Lack of Antibodies to SARS-CoV-2 among Blood Donors during COVID-19 Lockdown: A Study from Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Study Population
2.3. Immunoassays
2.3.1. In-House Enzyme-Linked Immunoassay (ELISA)
2.3.2. Chemiluminescence Immunoassay (CLIA)
2.3.3. Microneutralization (MN) Assay
2.4. Data Curation
3. Results
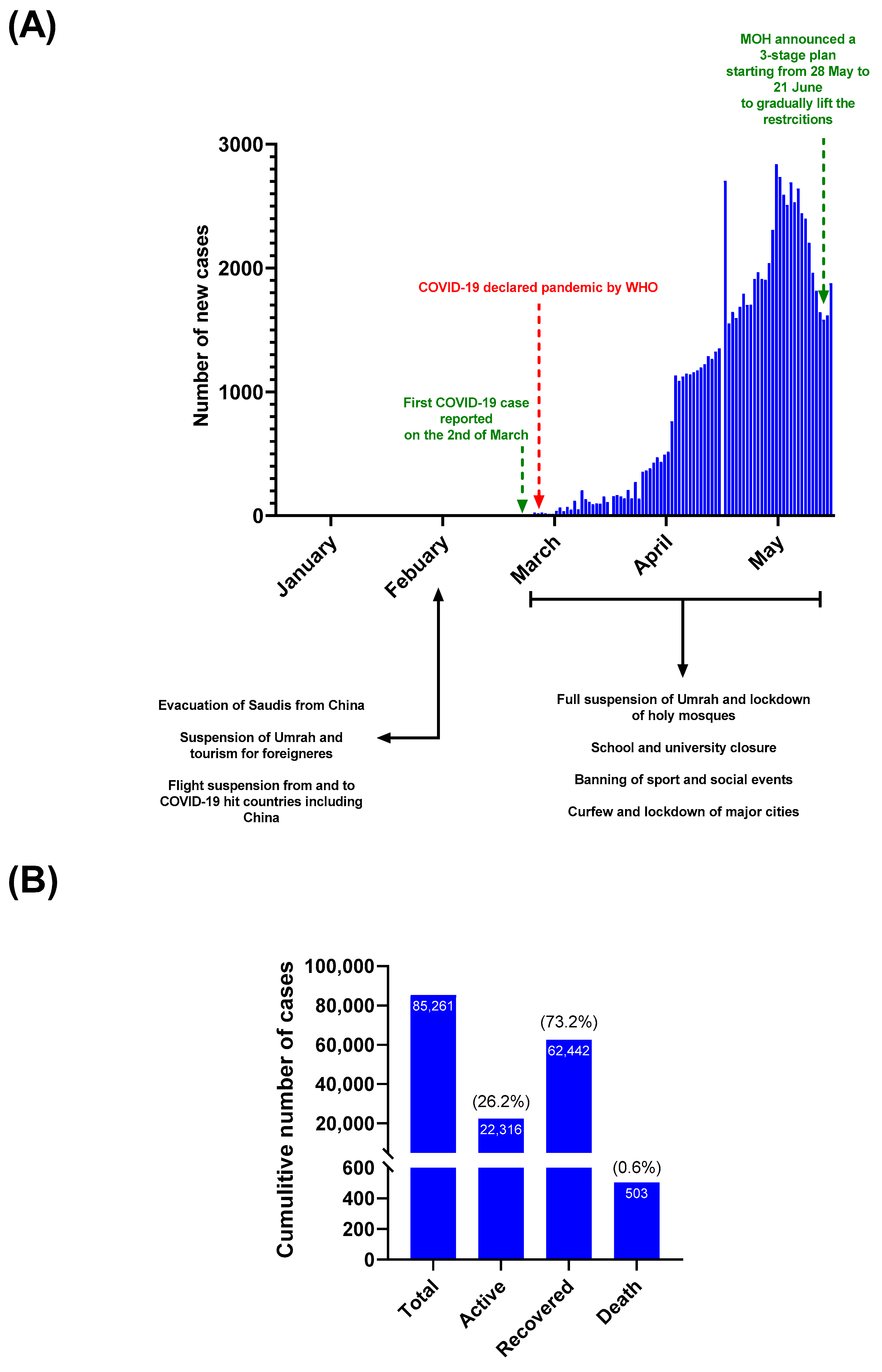
3.1. COVID-19 Status in SAUDI Arabia during the Study Period (1 January to 31 May 2020)
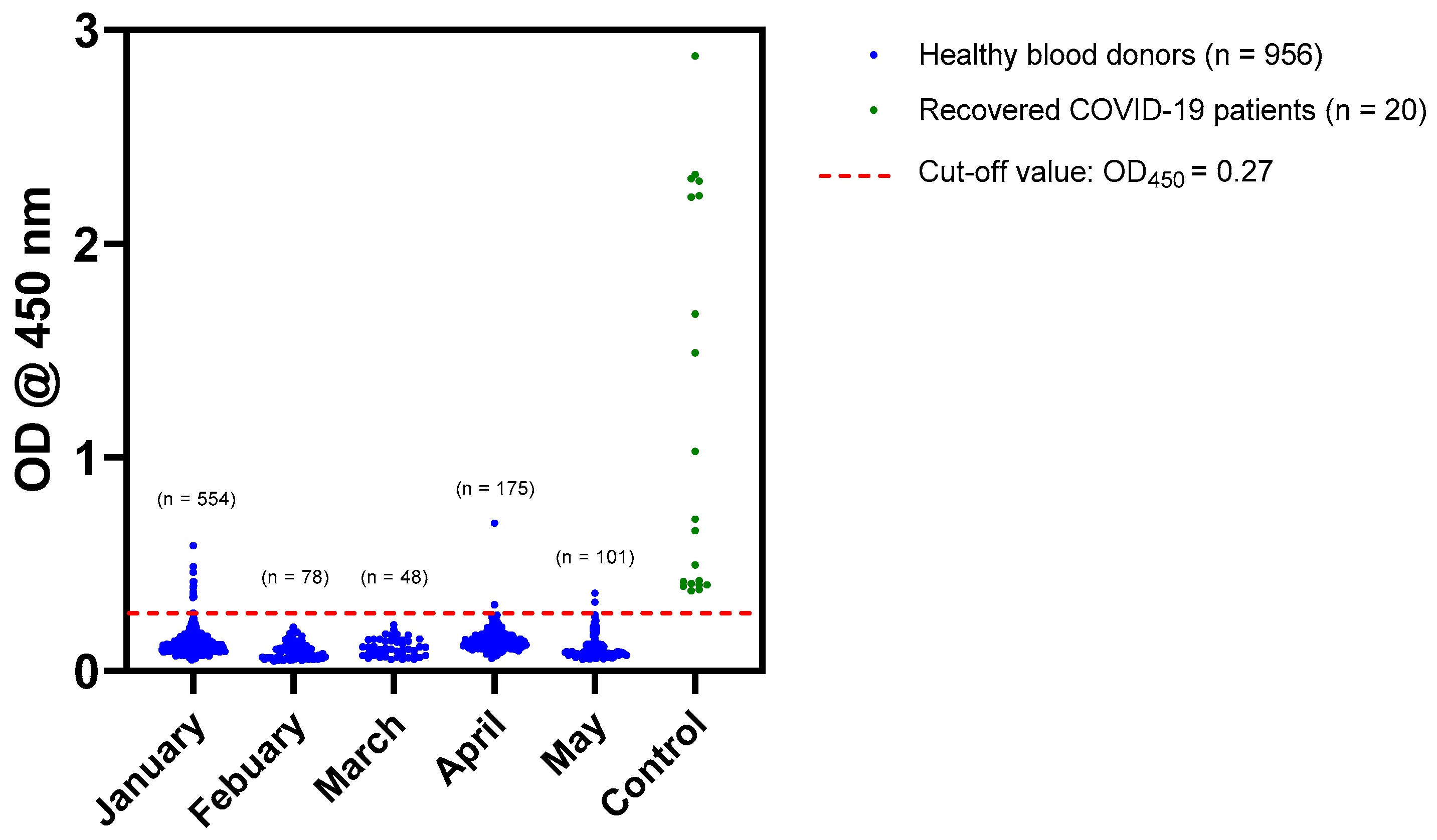
3.2. The Seroprevalence of SARS-CoV-2 among Blood Donors
3.2.1. Screening of Sera by In-House ELISA
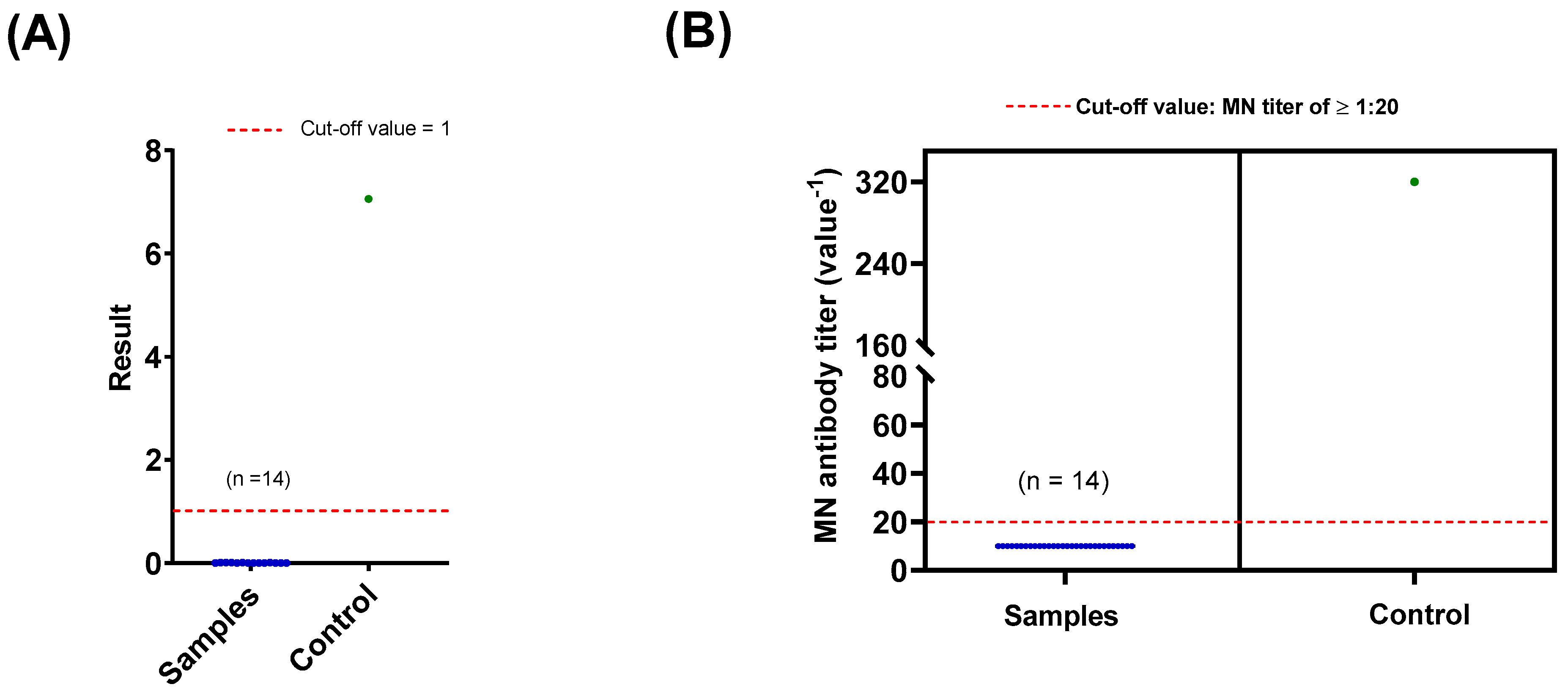
3.2.2. Confirmation of Sero Status by CLIA and MN Assay
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, L.-L.; Wang, Y.-M.; Wu, Z.-Q.; Xiang, Z.-C.; Guo, L.; Xu, T.; Jiang, Y.-Z.; Xiong, Y.; Li, Y.-J.; Li, X.-W.; et al. Identification of a novel coronavirus causing severe pneumonia in human: A descriptive study. Chin. Med. J. 2020, 133, 1015–1024. [Google Scholar] [CrossRef]
- World Health Organization (WHO). COVID-19 Dashboard. Available online: https://who.sprinklr.com (accessed on 8 November 2020).
- Alandijany, T.A.; Faizo, A.A.; Azhar, E.I. Coronavirus disease of 2019 (COVID-19) in the Gulf Cooperation Council (GCC) countries: Current status and management practices. J. Infect. Public Health 2020, 13, 839–842. [Google Scholar] [CrossRef]
- Siddiqui, A.F.; Wiederkehr, M.; Rozanova, L.; Flahault, A. Situation of India in the COVID-19 Pandemic: India’s Initial Pandemic Experience. Int. J. Environ. Res. Public Health 2020, 17, 8994. [Google Scholar] [CrossRef]
- Yoo, J.; Dutra, S.V.O.; Fanfan, D.; Sniffen, S.; Wang, H.; Siddiqui, J.; Song, H.-S.; Bang, S.H.; Kim, D.E.; Kim, S.; et al. Comparative analysis of COVID-19 guidelines from six countries: A qualitative study on the US, China, South Korea, the UK, Brazil, and Haiti. BMC Public Health 2020, 20, 1853. [Google Scholar] [CrossRef]
- Islam, N.; Sharp, S.J.; Chowell, G.; Shabnam, S.; Kawachi, I.; Lacey, B.; Massaro, J.M.; Sr, R.B.D.; White, M. Physical distancing interventions and incidence of coronavirus disease 2019: Natural experiment in 149 countries. BMJ 2020, 370, m2743. [Google Scholar] [CrossRef] [PubMed]
- May, T. Lockdown-type measures look effective against covid-19. BMJ 2020, 370, m2809. [Google Scholar] [CrossRef] [PubMed]
- Algaissi, A.A.; Alharbi, N.K.; Hassanain, M.; Hashem, A.M. Preparedness and response to COVID-19 in Saudi Arabia: Building on MERS experience. J. Infect. Public Health 2020, 13, 834–838. [Google Scholar] [CrossRef]
- Obied, D.; Alhamlan, F.S.; Al-Qahtani, A.; Al-Ahdal, M.N. Containment of COVID-19: The unprecedented response of Saudi Arabia. J. Infect. Dev. Ctries. 2020, 14, 699–706. [Google Scholar] [CrossRef]
- MOH-KSA. Ministry of Health, Kingdom of Saudi Arabia. Available online: https://www.moh.gov.sa/Ministry/MediaCenter/News/Pages/News-2020-03-02-002.aspx (accessed on 11 April 2020).
- MOH-KSA. Available online: https://twitter.com/SaudiMOH/status/1264992536453660673?s=20 (accessed on 27 May 2020).
- Peeling, R.W.; Wedderburn, C.J.; Garcia, P.J.; Boeras, D.; Fongwen, N.; Nkengasong, J.; Sall, A.; Tanuri, A.; Heymann, D.L. Serology testing in the COVID-19 pandemic response. Lancet Infect. Dis. 2020, 20, e245–e249. [Google Scholar] [CrossRef]
- Peto, J. Covid-19 mass testing facilities could end the epidemic rapidly. BMJ 2020, 368, m1163. [Google Scholar] [CrossRef]
- Ko, J.-H.; Joo, E.-J.; Park, S.-J.; Baek, J.Y.; Kim, W.D.; Jee, J.H.; Kim, C.J.; Jeong, C.; Kim, Y.-J.; Shon, H.J.; et al. Neutralizing Antibody Production in Asymptomatic and Mild COVID-19 Patients, in Comparison with Pneumonic COVID-19 Patients. J. Clin. Med. 2020, 9, 2268. [Google Scholar] [CrossRef]
- Nikolai, L.A.; Meyer, C.G.; Kremsner, P.G.; Velavan, T.P. Asymptomatic SARS Coronavirus 2 infection: Invisible yet invincible. Int. J. Infect. Dis. 2020, 100, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, D.; Xia, Y.; Wu, X.; Li, T.; Ou, X.; Zhou, L.; Liu, J. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect. Dis. 2020, 20, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Basteiro, A.L.; Moncunill, G.; Tortajada, M.; Vidal, M.; Guinovart, C.; Jiménez, A.; Santano, R.; Sanz, S.; Méndez, S.; Llupià, A.; et al. Seroprevalence of antibodies against SARS-CoV-2 among health care workers in a large Spanish reference hospital. Nat. Commun. 2020, 11, 3500. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.R.; Dbeibo, L.; Weaver, C.S.; Beeler, C.; Saysana, M.; Zimmerman, M.K.; Weaver, L. Seroprevalence of severe acute respiratory coronavirus virus 2 (SARS-CoV-2) antibodies among healthcare workers with differing levels of coronavirus disease 2019 (COVID-19) patient exposure. Infect. Control. Hosp. Epidemiol. 2020, 41, 1441–1442. [Google Scholar] [CrossRef]
- Korth, J.; Wilde, B.; Dolff, S.; Anastasiou, O.E.; Krawczyk, A.; Jahn, M.; Cordes, S.; Ross, B.; Esser, S.; Lindemann, M. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020, 14, 699–706. [Google Scholar] [CrossRef]
- Poustchi, H.; Darvishian, M.; Mohammadi, Z.; Shayanrad, A.; Delavari, A.; Bahadorimonfared, A.; Eslami, S.; Javanmard, S.H.; Shakiba, E.; Somi, M.H.; et al. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: A population-based cross-sectional study. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Buss, L.F.; Prete, C.A.; Abrahim, C.M.M.; Mendrone, A.; Salomon, T.; de Almeida-Neto, C.; França, R.F.O.; Belotti, M.C.; Carvalho, M.P.S.S.; Costa, A.G.; et al. Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science 2020, eabe9728. [Google Scholar] [CrossRef]
- Bajema, K.L.; Wiegand, R.E.; Cuffe, K.; Patel, S.V.; Iachan, R.; Lim, T.; Lee, A.; Moyse, D.; Havers, F.P.; Harding, L.; et al. Estimated SARS-CoV-2 Seroprevalence in the US as of September 2020. JAMA Intern. Med. 2020. [Google Scholar] [CrossRef]
- Alserehi, H.A.; Alqunaibet, A.M.; Al-Tawfiq, J.A.; Alharbi, N.K.; Alshukairi, A.N.; Alanazi, K.H.; Bin Saleh, G.M.; AlShehri, A.M.; Almasoud, A.; Hashem, A.M.; et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: Comparing case and control hospitals. Diagn. Microbiol. Infect. Dis. 2021, 99, 115273. [Google Scholar] [CrossRef]
- Alandijany, T.A.; El-Kafrawy, S.A.; Tolah, A.M.; Sohrab, S.S.; Faizo, A.A.; Hassan, A.M.; Alsubhi, T.L.; Othman, N.A.; Azhar, E.I. Development and Optimization of In-house ELISA for Detection of Human IgG Antibody to SARS-CoV-2 Full Length Spike Protein. Pathogens 2020, 9, 803. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 8 November 2020).
- MOH-KSA. The Saudi Ministry of Health. COVID-19 Dashboard. Available online: https://covid19.moh.gov.sa/ (accessed on 3 May 2020).
- GeurtsvanKessel, C.H.; Okba, N.M.; Iglὁi, Z.; Bogers, S.; Embregts, C.W.E.; Laksono, B.M.; Leijten, L.; Rokx, C.; Rijnders, B.; Rahamat-Langendoen, J. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat. Commun. 2020, 11, 3436. [Google Scholar] [CrossRef] [PubMed]
- Algaissi, A.; AlFaleh, M.A.; Hala, S.; Abujamel, T.S.; Alamri, S.S.; Almahboub, S.A.; Alluhaybi, K.A.; Hobani, H.I.; Alsulaiman, R.M.; Alharbi, R.H.; et al. SARS-CoV-2 S1 and N-based serological assays reveal rapid seroconversion and induction of specific antibody response in COVID-19 patients. Sci. Rep. 2020, 10, 16561. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Li, T.-D.; Zheng, S.-F.; Su, Y.-Y.; Li, Z.-Y.; Liu, W.; Yu, F.; Ge, S.-X.; Zou, Q.-D.; Yuan, Q.; et al. Serology characteristics of SARS-CoV-2 infection after exposure and post-symptom onset. Eur. Respir. J. 2020, 56. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alandijany, T.A.; El-Kafrawy, S.A.; Al-Ghamdi, A.A.; Qashqari, F.S.; Faizo, A.A.; Tolah, A.M.; Hassan, A.M.; Sohrab, S.S.; Hindawi, S.I.; Badawi, M.A.; et al. Lack of Antibodies to SARS-CoV-2 among Blood Donors during COVID-19 Lockdown: A Study from Saudi Arabia. Healthcare 2021, 9, 51. https://doi.org/10.3390/healthcare9010051
Alandijany TA, El-Kafrawy SA, Al-Ghamdi AA, Qashqari FS, Faizo AA, Tolah AM, Hassan AM, Sohrab SS, Hindawi SI, Badawi MA, et al. Lack of Antibodies to SARS-CoV-2 among Blood Donors during COVID-19 Lockdown: A Study from Saudi Arabia. Healthcare. 2021; 9(1):51. https://doi.org/10.3390/healthcare9010051
Chicago/Turabian StyleAlandijany, Thamir A., Sherif A. El-Kafrawy, Abrar A. Al-Ghamdi, Fadi S. Qashqari, Arwa A. Faizo, Ahmed M. Tolah, Ahmed M. Hassan, Sayed S. Sohrab, Salwa I. Hindawi, Maha A. Badawi, and et al. 2021. "Lack of Antibodies to SARS-CoV-2 among Blood Donors during COVID-19 Lockdown: A Study from Saudi Arabia" Healthcare 9, no. 1: 51. https://doi.org/10.3390/healthcare9010051
APA StyleAlandijany, T. A., El-Kafrawy, S. A., Al-Ghamdi, A. A., Qashqari, F. S., Faizo, A. A., Tolah, A. M., Hassan, A. M., Sohrab, S. S., Hindawi, S. I., Badawi, M. A., & Azhar, E. I. (2021). Lack of Antibodies to SARS-CoV-2 among Blood Donors during COVID-19 Lockdown: A Study from Saudi Arabia. Healthcare, 9(1), 51. https://doi.org/10.3390/healthcare9010051






