https://www.cdc.gov.tw/Category/DiseaseManual/bU9xd21vK0l5S3gwb3VUTldqdVNnQT09 (accessed on 23 September 2021).
- Hsieh, C.J.; Li, C.W.; Cheng, C.A.; Wu, D.C.; Wu, W.C.; Lin, F.H.; Chou, Y.C.; Yu, C.P. Epidemiologic Characteristics of Domestic Patients with Hemorrhagic Fever with Renal Syndrome in Taiwan: A 19-Year Retrospective Study. Int. J. Environ. Res. Public Health 2020, 17, 5291. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Hsieh, C.J.; Cheng, C.A.; Wu, D.C.; Wu, W.C.; Lin, F.H.; Yu, C.P. Epidemiologic Characteristics of Imported and Domestic Chikungunya Cases in Taiwan: A 13-Year Retrospective Study. Int. J. Environ. Res. Public Health 2020, 17, 3615. [Google Scholar] [CrossRef] [PubMed]
- Taiwan Centers for Disease control. Taiwan N. National Infectious Disease Statistics System. Available online: https://nidss.cdc.gov.tw/nndss/disease?id=0701 (accessed on 23 September 2021).
- GBD 2017 Typhoid and Paratyphoid Collaborators. The global burden of typhoid and paratyphoid fevers: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 369–381. [Google Scholar] [CrossRef]
- Das, J.K.; Hasan, R.; Zafar, A.; Ahmed, I.; Ikram, A.; Nizamuddin, S.; Fatima, S.; Akbar, N.; Sultan, F.; Bhutta, Z.A. Trends, Associations, and Antimicrobial Resistance of Salmonella Typhi and Paratyphi in Pakistan. Am. J. Trop. Med. Hyg 2018, 99, 48–54. [Google Scholar] [CrossRef]
- Reller, M.E.; Tauxe, R.V.; Kalish, L.A.; Mølbak, K. Excess salmonellosis in women in the United States: 1968–2000. Epidemiol. Infect. 2008, 136, 1109–1117. [Google Scholar] [CrossRef]
- Wagner, A.L.; Mubarak, M.Y.; Johnson, L.E.; Porth, J.M.; Yousif, J.E.; Boulton, M.L. Trends of vaccine-preventable diseases in Afghanistan from the Disease Early Warning System, 2009–2015. PLoS ONE 2017, 12, e0178677. [Google Scholar] [CrossRef]
- Dávalos-Becerril, E.; Correa-Morales, F.; González-Acosta, C.; Santos-Luna, R.; Peralta-Rodríguez, J.; Pérez-Rentería, C.; Ordoñez-Álvarez, J.; Huerta, H.; Carmona-Perez, M.; Díaz-Quiñonez, J.A. Urban and semi-urban mosquitoes of Mexico City: A risk for endemic mosquito-borne disease transmission. PLoS ONE 2019, 14, e0212987. [Google Scholar] [CrossRef]
- World Meteorogical Organization. 2019 Concludes a Decade of Exceptional Global Heat and High-Impact Weather. Available online: https://public.wmo.int/en/media/press-release/2019-concludes-decade-of-exceptional-global-heat-and-high-impact-weather (accessed on 23 September 2021).
- Taiwan Central Weather Bureau. Taiwan’s Average Temperature Reached a New High in 2019. Available online: https://www.cwb.gov.tw/Data/service/Newsbb/CH/20191231press.pdf (accessed on 23 September 2021).
- De Miguel Buckley, R.; Trigo, E.; de la Calle-Prieto, F.; Arsuaga, M.; Díaz-Menéndez, M. Social distancing to combat COVID-19 led to a marked decrease in food-borne infections and sexually transmitted diseases in Spain. J. Travel Med. 2020, 27. [Google Scholar] [CrossRef]
- Zhu, H.; Wei, L.; Niu, P. The novel coronavirus outbreak in Wuhan, China. Glob. Health Res. Policy 2020, 5, 1–3. [Google Scholar] [CrossRef]
- Seimenis, A.; Battelli, G. Main challenges in the control of zoonoses and related foodborne diseases in the South Mediterranean and Middle East region. Vet. Ital. 2018, 54, 97–106. [Google Scholar] [PubMed]
- Hirata, K.; Ogawa, T.; Fujikura, H.; Ogawa, Y.; Hirai, N.; Nakagawa-Onishi, T.; Uno, K.; Takeyama, M.; Kasahara, K.; Nakamura-Uchiyama, F.; et al. Characteristics of health problems in returned overseas travelers at a tertiary teaching hospital in a suburban area in Japan. J. Infect. Chemother. 2018, 24, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Antillón, M.; Warren, J.L.; Crawford, F.W.; Weinberger, D.M.; Kürüm, E.; Pak, G.D.; Marks, F.; Pitzer, V.E. The burden of typhoid fever in low- and middle-income countries: A meta-regression approach. PLoS Negl. Trop. Dis. 2017, 11, e0005376. [Google Scholar] [CrossRef]
- Marchello, C.S.; Hong, C.Y.; Crump, J.A. Global Typhoid Fever Incidence: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2019, 68, S105–S116. [Google Scholar] [CrossRef] [PubMed]
- Feasey, N.A.; Masesa, C.; Jassi, C.; Faragher, E.B.; Mallewa, J.; Mallewa, M.; MacLennan, C.A.; Msefula, C.; Heyderman, R.S.; Gordon, M.A. Three Epidemics of Invasive Multidrug-Resistant Salmonella Bloodstream Infection in Blantyre, Malawi, 1998–2014. Clin. Infect. Dis. 2015, 61, S363–S371. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Hatib, N.A.; Chong, C.Y.; Thoon, K.C.; Tee, N.W.; Krishnamoorthy, S.S.; Tan, N.W. Enteric Fever in a Tertiary Paediatric Hospital: A Retrospective Six-Year Review. Ann. Acad. Med. Singap. 2016, 45, 297–302. [Google Scholar]
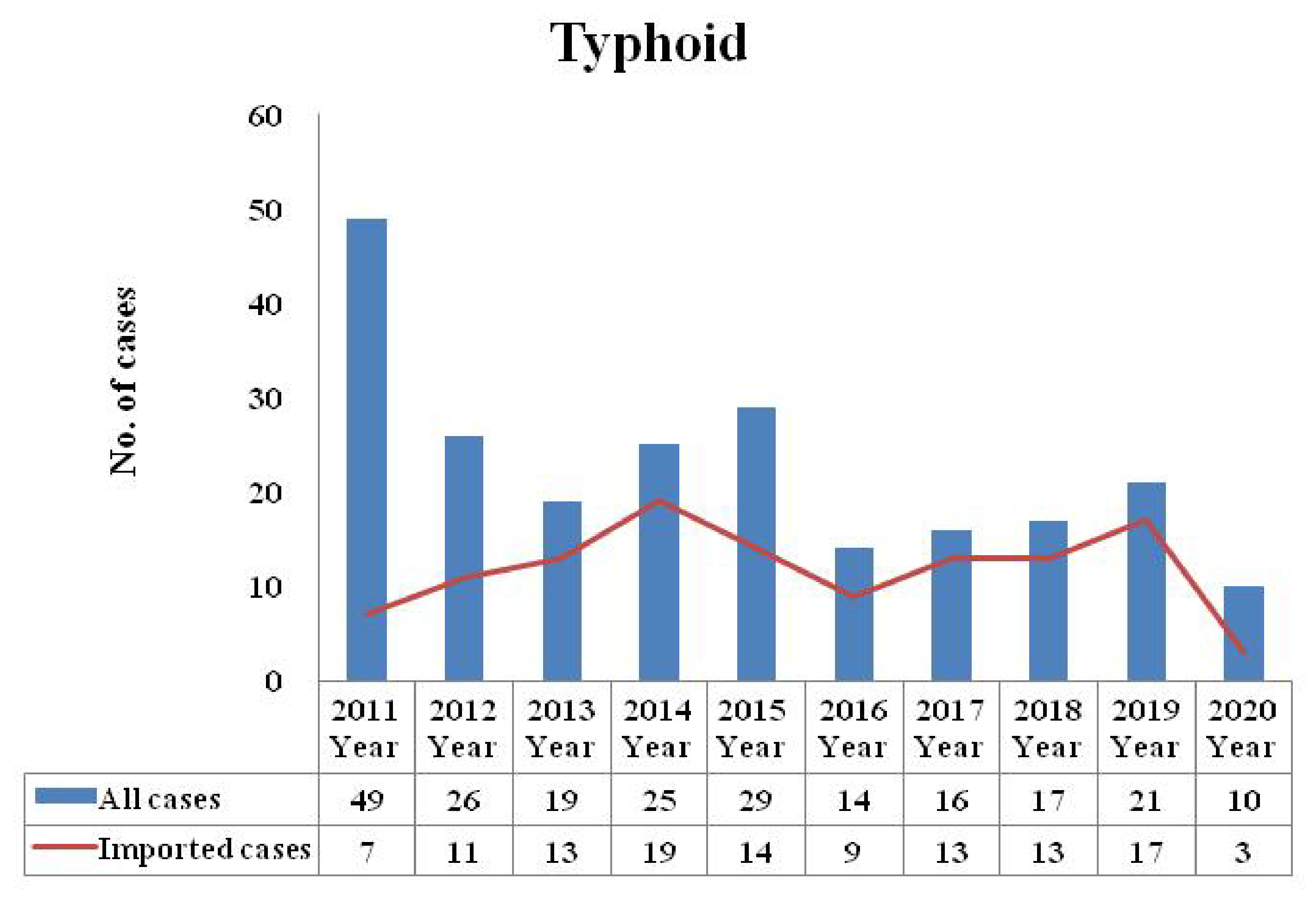
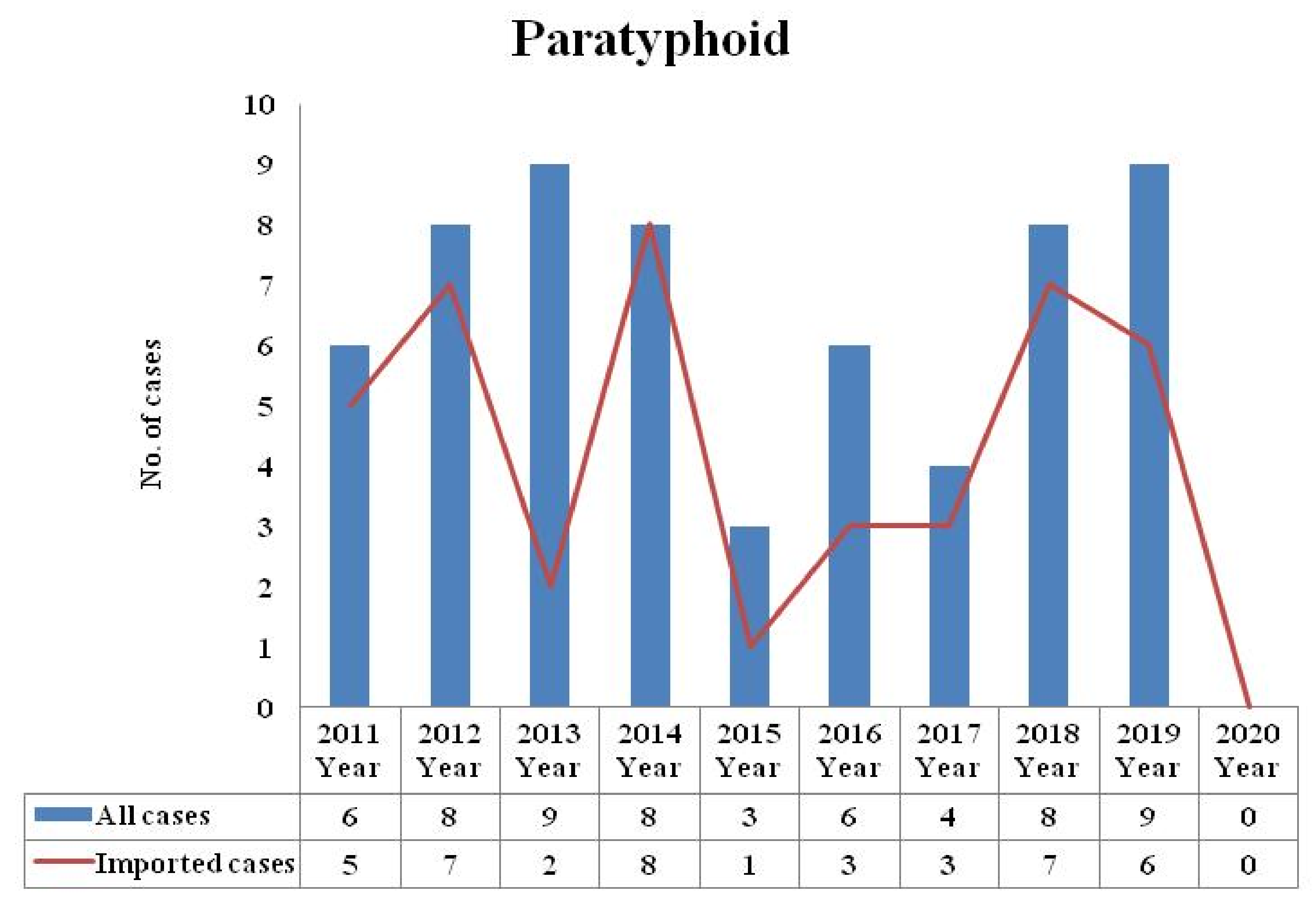
| Variables | All Cases | Domestic Cases | Imported Cases | |||
|---|---|---|---|---|---|---|
| n = 226 | % | n = 107 | % | n = 119 | % | |
| Sex | ||||||
| Male | 94 | 41.6 | 48 | 44.9 | 46 | 38.6 |
| Female | 132 | 58.4 | 59 | 55.1 | 73 | 61.4 |
| Age group | ||||||
| <20 | 46 | 20.3 | 24 | 22.4 | 22 | 18.5 |
| 20–39 | 123 | 54.4 | 47 | 43.9 | 76 | 63.9 |
| 40–59 | 32 | 14.2 | 15 | 14.0 | 17 | 14.3 |
| ≥60 | 25 | 11.1 | 21 | 19.6 | 4 | 3.3 |
| Year group | ||||||
| 2011–2015 | 148 | 65.5 | 84 | 78.5 | 64 | 53.8 |
| 2016–2020 | 78 | 34.5 | 23 | 21.5 | 55 | 46.2 |
| Season | ||||||
| Spring | 51 | 22.6 | 23 | 21.5 | 28 | 23.5 |
| Summer | 51 | 22.6 | 19 | 17.8 | 32 | 26.9 |
| Fall | 64 | 28.3 | 41 | 38.3 | 23 | 19.3 |
| Winter | 60 | 26.5 | 24 | 22.4 | 36 | 30.3 |
| Residency | ||||||
| Northern | 155 | 68.6 | 76 | 71.0 | 79 | 66.4 |
| Central | 27 | 11.9 | 10 | 9.3 | 17 | 14.3 |
| Southern | 42 | 18.6 | 20 | 18.7 | 22 | 18.5 |
| Eastern | 2 | 0.9 | 1 | 1.0 | 1 | 0.8 |
| Variables | All Cases | Domestic Cases | Imported Cases | |||
|---|---|---|---|---|---|---|
| n = 61 | % | n = 19 | % | n = 42 | % | |
| Sex | ||||||
| Male | 22 | 36.1 | 1 | 5.3 | 21 | 50 |
| Female | 39 | 63.9 | 18 | 94.7 | 21 | 50 |
| Age group | ||||||
| <20 | 4 | 6.6 | 0 | 0 | 4 | 9.5 |
| 20–39 | 36 | 59.0 | 10 | 52.6 | 26 | 61.9 |
| 40–59 | 16 | 26.2 | 6 | 31.6 | 10 | 23.8 |
| ≥60 | 5 | 8.2 | 3 | 15.8 | 2 | 4.8 |
| Year group | ||||||
| 2011–2015 | 34 | 55.7 | 11 | 57.9 | 23 | 54.8 |
| 2016–2020 | 27 | 44.3 | 8 | 42.1 | 19 | 45.2 |
| Season | ||||||
| Spring | 22 | 36.1 | 8 | 42.1 | 14 | 33.3 |
| Summer | 20 | 32.8 | 7 | 36.8 | 13 | 31.0 |
| Fall | 10 | 16.4 | 2 | 10.5 | 8 | 19.0 |
| Winter | 9 | 14.7 | 2 | 10.5 | 7 | 16.7 |
| Residency | ||||||
| Northern | 39 | 63.9 | 12 | 63.2 | 27 | 64.3 |
| Central | 15 | 24.6 | 5 | 26.3 | 10 | 23.8 |
| Southern | 7 | 11.5 | 2 | 10.5 | 5 | 11.9 |
| Eastern | 0 | 0 | 0 | 0 | 0 | 0 |
| Variables | Typhoid Cases | Paratyphoid Cases | p | ||
|---|---|---|---|---|---|
| n = 226 | % | n = 61 | % | ||
| Sex | |||||
| Male | 94 | 41.6 | 22 | 36.1 | 0.435 |
| Female | 132 | 58.4 | 39 | 63.9 | |
| Age group | |||||
| <20 | 46 | 20.3 | 4 | 6.6 | 0.019 |
| 20–39 | 123 | 54.4 | 36 | 59.0 | |
| 40–59 | 32 | 14.2 | 16 | 26.2 | |
| ≥60 | 25 | 11.1 | 5 | 8.2 | |
| Year group | |||||
| 2011–2015 | 148 | 65.5 | 34 | 55.7 | 0.161 |
| 2016–2020 | 78 | 34.5 | 27 | 44.3 | |
| Season | |||||
| Spring | 51 | 22.6 | 22 | 36.1 | 0.012 |
| Summer | 51 | 22.6 | 20 | 32.8 | |
| Fall | 64 | 28.3 | 10 | 16.4 | |
| Winter | 60 | 26.5 | 9 | 14.7 | |
| Residency | |||||
| Northern | 155 | 68.6 | 39 | 63.9 | 0.061 |
| Central | 27 | 11.9 | 15 | 24.6 | |
| Southern | 42 | 18.6 | 7 | 11.5 | |
| Eastern | 2 | 0.9 | 0 | 0 | |
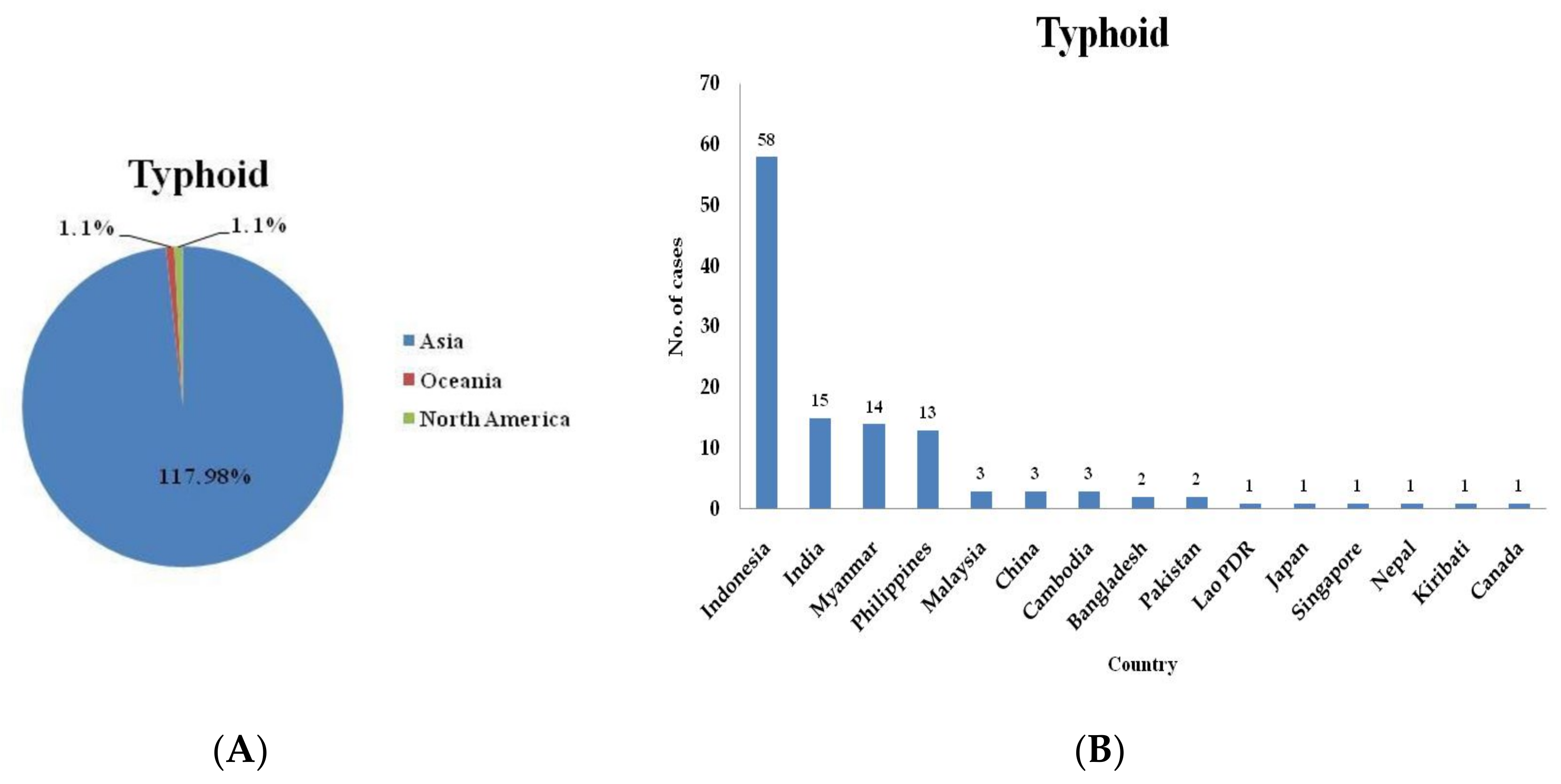
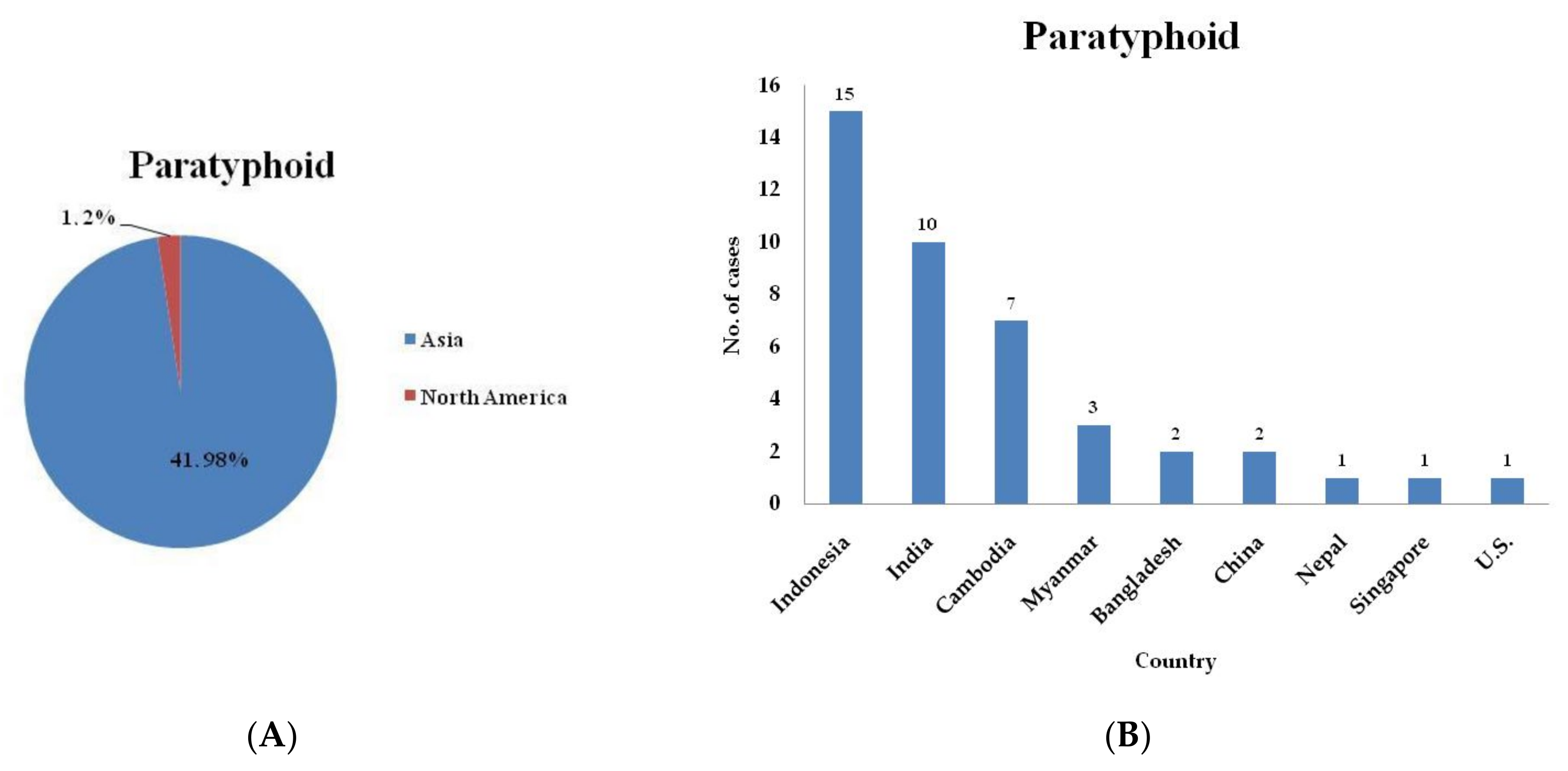
| Country of Destination | No. Cases (%) | No. of Air Passengers (100,000) | RR |
|---|---|---|---|
| Typhoid | |||
| China | 3 | 271.82 | Reference |
| Indonesia | 58 | 17.27 | 304.30 |
| India | 15 | 2.87 | 473.55 |
| Myanmar | 14 | 0.94 | 1349.46 |
| Philippines | 13 | 20.52 | 57.40 |
| Malaysia | 3 | 40.53 | 6.71 |
| Cambodia | 3 | 0.51 | 532.98 |
| Paratyphoid | |||
| China | 2 | 271.82 | Reference |
| Indonesia | 15 | 17.27 | 118.05 |
| India | 10 | 2.87 | 473.55 |
| Cambodia | 7 | 0.51 | 1865.43 |
| Myanmar | 3 | 0.94 | 433.76 |
| Bangladesh | 2 | 0.10 | 2718.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, F.-H.; Chen, B.-C.; Chou, Y.-C.; Hsieh, C.-J.; Yu, C.-P. Incidence and Risk Factors for Notifiable Typhoid and Paratyphoid in Taiwan during the Period 2011–2020. Healthcare 2021, 9, 1316. https://doi.org/10.3390/healthcare9101316
Lin F-H, Chen B-C, Chou Y-C, Hsieh C-J, Yu C-P. Incidence and Risk Factors for Notifiable Typhoid and Paratyphoid in Taiwan during the Period 2011–2020. Healthcare. 2021; 9(10):1316. https://doi.org/10.3390/healthcare9101316
Chicago/Turabian StyleLin, Fu-Huang, Bao-Chung Chen, Yu-Ching Chou, Chi-Jeng Hsieh, and Chia-Peng Yu. 2021. "Incidence and Risk Factors for Notifiable Typhoid and Paratyphoid in Taiwan during the Period 2011–2020" Healthcare 9, no. 10: 1316. https://doi.org/10.3390/healthcare9101316
APA StyleLin, F.-H., Chen, B.-C., Chou, Y.-C., Hsieh, C.-J., & Yu, C.-P. (2021). Incidence and Risk Factors for Notifiable Typhoid and Paratyphoid in Taiwan during the Period 2011–2020. Healthcare, 9(10), 1316. https://doi.org/10.3390/healthcare9101316






