Allergic Rhinitis and Laryngeal Pathology: Real-World Evidence
Abstract
1. Introduction
2. Materials and Methods
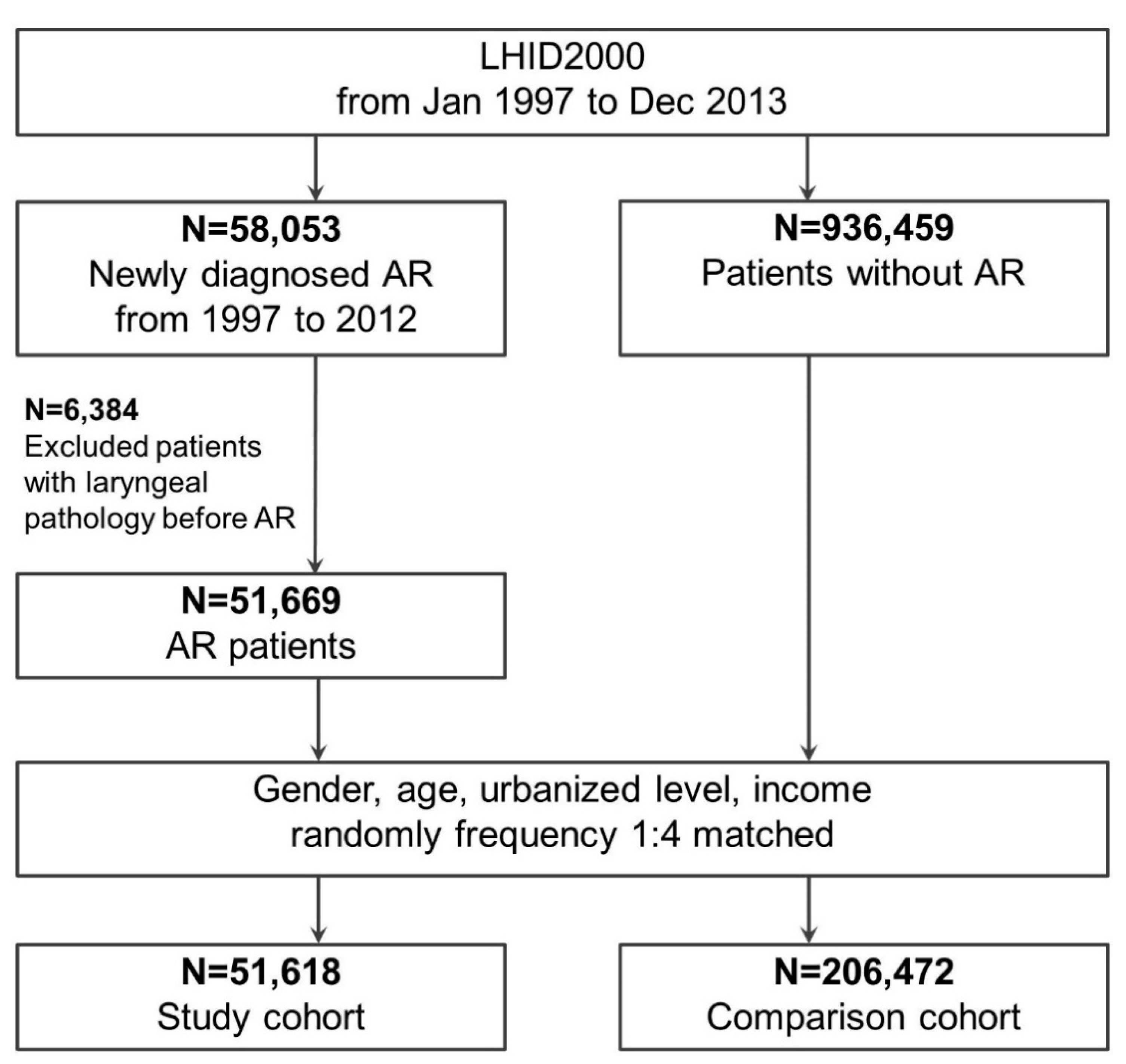
2.1. Design and Data
2.2. Study Groups: With and without AR
2.3. Outcome and Covariate Measurements
2.4. Statistical Analysis
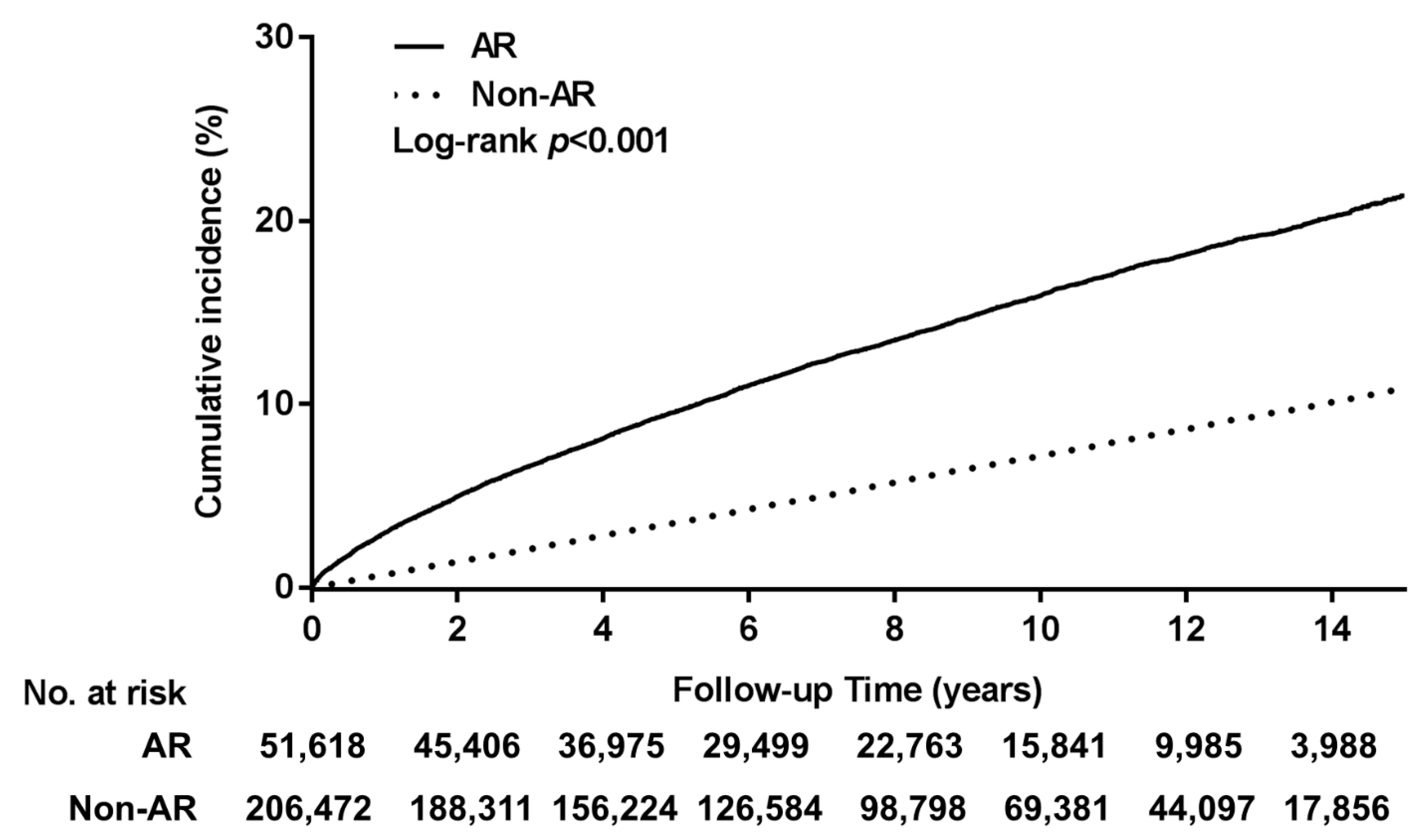
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, D.A. Allergic rhinitis and asthma: Epidemiology and common pathophysiology. Allergy Asthma Proc. 2014, 35, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J. One airway, one disease. Chest 1997, 111, 11s–16s. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C. Sinusitis, Rhinitis, Asthma, and the Single Airway Hypothesis. Dis. Sinuses 2013, 173–194. [Google Scholar] [CrossRef]
- Bertelsen, C.; Zhou, S.; Hapner, E.R.; Johns, M.M., 3rd. Sociodemographic Characteristics and Treatment Response among Aging Adults with Voice Disorders in the United States. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, K.E.; Roy, N.; Losonczy, K.G.; Hoffman, H.J.; Cohen, S.M. Voice disorders and associated risk markers among young adults in the United States. Laryngoscope 2017, 127, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Hah, J.H.; Sim, S.; An, S.Y.; Sung, M.W.; Choi, H.G. Evaluation of the prevalence of and factors associated with laryngeal diseases among the general population. Laryngoscope 2015, 125, 2536–2542. [Google Scholar] [CrossRef] [PubMed]
- Spantideas, N.; Bougea, A.; Drosou, E.; Assimakopoulos, D. The Role of Allergy in Phonation. J. Voice 2019, 33, 811.e819–811.e827. [Google Scholar] [CrossRef]
- Ohlsson, A.C.; Drevsater, A.; Brynnel, M.; Johansson, I. Allergic rhinitis and voice change. Logoped. Phoniatr. Vocol. 2016, 41, 143–148. [Google Scholar] [CrossRef]
- Turley, R.; Cohen, S.M.; Becker, A.; Ebert, C.S., Jr. Role of rhinitis in laryngitis: Another dimension of the unified airway. Annals Otol. Rhinol. Laryngol. 2011, 120, 505–510. [Google Scholar] [CrossRef]
- Jackson-Menaldi, C.A.; Dzul, A.I.; Holland, R.W. Allergies and vocal fold edema: A preliminary report. J. Voice 1999, 13, 113–122. [Google Scholar] [CrossRef]
- Tsai, M.S.; Lee, L.A.; Tsai, Y.T.; Yang, Y.H.; Liu, C.Y.; Lin, M.H.; Hsu, C.M.; Chen, C.K.; Li, H.Y. Sleep apnea and risk of vertigo: A nationwide population-based cohort study. Laryngoscope 2018, 128, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.-H.; Su, Y.-C.; Lin, K.-M.; Liu, C.-Y.; Yang, Y.-H.; Chang, P.-J.; Lin, M.-H.; Lee, C.-P.; Hsu, C.-M.; Tsai, Y.-T.; et al. Deep Neck Infection in Systemic Lupus Erythematosus Patients: Real-World Evidence. Sci. Rep. 2020, 10, 4133. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Chen, Y.J.; Ho, H.J.; Hsu, Y.C.; Kuo, K.N.; Wu, M.S.; Lin, J.T. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012, 308, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Lee, C.H.; Chen, P.S.; Li, Y.H.; Lin, S.J.; Yang, Y.H. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J. Epidemiol. 2014, 24, 500–507. [Google Scholar] [CrossRef]
- Chang, G.H.; Ding, M.C.; Chen, Y.C.; Yang, Y.H.; Liu, C.Y.; Chang, P.J.; Lee, C.P.; Lin, M.H.; Hsu, C.M.; Wu, C.Y. Real-world evidence for increased deep neck infection risk in patients with rheumatoid arthritis. Laryngoscope 2020, 130, 1402–1407. [Google Scholar] [CrossRef]
- Tsai, M.S.; Li, H.Y.; Huang, C.G.; Wang, R.Y.; Chuang, L.P.; Chen, N.H.; Liu, C.H.; Yang, Y.H.; Liu, C.Y.; Hsu, C.M. Risk of Alzheimer’s disease in obstructive sleep apnea patients with or without treatment: Real-world evidence. Laryngoscope 2020, 130, 2292–2298. [Google Scholar] [CrossRef]
- Tsai, M.S.; Chang, G.H.; Chen, W.M.; Liu, C.Y.; Lin, M.H.; Chang, P.J.; Huang, T.Y.; Tsai, Y.T.; Wu, C.Y.; Hsu, C.M.; et al. The Association between Decompensated Liver Cirrhosis and Deep Neck Infection: Real-World Evidence. Int. J. Environ. Res. Public Health 2019, 16, 3863. [Google Scholar] [CrossRef]
- Tsai, M.S.; Yang, Y.H.; Liu, C.Y.; Lin, M.H.; Chang, G.H.; Tsai, Y.T.; Li, H.Y.; Tsai, Y.H.; Hsu, C.M. Unilateral Vocal Fold Paralysis and Risk of Pneumonia: A Nationwide Population-Based Cohort Study. Otolaryngol. Head Neck Surg. 2018, 158, 896–903. [Google Scholar] [CrossRef]
- Fabbri, L.; Peters, S.P.; Pavord, I.; Wenzel, S.E.; Lazarus, S.C.; Macnee, W.; Lemaire, F.; Abraham, E. Allergic rhinitis, asthma, airway biology, and chronic obstructive pulmonary disease in AJRCCM in 2004. Am. J. Respir. Crit. Care Med. 2005, 171, 686–698. [Google Scholar] [CrossRef][Green Version]
- Kung, Y.M.; Tsai, P.Y.; Chang, Y.H.; Wang, Y.K.; Hsieh, M.S.; Hung, C.H.; Kuo, C.H. Allergic rhinitis is a risk factor of gastro-esophageal reflux disease regardless of the presence of asthma. Sci. Rep. 2019, 9, 15535. [Google Scholar] [CrossRef]
- De Labio, R.B.; Tavares, E.L.; Alvarado, R.C.; Martins, R.H. Consequences of chronic nasal obstruction on the laryngeal mucosa and voice quality of 4- to 12-year-old children. J. Voice 2012, 26, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.H.; Ding, M.C.; Yang, Y.H.; Lin, Y.H.; Liu, C.Y.; Lin, M.H.; Wu, C.Y.; Hsu, C.M.; Tsai, M.S. High Risk of Deep Neck Infection in Patients with Type 1 Diabetes Mellitus: A Nationwide Population-Based Cohort Study. J. Clin. Med. 2018, 7, 385. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.N.; Hellings, P.W.; Rotiroti, G.; Scadding, G.K. Allergic rhinitis. Lancet 2011, 378, 2112–2122. [Google Scholar] [CrossRef]
- Stachler, R.J.; Dworkin-Valenti, J.P. Allergic laryngitis: Unraveling the myths. Curr. Opin. Otolaryngol. Head Neck Surg. 2017, 25, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Krouse, J.H.; Altman, K.W. Rhinogenic laryngitis, cough, and the unified airway. Otolaryngol. Clin. N. Am. 2010, 43, 111–121. [Google Scholar] [CrossRef]
- Krouse, J.H. Allergy and laryngeal disorders. Curr. Opin. Otolaryngol. Head Neck Surg. 2016, 24, 221–225. [Google Scholar] [CrossRef]
- Hamdan, A.L.; Sibai, A.; Youssef, M.; Deeb, R.; Zaitoun, F. The use of a screening questionnaire to determine the incidence of allergic rhinitis in singers with dysphonia. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 547–549. [Google Scholar] [CrossRef]
- Byeon, H. The association between lifetime cigarette smoking and dysphonia in the Korean general population: Findings from a national survey. PeerJ 2015, 3, e912. [Google Scholar] [CrossRef]
- Akbulut, S.; Gartner-Schmidt, J.L.; Gillespie, A.I.; Young, V.N.; Smith, L.J.; Rosen, C.A. Voice outcomes following treatment of benign midmembranous vocal fold lesions using a nomenclature paradigm. Laryngoscope 2016, 126, 415–420. [Google Scholar] [CrossRef]
| Variables | Allergic Rhinitis | Non-Allergic | p-Value | ||
|---|---|---|---|---|---|
| (N = 51,618) | (N = 206,472) | ||||
| n | % | n | % | ||
| Gender | 1.000 | ||||
| Male | 23,736 | 46.0 | 94,944 | 46.0 | |
| Female | 27,882 | 54.0 | 111,528 | 54.0 | |
| Age (years) | 1.000 | ||||
| <30 | 24,549 | 47.6 | 98,196 | 47.6 | |
| 30–60 | 15,926 | 30.9 | 63,704 | 30.9 | |
| >60 | 11,143 | 21.6 | 44,572 | 21.6 | |
| Urbanization level | 1.000 | ||||
| 1 (City) | 17,219 | 33.4 | 68,876 | 33.4 | |
| 2 | 24,290 | 47.1 | 97,160 | 47.1 | |
| 3 | 7004 | 13.6 | 28,016 | 13.6 | |
| 4 (Villages) | 3105 | 6.0 | 12,420 | 6.0 | |
| Monthly income (NT$) | 1.000 | ||||
| 0 | 27,925 | 54.1 | 111,700 | 54.1 | |
| 1–15,840 | 5964 | 11.6 | 23,856 | 11.6 | |
| 15,841–25,000 | 10,564 | 20.5 | 42,256 | 20.5 | |
| ≥25,001 | 7165 | 13.9 | 28,660 | 13.9 | |
| Comorbidity | |||||
| Asthma | 9392 | 18.2 | 16,087 | 7.8 | <0.001 |
| COPD | 5680 | 11.0 | 11,976 | 5.8 | <0.001 |
| CRS | 5859 | 11.4 | 1997 | 1.0 | <0.001 |
| GERD | 6807 | 13.2 | 12,499 | 6.1 | <0.001 |
| NSD | 6368 | 12.3 | 1316 | 0.6 | <0.001 |
| Outcome | 6854 | 13.3 | 12,014 | 5.8 | <0.001 |
| Variables | Adjusted HR | 95% CI | p-Value | |
|---|---|---|---|---|
| Main Model * | 2.43 | 2.36 | 2.50 | <0.001 |
| Additional Covariates † | ||||
| Main model + asthma | 2.45 | 2.38 | 2.53 | <0.001 |
| Main model + COPD | 2.43 | 2.36 | 2.50 | <0.001 |
| Main model + CRS | 2.43 | 2.36 | 2.50 | <0.001 |
| Main model + GERD | 2.45 | 2.38 | 2.53 | <0.001 |
| Main model + NSD | 2.43 | 2.36 | 2.51 | <0.001 |
| Subgroup Effects ‡ | ||||
| Gender | ||||
| Male | 2.70 | 2.58 | 2.82 | <0.001 |
| Female | 2.24 | 2.15 | 2.33 | <0.001 |
| Age (yrs) | ||||
| <30 | 1.99 | 1.90 | 2.09 | <0.001 |
| 30–60 | 2.73 | 2.60 | 2.87 | <0.001 |
| >60 | 2.86 | 2.69 | 3.05 | <0.001 |
| Asthma | ||||
| Yes | 2.04 | 1.86 | 2.23 | <0.001 |
| No | 2.51 | 2.43 | 2.59 | <0.001 |
| GERD | ||||
| Yes | 2.12 | 1.92 | 2.34 | <0.001 |
| No | 2.49 | 2.41 | 2.57 | <0.001 |
| COPD | ||||
| Yes | 2.53 | 2.28 | 2.80 | <0.001 |
| No | 2.42 | 2.35 | 2.50 | <0.001 |
| CRS | ||||
| Yes | 1.49 | 1.27 | 1.75 | <0.001 |
| No | 2.47 | 2.39 | 2.54 | <0.001 |
| NSD | ||||
| Yes | 1.21 | 1.00 | 1.46 | 0.047 |
| No | 2.47 | 2.39 | 2.55 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-T.; Chang, G.-H.; Yang, Y.-H.; Liu, C.-Y.; Tsai, Y.-T.; Hsu, C.-M.; Lee, Y.-C.; Lee, L.-A.; Yang, P.-R.; Tsai, M.-S.; et al. Allergic Rhinitis and Laryngeal Pathology: Real-World Evidence. Healthcare 2021, 9, 36. https://doi.org/10.3390/healthcare9010036
Wang Y-T, Chang G-H, Yang Y-H, Liu C-Y, Tsai Y-T, Hsu C-M, Lee Y-C, Lee L-A, Yang P-R, Tsai M-S, et al. Allergic Rhinitis and Laryngeal Pathology: Real-World Evidence. Healthcare. 2021; 9(1):36. https://doi.org/10.3390/healthcare9010036
Chicago/Turabian StyleWang, Yun-Ting, Geng-He Chang, Yao-Hsu Yang, Chia-Yen Liu, Yao-Te Tsai, Cheng-Ming Hsu, Yi-Chan Lee, Li-Ang Lee, Pei-Rung Yang, Ming-Shao Tsai, and et al. 2021. "Allergic Rhinitis and Laryngeal Pathology: Real-World Evidence" Healthcare 9, no. 1: 36. https://doi.org/10.3390/healthcare9010036
APA StyleWang, Y.-T., Chang, G.-H., Yang, Y.-H., Liu, C.-Y., Tsai, Y.-T., Hsu, C.-M., Lee, Y.-C., Lee, L.-A., Yang, P.-R., Tsai, M.-S., & Li, H.-Y. (2021). Allergic Rhinitis and Laryngeal Pathology: Real-World Evidence. Healthcare, 9(1), 36. https://doi.org/10.3390/healthcare9010036









