Continuum of Care UNAIDS Fast-Track Targets Evaluation of Patients Living with Human Immunodeficiency Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Procedures, Study Population, Outcomes, and Other Variables
- (1)
- regardless of age and gender;
- (2)
- enrolled in care at the Cluj-Napoca AIDS Center, Romania in December 2016 and, respectively, December 2020;
- (3)
- with domicile in Cluj County;
- (4)
- and having complete clinical routine data regarding diagnosis, ART(s) regimen, and/or viral load.
2.3. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gayle, H.D.; Hill, G.L. Global impact of human immunodeficiency virus and AIDS. Clin. Microbiol. Rev. 2001, 14, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borre, E.D.; Hyle, E.P.; Paltiel, A.D.; Neilan, A.M.; Sax, P.E.; Freedberg, K.A.; Weinstein, M.C.; Walensky, R.P. The Clinical and Economic Impact of Attaining National HIV/AIDS Strategy Treatment Targets in the United States. J. Infect Dis. 2017, 216, 798–807. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. HIV/AIDS. Available online: https://www.who.int/health-topics/hiv-aids/#tab=tab_1 (accessed on 20 March 2021).
- Compartment for Monitoring and Evaluation of HIV/AIDS Data in Romania—INBI “Prof. Dr. Matei Bals”. Available online: http://cnlas.ro/images/doc/30062020_eng.pdf (accessed on 20 March 2021).
- UNAIDS. 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic. 2014. Available online: www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf (accessed on 20 March 2021).
- Lazarus, J.V.; Safreed-Harmon, K.; Barton, S.E.; Costagliola, D.; Dedes, N.; del Amo Valero, J.; Gatell, J.M.; Baptista-Leite, R.; Mendão, L.; Porter, K.; et al. Beyond viral suppression of HIV—The new quality of life frontier. BMC Med. 2016, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. 2015, p. 24. Available online: https://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/ (accessed on 20 March 2021).
- Ford, N.; Meintjes, G.; Vitoria, M.; Greene, G.; Chiller, T. The evolving role of CD4 cell counts in HIV care. Curr Opin HIV AIDS 2017, 12, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Max, B. Update on HIV integrase inhibitors for the treatment of HIV-1 infection. Future Virol. 2019, 14, 693–709. [Google Scholar] [CrossRef]
- Brehm, T.T.; Franz, M.; Hüfner, A.; Hertling, S.; Schmiedel, S.; Degen, O.; Kreuels, B.; Schulze Zur Wiesch, J. Safety and efficacy of elvitegravir, dolutegravir, and raltegravir in a real-world cohort of treatment-naive and -experienced patients. Medicine 2019, 98, e16721. [Google Scholar] [CrossRef] [PubMed]
- Kolakowska, A.; Maresca, A.F.; Collins, I.J.; Cailhol, J. Update on Adverse Effects of HIV Integrase Inhibitors. Curr. Treat. Options Infect Dis. 2019, 11, 372–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levi, J.; Raymond, A.; Pozniak, A.; Vernazza, P.; Kohler, P.; Hill, A. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Glob. Health 2016, 1, e000010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerga, H.; Van Cutsem, G.; Ben Farhat, J.; Puren, A.; Bouhenia, M.; Wiesner, L.; Dlamini, L.; Maman, D.; Ellman, T.; Etard, J.F. Progress towards the UNAIDS 90-90-90 goals by age and gender in a rural area of KwaZulu-Natal, South Africa: A household-based community cross-sectional survey. BMC Public Health 2018, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Ssekalembe, G.; Isfandiari, M.A.; Suprianto, H. Current Status Towards 90-90-90 UNAIDS Target and Factors Associated with HIV Viral Load Suppression in Kediri City, Indonesia. HIV AIDS 2020, 12, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Ross, J.; Ribakare, M.; Remera, E.; Murenzi, G.; Munyaneza, A.; Hoover, D.R.; Shi, Q.; Nsanzimana, S.; Yotebieng, M.; Nash, D.; et al. High levels of viral load monitoring and viral suppression under Treat All in Rwanda—A cross-sectional study. J. Int. AIDS Soc. 2020, 23, e25543. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.E.; Hayes, R.; Noori, T.; Azad, Y.; Amato-Gauci, A.J.; Pharris, A.; Delpech, V.C. The ECDC Dublin Declaration Monitoring Network. HIV in Europe and Central Asia: Progress in 2018 towards meeting the UNAIDS 90-90-90 targets. Eurosurveillance 2018, 23, 1800622. [Google Scholar] [CrossRef] [PubMed]
- Streinu-Cercel, A. Specific Challenges in Key Population in Romania and in Central and Eastern Europe. Available online: https://www.eacsociety.org/files/soc2019_specific_challenges_in_key_population_in_romania_and_central-eastern_europe_a.streinu-cercel.pdf (accessed on 20 February 2021).
- Felicia, A. Trends of HIV/AIDS Phenomenon Dynamics in Romania from 2017–2027. Iran. J. Public Health 2019, 48, 1903–1909. [Google Scholar] [CrossRef] [PubMed]
- Hersh, B.S.; Popovici, F.; Apetrei, R.C.; Zolotusca, L.; Beldescu, N.; Calomfirescu, A.; Jezek, Z.; Oxtoby, M.J.; Gromyko, A.; Heymann, D.L. Acquired immunodeficiency syndrome in Romania. Lancet 1991, 338, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Global HIV 90-90-90 Watch. [last updated May 2017]. Available online: http://hiv90-90-90watch.org/ (accessed on 20 February 2021).
- CDC. 1993 Revised Classification System for HIV Infection and Expanded Surveillance Case Definition for AIDS among Adolescents and Adults. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00018871.htm (accessed on 20 February 2021). [CrossRef]
- European Centre for Disease Prevention and Control, WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2018–2017 Data; WHO Regional Office for Europe: Copenhagen, Denmark, 2018; Available online: https://ecdc.europa.eu/sites/portal/files/documents/hiv-aids-surveillance-europe-2018.pdf (accessed on 23 February 2021).
- Compartment for Monitoring and Evaluation of HIV/AIDS data in Romania—INBI “Prof. Dr. Matei Bals”. Available online: http://cnlas.ro/images/doc/15092020_2.pdf (accessed on 23 February 2021).
- Updated Recommendations on HIV Prevention, Infant Diagnosis, Antiretroviral Initiation and Monitoring; World Health Organization: Geneva, Switzerland, 2021; 2021.Licence: CC BY-NC-SA 3.0 IGO; Available online: https://www.who.int/publications/i/item/9789240022232 (accessed on 20 July 2021).
- One-Sample Proportion Test. 2021. Available online: https://stats.libretexts.org/@go/page/5200 (accessed on 20 July 2021).
- World Health Organization. Global Health Observatory Data Repository. Incidence Data by Country. 2020. Available online: http://apps.who.int/gho/data/view.main.57040ALL (accessed on 23 February 2021).
- ECDC Special Report, The Status of the HIV Response in the European Union/European Economic Area, 2016. Dublin Declaration Report. Available online: https://www.ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/Status-of-HIV-response-in-EU-EEA-2016-30-jan-2017.pdf (accessed on 23 February 2021).
- HIV Combination Prevention–Monitoring Implementation of the Dublin Declaration on Partnership to Fight HIV/AIDS in Europe and Central Asia (2018 Progress Report). 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/hiv-combination-prevention-monitoring-implementation-dublin-declaration (accessed on 25 February 2021).
- European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS Surveillance in Europe 2019—2018 Data. 2019. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/hiv-surveillance-report-2019.pdf (accessed on 25 February 2021).
- Jianu, C.; Bolboacă, S.D.; Topan, A.V.; Filipescu, I.; Jianu, M.E.; Itu-Mureșan, C. A View of Human Immunodeficiency Virus Infections in the North-West Region of Romania. Medicina 2019, 55, 765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, S.K.; Mayer, K.H. Providers should discuss U=U with all patients living with HIV. Lancet HIV 2019, 6, e21–e213. [Google Scholar] [CrossRef]
- World Health Organization. Global Health Observatory Data Repository. Number of New HIV Infections Data by Country. Available online: http://apps.who.int/gho/data/node.main.HIVINCIDENCE?lang=en (accessed on 25 February 2021).
- Continuum of HIV Care: Monitoring Implementation of the Dublin Declaration on Partenership to Fight HIV/AIDS in Europe and Central Asia: 2018 Progress Report. 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/continuum-hiv-care-monitoring-implementation-dublin-declaration-2018-progress (accessed on 25 March 2021).
- European AIDS Clinical Society. EACS Guidelines 10.0. 2019. Available online: https://www.eacsociety.org/files/2019_guidelines-10.0_final.pdf (accessed on 25 February 2021).
- Brooks, K.M.; Sherman, E.M.; Egelund, E.F.; Brotherton, A.; Durham, S.; Badowski, M.E.; Cluck, D.B. Integrase Inhibitors: After 10 Years of Experience, Is the Best Yet to Come? Pharmacotherapy 2019, 39, 576–598. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Osswald, H.L.; Prato, G. Recent Progress in the Development of HIV-1 Protease Inhibitors for the Treatment of HIV/AIDS. J. Med. Chem. 2016, 59, 172–5208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; De Clercq, E.; Li, G. Current and emerging non-nucleoside reverse transcriptase inhibitors (NNRTIs) for HIV-1 treatment. Expert Opin. Drug Metab. Toxicol. 2019, 15, 813–829. [Google Scholar] [CrossRef] [PubMed]
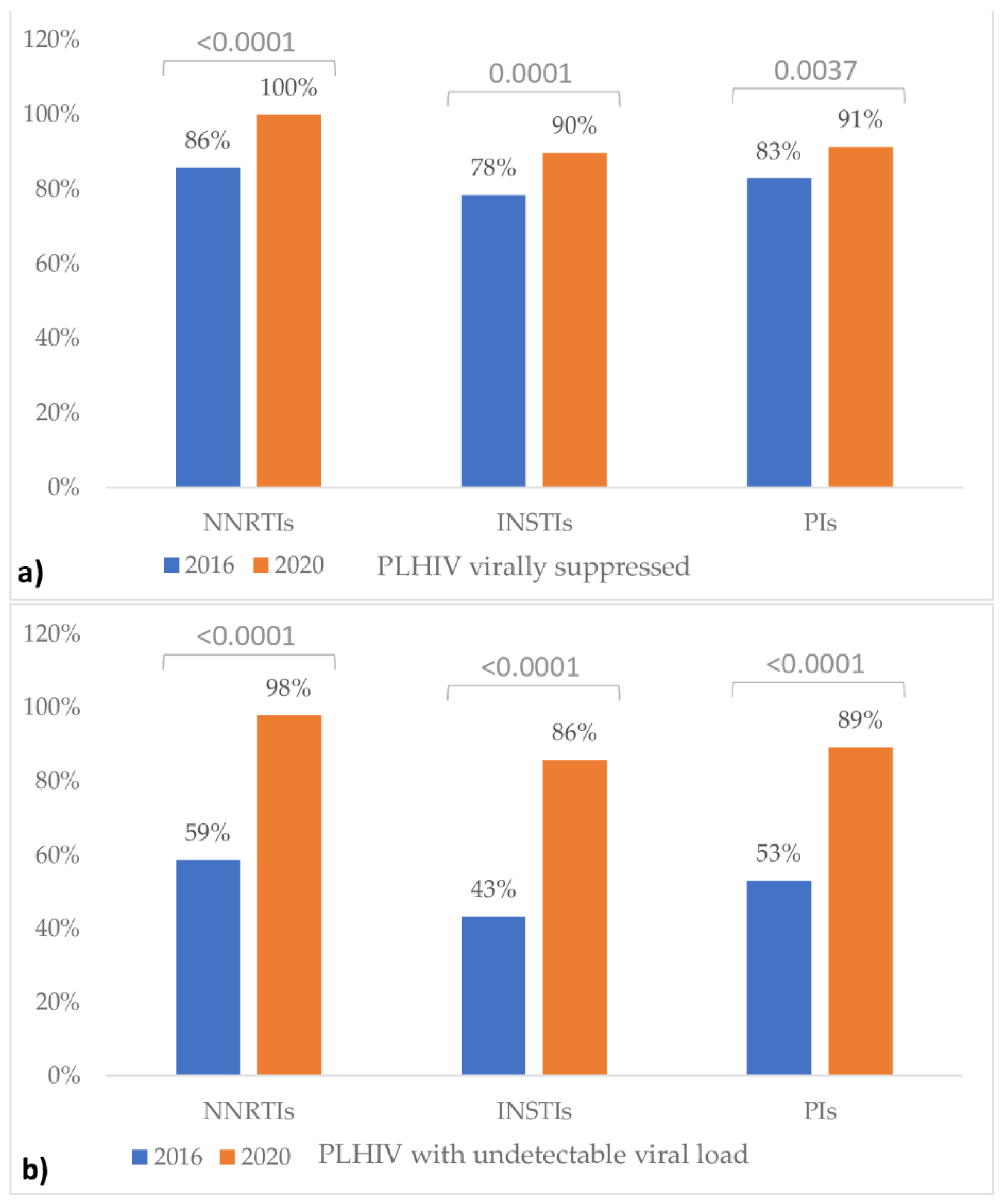
| Characteristic | December 2016 (n = 254) | December 2020 (n = 368) |
|---|---|---|
| Gender, no. (%) Men Women | 182 (71.7) 72 (28.3) | 280 (76.1) 88 (23.9) |
| Way of HIV infection, no. (%) Heterosexual MSM Romanian cohort Parenteral not IDU Mother to child IDU | 147 (57.9) 79 (31.1) 21 (8.3) 4 (1.6) 5 (2) 1 (0.4) | 194 (52.7) 138 (37.5) 23 (6.3) 5 (1.4) 5 (1.4) 3 (0.8) |
| Patients under ART | December 2016 (n = 254) | December 2020 (n = 368) |
|---|---|---|
| No. (%) | 226 (88.98) a | 358 (97.28) b |
| Age, years | 36 (29 to 48) {7 to 75} | 38 (32 to 47) {8 to 79} |
| Men | 168 (74.3) | 280 (76.1) |
| Living in rural areas | 56 (24.8) | 95 (25.8) |
| Disease duration, years | 5 (2 to 9) {0 to 23} # | 6 (2 to 10) {0 to 27} |
| CD4 count/mm3 | 562 (384 to 789) | 644 (475 to 897) |
| Viral load | ||
| undetectable * | 170 (75.2) | 326 (91.1) |
| between 50 and 200 copies/mL | 15 (7.5) | 11 (3.1) |
| >200 copies/mL | 38 (16.8) | 31 (8.7) |
| without virological evaluation | 2 (0.9) | 0 (0.0) |
| ART | December 2016 (n = 254) | December 2020 (n = 368) | Χ2 (p-Value) |
|---|---|---|---|
| Protease inhibitors (PIs) | 117 (51.8) | 140 (39.1) | 9.0 (0.0027) |
| Non-nucleoside reverse transcriptase inhibitors (NNRTIs) | 70 (31.0) | 54 (15.1) | 20.9 (<0.0001) |
| Integrase strand transfer inhibitors (INSTIs) | 39 (17.3) | 164 (45.8) | 49.8 (<0.0001) |
| single tablet regimen | 0 (0.00) | 20 (5.58) | n.a. (0.2602) * |
| Region | Diagnosed | Patients on ART | Virally Suppressed | Virally Suppressed of All Patients |
|---|---|---|---|---|
| Target | 90 | 90 | 90 | 73 |
| Central Europe (2018) [31] | 83 | 73 | 75 | 46 |
| Romania (2019) [4] | 83.16 * | 91.3 | 81.2 | 58.2 |
| Cluj County (2020)—the current study | 95.58 ** | 97.28 | 94.1 | 91.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jianu, C.; Itu-Mureşan, C.; Topan, A.V.; Filipescu, I.; Jianu, M.E.; Melincovici, C.S.; Mihu, C.M.; Bolboacă, S.D. Continuum of Care UNAIDS Fast-Track Targets Evaluation of Patients Living with Human Immunodeficiency Virus Infection. Healthcare 2021, 9, 1249. https://doi.org/10.3390/healthcare9101249
Jianu C, Itu-Mureşan C, Topan AV, Filipescu I, Jianu ME, Melincovici CS, Mihu CM, Bolboacă SD. Continuum of Care UNAIDS Fast-Track Targets Evaluation of Patients Living with Human Immunodeficiency Virus Infection. Healthcare. 2021; 9(10):1249. https://doi.org/10.3390/healthcare9101249
Chicago/Turabian StyleJianu, Cristian, Corina Itu-Mureşan, Adriana Violeta Topan, Irina Filipescu, Mihaela Elena Jianu, Carmen Stanca Melincovici, Carmen Mihaela Mihu, and Sorana D. Bolboacă. 2021. "Continuum of Care UNAIDS Fast-Track Targets Evaluation of Patients Living with Human Immunodeficiency Virus Infection" Healthcare 9, no. 10: 1249. https://doi.org/10.3390/healthcare9101249
APA StyleJianu, C., Itu-Mureşan, C., Topan, A. V., Filipescu, I., Jianu, M. E., Melincovici, C. S., Mihu, C. M., & Bolboacă, S. D. (2021). Continuum of Care UNAIDS Fast-Track Targets Evaluation of Patients Living with Human Immunodeficiency Virus Infection. Healthcare, 9(10), 1249. https://doi.org/10.3390/healthcare9101249







