Cognitive Reserve Characteristics and Occupational Performance Implications in People with Mild Cognitive Impairment
Abstract
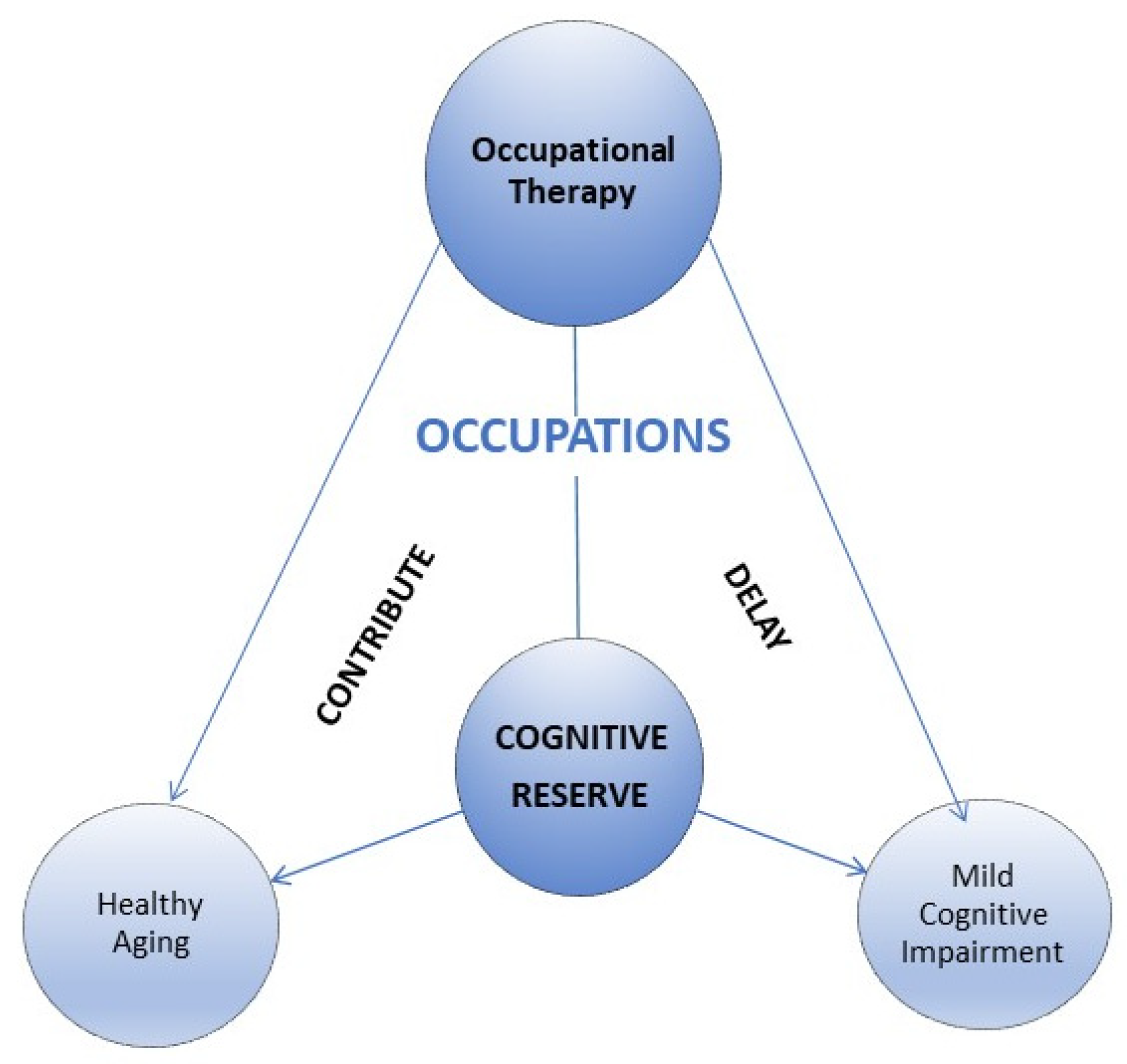
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethical Considerations
2.3. Study Tools
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alzheimer’s Disease International; Wimo, A.; Ali, G.-C.; Guerchet, M.; Prince, M.; Prina, M.; Wu, Y.-T. World Alzheimer Report 2015: The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- INE. Instituto Nacional de Estadística. Available online: https://www.ine.es/ (accessed on 26 July 2021).
- Kang, J.M.; Cho, Y.-S.; Park, S.; Lee, B.H.; Sohn, B.K.; Choi, C.H.; Choi, J.-S.; Jeong, H.Y.; Cho, S.-J.; Lee, J.-H.; et al. Montreal Cognitive Assessment Reflects Cognitive Reserve. BMC Geriatr. 2018, 18, 261. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Knopman, D.S.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Roberts, R.O.; Jack, C.R. Mild Cognitive Impairment: Ten Years Later. Arch. Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R.C. Mild Cognitive Impairment as a Diagnostic Entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y. Cognitive Reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Colangeli, S.; Boccia, M.; Verde, P.; Guariglia, P.; Bianchini, F.; Piccardi, L. Cognitive Reserve in Healthy Aging and Alzheimer’s Disease: A Meta-Analysis of FMRI Studies. Am. J. Alzheimers Dis. Other Demen. 2016, 31, 443–449. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y. Cognitive Reserve and Lifestyle. J. Clin. Exp. Neuropsychol. 2003, 25, 625–633. [Google Scholar] [CrossRef]
- Stern, Y. Cognitive Reserve: Implications for Assessment and Intervention. Folia Phoniatr. Logop. Off. Organ Int. Assoc. Logop. Phoniatr. IALP 2013, 65, 49–54. [Google Scholar] [CrossRef] [Green Version]
- Wilcock, A.A.; Chelin, M.; Hall, M.; Hamley, N.; Morrison, B.; Scrivener, L.; Townsend, M.; Treen, K. The Relationship between Occupational Balance and Health: A Pilot Study. Occup. Ther. Int. 1997, 4, 17–30. [Google Scholar] [CrossRef]
- Anaby, D.R.; Backman, C.L.; Jarus, T. Measuring Occupational Balance: A Theoretical Exploration of Two Approaches. Can. J. Occup. Ther. 2010, 77, 280–288. [Google Scholar] [CrossRef]
- Hammell, K.W. Opportunities for Well-Being: The Right to Occupational Engagement. Can. J. Occup. Ther. 2017, 84, E1–E14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallahpour, M.; Borell, L.; Luborsky, M.; Nygård, L. Leisure-Activity Participation to Prevent Later-Life Cognitive Decline: A Systematic Review. Scand. J. Occup. Ther. 2016, 23, 162–197. [Google Scholar] [CrossRef] [PubMed]
- Hammell, K.W. Dimensions of Meaning in the Occupations of Daily Life. Can. J. Occup. Ther. 2004, 71, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Hammell, K.W. Self-Care, Productivity, and Leisure, or Dimensions of Occupational Experience? Rethinking Occupational “Categories”. Can. J. Occup. Ther. 2009, 76, 107–114. [Google Scholar] [CrossRef]
- Clare, L.; Wu, Y.-T.; Teale, J.C.; MacLeod, C.; Matthews, F.; Brayne, C.; Woods, B.; CFAS-Wales Study Team. Potentially Modifiable Lifestyle Factors, Cognitive Reserve, and Cognitive Function in Later Life: A Cross-Sectional Study. PLoS Med. 2017, 14, e1002259. [Google Scholar] [CrossRef]
- Wilson, R.S.; Yu, L.; Lamar, M.; Schneider, J.A.; Boyle, P.A.; Bennett, D.A. Education and Cognitive Reserve in Old Age. Neurology 2019, 92, e1041–e1050. [Google Scholar] [CrossRef] [PubMed]
- Scarmeas, N.; Zarahn, E.; Anderson, K.E.; Habeck, C.G.; Hilton, J.; Flynn, J.; Marder, K.S.; Bell, K.L.; Sackeim, H.A.; Van Heertum, R.L.; et al. Association of Life Activities with Cerebral Blood Flow in Alzheimer Disease: Implications for the Cognitive Reserve Hypothesis. Arch. Neurol. 2003, 60, 359–365. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y. Cognitive Reserve: Implications for Diagnosis and Prevention of Alzheimer’s Disease. Curr. Neurol. Neurosci. Rep. 2004, 4, 374–380. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Li, C.; Wang, A.; Qi, Y.; Feng, W.; Hou, C.; Tao, L.; Liu, X.; Li, X.; Wang, W.; et al. Associations between Social and Intellectual Activities with Cognitive Trajectories in Chinese Middle-Aged and Older Adults: A Nationally Representative Cohort Study. Alzheimers Res. Ther. 2020, 12, 115. [Google Scholar] [CrossRef]
- Park, S.; Kwon, E.; Lee, H. Life Course Trajectories of Later-Life Cognitive Functions: Does Social Engagement in Old Age Matter? Int. J. Environ. Res. Public Health 2017, 14, 393. [Google Scholar] [CrossRef] [Green Version]
- Steffener, J.; Stern, Y. Exploring the Neural Basis of Cognitive Reserve in Aging. Biochim. Biophys. Acta 2012, 1822, 467–473. [Google Scholar] [CrossRef] [Green Version]
- Barulli, D.; Stern, Y. Efficiency, Capacity, Compensation, Maintenance, Plasticity: Emerging Concepts in Cognitive Reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Stern, Y.; Arenaza-Urquijo, E.M.; Bartrés-Faz, D.; Belleville, S.; Cantilon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and Investigating Cognitive Reserve, Brain Reserve, and Brain Maintenance. Alzheimers Dement. J. Alzheimers Assoc. 2020, 16, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Kartschmit, N.; Mikolajczyk, R.; Schubert, T.; Lacruz, M.E. Measuring Cognitive Reserve (CR)—A Systematic Review of Measurement Properties of CR Questionnaires for the Adult Population. PLoS ONE 2019, 14, e0219851. [Google Scholar] [CrossRef]
- Nucci, M.; Mapelli, D.; Mondini, S. Cognitive Reserve Index Questionnaire (CRIq): A New Instrument for Measuring Cognitive Reserve. Aging Clin. Exp. Res. 2012, 24, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Mourao, R.J.; Mansur, G.; Malloy-Diniz, L.F.; Castro Costa, E.; Diniz, B.S. Depressive Symptoms Increase the Risk of Progression to Dementia in Subjects with Mild Cognitive Impairment: Systematic Review and Meta-Analysis. Int. J. Geriatr. Psychiatry 2016, 31, 905–911. [Google Scholar] [CrossRef]
- Lara, E.; Martín-María, N.; Miret, M.; Olaya, B.; Haro, J.M.; Ayuso-Mateos, J.L. Is There a Combined Effect of Depression and Cognitive Reserve on Cognitive Function? Findings from a Population-Based Study. Psychol. Health 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.S.; Kanellopoulos, D.; Manning, K.J.; Alexopoulos, G.S. Diagnosis and Treatment of Depression and Cognitive Impairment in Late Life. Ann. N. Y. Acad. Sci. 2015, 1345, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Yesavage, J.A. Geriatric Depression Scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar]
- van Loenhoud, A.C.; van der Flier, W.M.; Wink, A.M.; Dicks, E.; Groot, C.; Twisk, J.; Barkhof, F.; Scheltens, P.; Ossenkoppele, R.; for the Alzheimer’s Disease Neuroimaging Initiative. Cognitive Reserve and Clinical Progression in Alzheimer Disease: A Paradoxical Relationship. Neurology 2019, 93, e334–e346. [Google Scholar] [CrossRef] [Green Version]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The Global Deterioration Scale for Assessment of Primary Degenerative Dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef] [Green Version]
- Berezuk, C.; Zakzanis, K.K.; Ramirez, J.; Ruocco, A.C.; Edwards, J.D.; Callahan, B.L.; Black, S.E. Alzheimer’s Disease Neuroimaging Initiative Functional Reserve: Experience Participating in Instrumental Activities of Daily Living Is Associated with Gender and Functional Independence in Mild Cognitive Impairment. J. Alzheimers Dis. JAD 2017, 58, 425–434. [Google Scholar] [CrossRef]
- Mielke, M.M.; Vemuri, P.; Rocca, W.A. Clinical Epidemiology of Alzheimer’s Disease: Assessing Sex and Gender Differences. Clin. Epidemiol. 2014, 6, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Kim, M.J.; Kim, S.; Kang, H.S.; Lim, S.W.; Myung, W.; Lee, Y.; Hong, C.H.; Choi, S.H.; Na, D.L.; et al. Gender Differences in Risk Factors for Transition from Mild Cognitive Impairment to Alzheimer’s Disease: A CREDOS Study. Compr. Psychiatry 2015, 62, 114–122. [Google Scholar] [CrossRef]
- Proust-Lima, C.; Amieva, H.; Letenneur, L.; Orgogozo, J.-M.; Jacqmin-Gadda, H.; Dartigues, J.-F. Gender and Education Impact on Brain Aging: A General Cognitive Factor Approach. Psychol. Aging 2008, 23. [Google Scholar] [CrossRef] [Green Version]
- Hebert, L.E.; Weuve, J.; Scherr, P.A.; Evans, D.A. Alzheimer Disease in the United States (2010–2050) Estimated Using the 2010 Census. Neurology 2013, 80, 1778–1783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, S.L.; Hu, T.; Fava, N.M.; Li, T.; Rodriguez, M.J.; Schuldiner, K.L.; Burgess, A.; Laird, A. Sex Differences in the Development of Mild Cognitive Impairment and Probable Alzheimer’s Disease as Predicted by Hippocampal Volume or White Matter Hyperintensities. J. Women Aging 2019, 31, 140–164. [Google Scholar] [CrossRef] [PubMed]
- Au, B.; Dale-McGrath, S.; Tierney, M.C. Sex Differences in the Prevalence and Incidence of Mild Cognitive Impairment: A Meta-Analysis. Ageing Res. Rev. 2017, 35, 176–199. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, L.D.; Peters, M.E.; Lyketsos, C.G.; Marano, C.M. Depression in Cognitive Impairment. Curr. Psychiatry Rep. 2013, 15, 384. [Google Scholar] [CrossRef] [Green Version]
- Liew, T.M. Subjective Cognitive Decline, Anxiety Symptoms, and the Risk of Mild Cognitive Impairment and Dementia. Alzheimers Res. Ther. 2020, 12, 107. [Google Scholar] [CrossRef]
- Haaksma, M.L.; Vilela, L.R.; Marengoni, A.; Calderón-Larrañaga, A.; Leoutsakos, J.-M.S.; Olde Rikkert, M.G.M.; Melis, R.J.F. Comorbidity and Progression of Late Onset Alzheimer’s Disease: A Systematic Review. PLoS ONE 2017, 12, e0177044. [Google Scholar] [CrossRef] [Green Version]
- Sundermann, E.E.; Katz, M.J.; Lipton, R.B. Sex Differences in the Relationship between Depressive Symptoms and Risk of Amnestic Mild Cognitive Impairment. Am. J. Geriatr. Psychiatry 2017, 25, 13–22. [Google Scholar] [CrossRef] [Green Version]
- LaMonica, H.M.; Biddle, D.J.; Naismith, S.L.; Hickie, I.B.; Maruff, P.; Glozier, N. The Relationship between Depression and Cognitive Function in Adults with Cardiovascular Risk: Evidence from a Randomised Attention-Controlled Trial. PLoS ONE 2018, 13, e0203343. [Google Scholar] [CrossRef] [PubMed]
- Noale, M.; Limongi, F.; Zambon, S.; Crepaldi, G.; Maggi, S.; ILSA Working Group. Incidence of Dementia: Evidence for an Effect Modification by Gender. The ILSA Study. Int. Psychogeriatr. 2013, 25, 1867–1876. [Google Scholar] [CrossRef]
- Gabryelewicz, T.; Styczynska, M.; Luczywek, E.; Barczak, A.; Pfeffer, A.; Androsiuk, W.; Chodakowska-Zebrowska, M.; Wasiak, B.; Peplonska, B.; Barcikowska, M. The Rate of Conversion of Mild Cognitive Impairment to Dementia: Predictive Role of Depression. Int. J. Geriatr. Psychiatry 2007, 22, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Occupational Therapy Practice Framework: Domain and Process—Fourth Edition. Am. J. Occup. Ther. 2020, 74, 7412410010p1–7412410010p87. [CrossRef]
- Townsend, E.; Wilcock, A.A. Occupational Justice and Client-Centred Practice: A Dialogue in Progress. Can. J. Occup. Ther. Rev. Can. Ergother. 2004, 71, 75–87. [Google Scholar] [CrossRef] [PubMed]

| Variable | Male (n = 56) | Female (n = 69) | Total (n = 125) | p-Value |
|---|---|---|---|---|
| Age, mean ± SD | 74.04 ± 7.42 | 74.43 ± 5.98 | 74.26 ± 6.64 | ns |
| Age group, n (%) | ||||
| 60–74 | 22 (39.3) | 33 (47.8) | 55 (44.0) | |
| 75–90 | 34 (60.7) | 36 (52.2) | 70 (56.0) | |
| Study level, n (%) | ||||
| No study | 2 (3.6) | 2 (2.9) | 4 (3.2) | |
| Basic | 28 (50.0) | 39 (56.5) | 67 (53.6) | |
| Primary | 11 (19.6) | 17 (24.6) | 28 (22.4) | |
| Secondary | 7 (12.5) | 4 (5.8) | 11 (8.8) | |
| University | 8 (14.3) | 7 (10.1) | 15 (12.0) | |
| Marital status, n (%) | ||||
| Single | 1 (1.8) | 4 (5.8) | 5 (4.0) | |
| Married | 51 (91.1) | 43 (62.3) | 94 (75.2) | |
| Widowed | 4 (7.1) | 19 (27.5) | 23 (18.4) | |
| Divorced | - | 3 (4.3) | 3 (2.4) | |
| Living status, n (%) | ||||
| Alone | 3 (5.4) | 14 (20.3) | 17 (13.6) | |
| Couple | 49 (87.5) | 43 (62.3) | 92 (73.6) | |
| Daughter/son | - | 3 (4.3) | 3 (2.4) | |
| Professional care | 1 (1.8) | 2 (2.9) | 3 (2.4) | |
| Relatives | 3 (5.4) | 7 (10.1) | 10 (8.0) | |
| Location, n (%) | ||||
| Urban | 28 (50.0) | 33 (47.8) | 61 (48.8) | |
| Intermediate | 15 (26.8) | 20 (29.0) | 35 (28.0) | |
| Rural | 13 (23.2) | 16 (23.2) | 29 (23.2) | |
| GDS 1, n (%) | ||||
| 3 | 40 (71.5) | 48 (69.6) | 88 (70.4) | |
| 4 | 15 (26.8) | 20 (28.9) | 35 (28.0) | |
| 5 | 1 (1.8) | 1 (1.4) | 2 (1.6) | |
| Type of MCI 2 | ||||
| MCI | 14 (25.0) | 21(30.4) | 35 (28.0) | |
| MCI mixed | 3 (5.4) | 6 (8.7) | 9 (7.2) | |
| MCI vascular | 2 (3.6) | 3 (4.3) | 5 (4.0) | |
| MCI amnesic /probable AD 3 | 16 (28.6) | 19 (27.5) | 35 (28.0) | |
| Others | 10 (17.8) | 4 (5.7) | 14 (11.2) | |
| Unknown | 11(19.6) | 16 (23.2) | 27 (21.6) | |
| Geriatric depression Scale, mean ± SD | 4.04 ± 3.24 | 4.93 ± 3.07 | 4.53 ± 3.16 | ns |
| Geriatric depression Scale group, n (%) | ||||
| No depression | 39 (69.6) | 37 (53.6) | 76 (60.8) | |
| Depression probably | 12 (21.4) | 27 (39.1) | 39 (31.2) | |
| Established depression | 5 (8.9) | 5 (7.2) | 10 (8.0) | |
| MoCA 4, mean ± SD | 18.96 ± 4.8 | 16.81 ± 5.0 | 17.78 ± 5.01 | 0.016 |
| CRIq 5, mean ± SD | ||||
| CRI Index | 99.1 ± 19.4 | 88.3 ± 17.0 | 93.2 ± 18.9 | 0.001 |
| CRI-Education | 101.7 ± 19.7 | 98.6 ± 16.2 | 100.0 ± 17.9 | ns |
| CRI-WorkingActivity | 104.1 ± 17.9 | 86.6 ± 16.4 | 94.5 ± 19.1 | <0.001 |
| CRI-LeisureTime | 92.6 ± 21.1 | 88.5 ± 21.0 | 90.3 ± 21.0 | ns |
| Male (n = 56) | Female (n = 69) | |||||
|---|---|---|---|---|---|---|
| Variable | Basic | Primary | Higher | Basic | Primary | Higher |
| Age year | ||||||
| 60–74 | ||||||
| N | 8 | 7 | 7 | 18 | 9 | 6 |
| Mean | 19.75 | 19.14 | 21.00 | 16.00 | 17.67 | 17.50 |
| SD | 5.17 | 7.08 | 5.38 | 4.899 | 5.657 | 6.80 |
| 75–90 | ||||||
| N | 22 | 4 | 8 | 23 | 8 | 5 |
| Mean | 17.32 | 20.00 | 20.25 | 15.78 | 18.63 | 19.20 |
| SD | 3.63 | 5.03 | 4.528 | 4.85 | 4.17 | 4.02 |
| Variable | Age | Gender | Level of Education |
|---|---|---|---|
| MoCA 1 | r = −0.139 | r = −0.215 | r = 0.290 |
| p-value | p = 0.123 | p = 0.016 | p = 0.001 |
| Variable | CRIq 2 | CRI-Education | CRI-WorkingActivity | CRI-LeisureTime |
|---|---|---|---|---|
| MoCA 1 | r = 0.385 | r = 0.231 | r = 0.237 | r = 0.319 |
| 95% Confidence Interval | 0.21–0.53 | 0.06–0.39 | 0.06–0.40 | 0.15–0.47 |
| p-value | p < 0.001 | p = 0.010 | p = 0.008 | p < 0.001 |
| CRIq 2 | CRI-Education | CRI-WorkingActivity | CRI-LeisureTime | |
|---|---|---|---|---|
| MoCA 1 | r = 0.319 | r = 0.254 | r = 0.126 | r = 0.298 |
| p-value | p < 0.001 | p = 0.005 | p = 0.165 | p = 0.001 |
| Dependent Variable | Independent Variable | B | Standard Error | T | p-Value |
|---|---|---|---|---|---|
| MoCA 1 | CRIq 2 | 0.277 | 0.215 | 1.288 | 0.200 |
| CRI 3-Education | −0.076 | 0.098 | −0.778 | 0.438 | |
| CRI 3-WorkingActivity | −0.100 | 0.096 | −1.045 | 0.298 | |
| CRI 3-LeisureTime | −0.058 | 0.096 | −0.609 | 0.544 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Holgado, C.; Lavado-García, J.; López-Espuela, F.; Roncero-Martín, R.; Canal-Macías, M.L.; Vera, V.; Aliaga, I.; Rey-Sánchez, P.; Pedrera-Zamorano, J.D.; Moran, J.M. Cognitive Reserve Characteristics and Occupational Performance Implications in People with Mild Cognitive Impairment. Healthcare 2021, 9, 1266. https://doi.org/10.3390/healthcare9101266
Mendoza-Holgado C, Lavado-García J, López-Espuela F, Roncero-Martín R, Canal-Macías ML, Vera V, Aliaga I, Rey-Sánchez P, Pedrera-Zamorano JD, Moran JM. Cognitive Reserve Characteristics and Occupational Performance Implications in People with Mild Cognitive Impairment. Healthcare. 2021; 9(10):1266. https://doi.org/10.3390/healthcare9101266
Chicago/Turabian StyleMendoza-Holgado, Cristina, Jesús Lavado-García, Fidel López-Espuela, Raúl Roncero-Martín, María Luz Canal-Macías, Vicente Vera, Ignacio Aliaga, Purificación Rey-Sánchez, Juan Diego Pedrera-Zamorano, and Jose M. Moran. 2021. "Cognitive Reserve Characteristics and Occupational Performance Implications in People with Mild Cognitive Impairment" Healthcare 9, no. 10: 1266. https://doi.org/10.3390/healthcare9101266
APA StyleMendoza-Holgado, C., Lavado-García, J., López-Espuela, F., Roncero-Martín, R., Canal-Macías, M. L., Vera, V., Aliaga, I., Rey-Sánchez, P., Pedrera-Zamorano, J. D., & Moran, J. M. (2021). Cognitive Reserve Characteristics and Occupational Performance Implications in People with Mild Cognitive Impairment. Healthcare, 9(10), 1266. https://doi.org/10.3390/healthcare9101266








