Clinical Usefulness of Bright White Light Therapy for Depressive Symptoms in Cancer Survivors: Results from a Series of Personalized (N-of-1) Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Customization of N-of-1Trial Smartphone Application and Electronic Platform
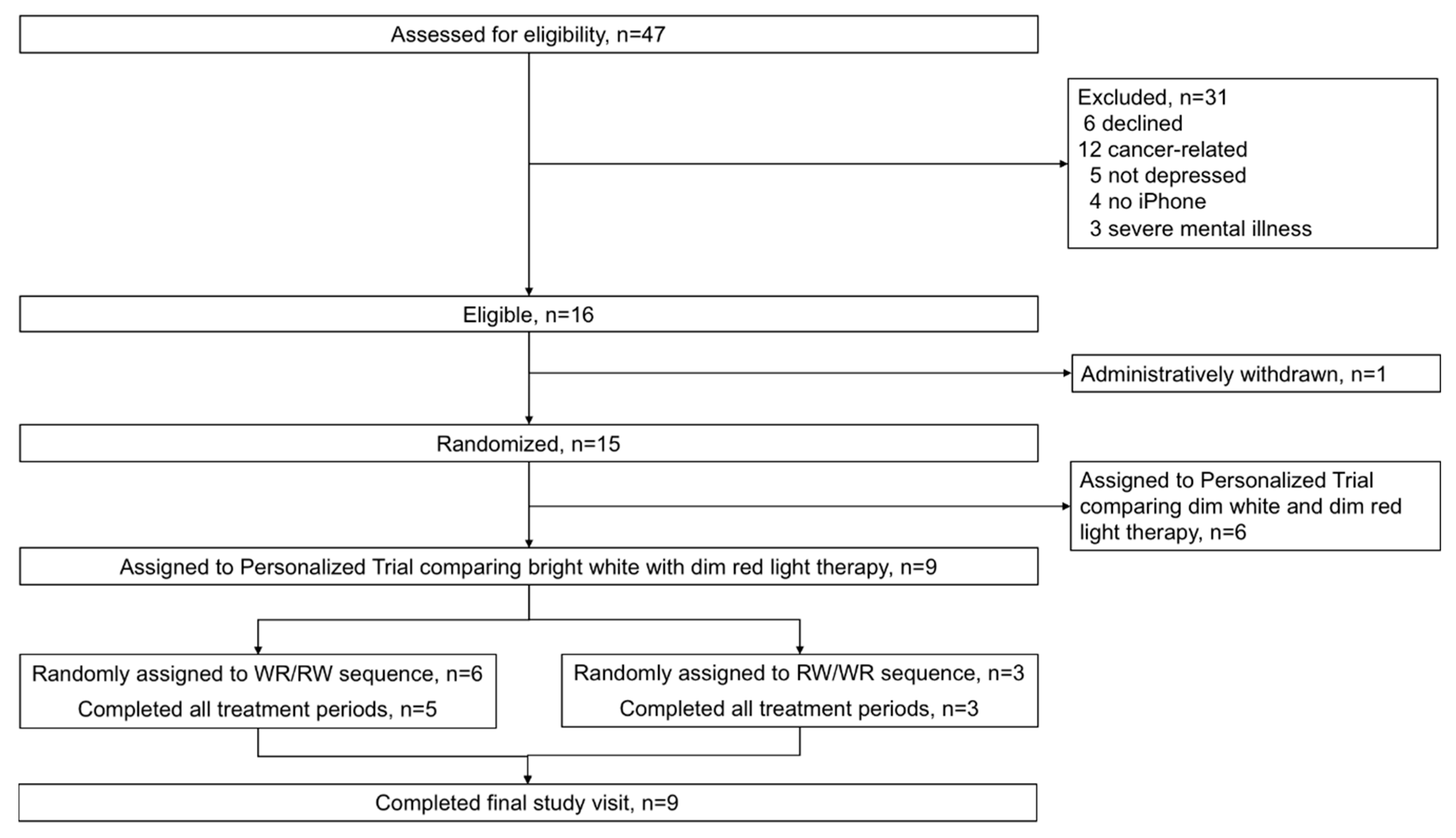
2.2. Eligibility, Recruitment, and Consent
2.3. Personalized Trial Protocol
2.4. Randomization and Blinding
2.5. Outcomes
2.5.1. Depressive (Primary) and Fatigue Symptoms
2.5.2. Side Effects
2.5.3. Lightbox Preference
2.5.4. Other Measures
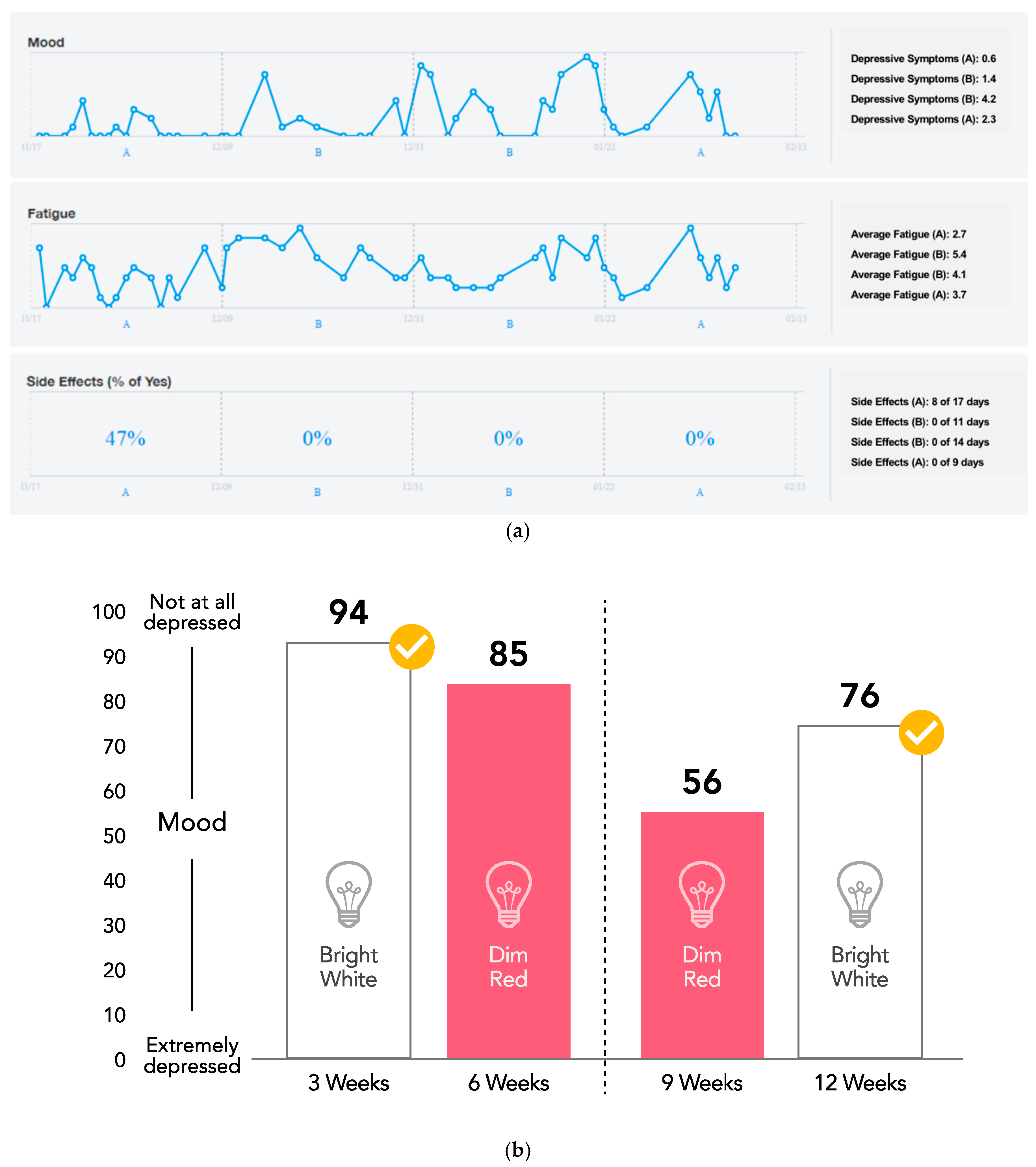
2.6. Data Visualization
2.7. Sample Size Estimate
2.8. Statistical Analyses
2.9. Data Availability
3. Results
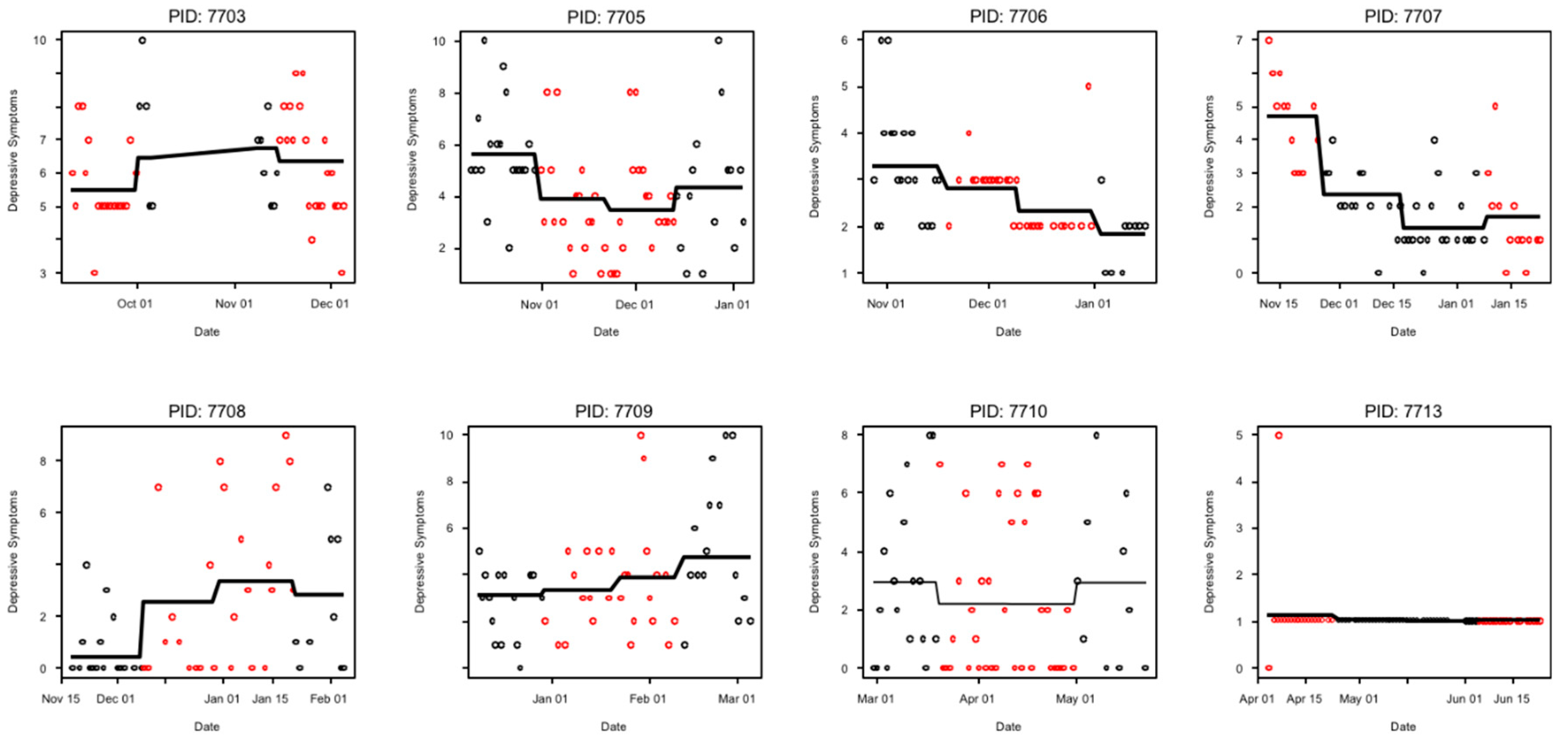
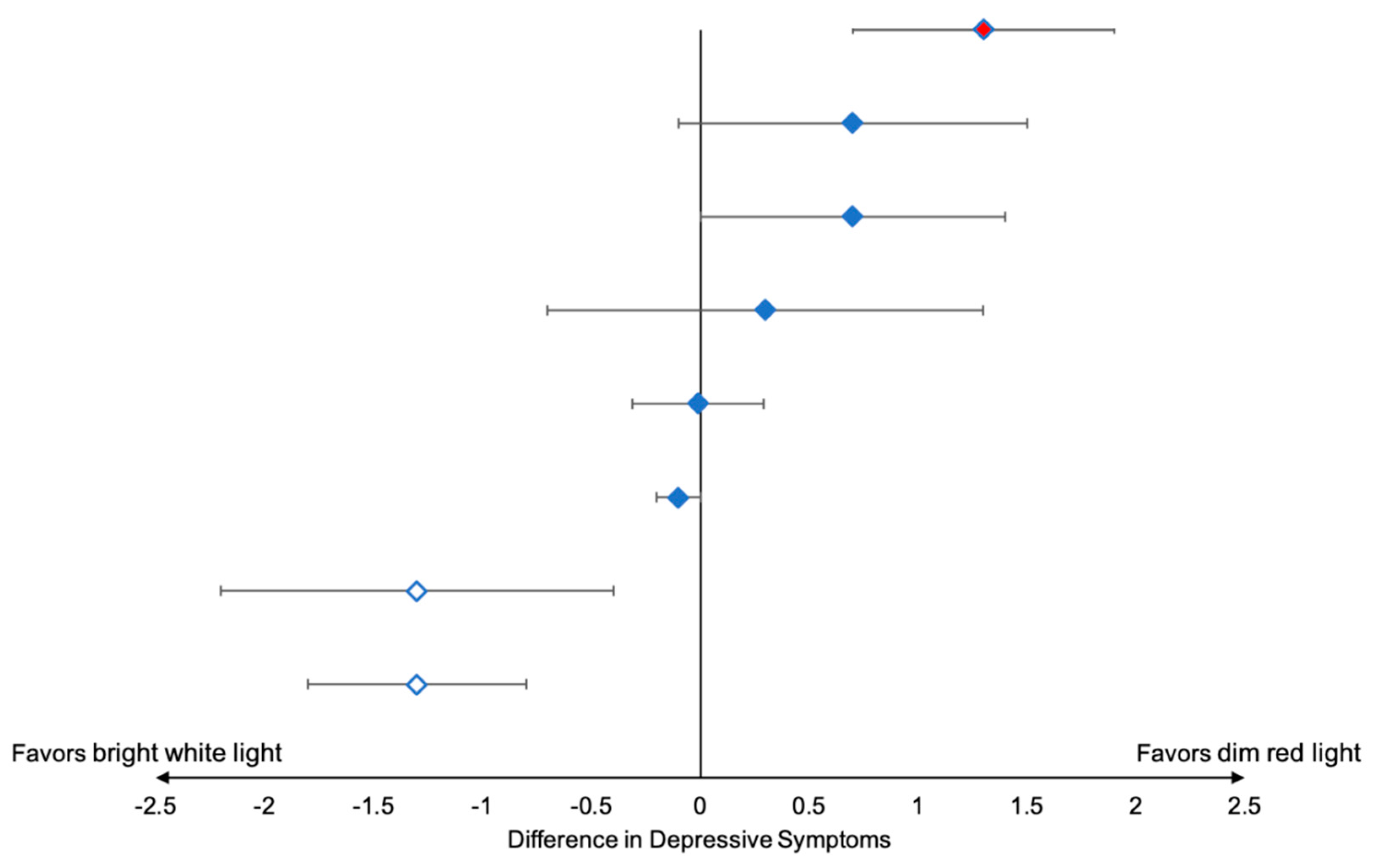
3.1. Outcomes and Estimation
3.2. Harms
3.3. Light Therapy Preferences and Overall Satisfaction with Personalized Trial
4. Discussion
4.1. Limitations
4.2. Generalizability
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Mitchell, A.J.; Chan, M.; Bhatti, H.; Halton, M.; Grassi, L.; Johansen, C.; Meader, N. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: A meta-analysis of 94 interview-based studies. Lancet Oncol. 2011, 12, 160–174. [Google Scholar] [CrossRef]
- Linden, W.; Vodermaier, A.; Mackenzie, R.; Greig, D. Anxiety and depression after cancer diagnosis: Prevalence rates by cancer type, gender, and age. J. Affect. Disord. 2012, 141, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Pirl, W.F. Evidence report on the occurrence, assessment, and treatment of depression in cancer patients. JNCI Monogr. 2004, 32, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, Ó.; Angulo, L.P.; Núñez-Valencia, C.; Dorantes-Gallareta, Y.; Macedo, E.O.; Martínez-López, D.; Alvarado, S.; Corona-Cruz, J.F.; Oñate-Ocaña, L.F. Association of depression and anxiety on quality of life, treatment adherence, and prognosis in patients with advanced non-small cell lung cancer. Ann. Surg. Oncol. 2013, 20, 1941–1948. [Google Scholar] [CrossRef] [PubMed]
- Mausbach, B.T.; Schwab, R.B.; Irwin, S.A. Depression as a predictor of adherence to adjuvant endocrine therapy (AET) in women with breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2015, 152, 239–246. [Google Scholar] [CrossRef] [PubMed]
- DiMatteo, M.R.; Lepper, H.S.; Croghan, T.W. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch. Intern. Med. 2000, 160, 2101–2107. [Google Scholar] [CrossRef]
- Pinquart, M.; Duberstein, P.R. Depression and cancer mortality: A meta-analysis. Psychol. Med. 2010, 40, 1797–1810. [Google Scholar] [CrossRef]
- Satin, J.R.; Linden, W.; Phillips, M.J. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer 2009, 115, 5349–5361. [Google Scholar] [CrossRef]
- Pan, X.; Sambamoorthi, U. Health care expenditures associated with depression in adults with cancer. J. Community Support Oncol. 2015, 13, 240–247. [Google Scholar] [CrossRef]
- Richardson, M.A.; Sanders, T.; Palmer, J.L.; Greisinger, A.; Singletary, S.E. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J. Clin. Oncol. 2000, 18, 2505–2514. [Google Scholar] [CrossRef]
- Owen, D.K.; Lewith, G.; Stephens, C.R. Can doctors respond to patients’ increasing interest in complementary and alternative medicine? BMJ 2001, 322, 154–158. [Google Scholar] [CrossRef]
- Mao, J.J.; Palmer, C.S.; Healy, K.E.; Desai, K.; Amsterdam, J. Complementary and alternative medicine use among cancer survivors: A population-based study. J. Cancer Surviv. 2011, 5, 8–17. [Google Scholar] [CrossRef]
- Golden, R.N.; Gaynes, B.N.; Ekstrom, R.D.; Hamer, R.M.; Jacobsen, F.M.; Suppes, T.; Wisner, K.L.; Nemeroff, C.B. The efficacy of light therapy in the treatment of mood disorders: A review and meta-analysis of the evidence. Am. J. Psychiatry 2005, 162, 656–662. [Google Scholar] [CrossRef]
- Johnson, J.A.; Garland, S.N.; Carlson, L.E.; Savard, J.; Simpson, J.S.A.; Ancoli-Israel, S.; Campbell, T.S. Bright light therapy improves cancer-related fatigue in cancer survivors: A randomized controlled trial. J. Cancer Surviv. 2018, 12, 206–215. [Google Scholar] [CrossRef]
- Valdimarsdottir, H.B.; Figueiro, M.G.; Holden, W.; Lutgendorf, S.; Wu, L.M.; Ancoli-Israel, S.; Chen, J.; Hoffman-Peterson, A.; Granski, J.; Prescott, N.; et al. Programmed environmental illumination during autologous stem cell transplantation hospitalization for the treatment of multiple myeloma reduces severity of depression: A preliminary randomized controlled trial. Cancer Med. 2018, 7, 4345–4353. [Google Scholar] [CrossRef]
- Redd, W.H.; Valdimarsdottir, H.; Wu, L.; Winkel, G.; Byrne, E.E.; Beltre, M.A.; Liebman, E.S.; Erazo, T.; Hayes, J.A.; Isola, L.; et al. Systematic light exposure in the treatment of cancer-related fatigue: A preliminary study. Psychooncology 2014, 23, 1431–1434. [Google Scholar] [CrossRef]
- Kronish, I.M.; Alcántara, C.; Duer-Hefele, J.; Onge, T.S.; Davidson, K.W.; Carter, E.J.; Medina, V.; Cohn, E.; Moise, N. Patients and primary care providers identify opportunities for personalized (N-of-1) trials in the mobile health era. J. Clin. Epidemiol. 2017, 89, 236–237. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duan, N.; Kravitz, R.L.; Schmid, C.H. Single-patient (n-of-1) trials: A pragmatic clinical decision methodology for patient-centered comparative effectiveness research. J. Clin. Epidemiol. 2013, 66, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Duan, N.; Eslick, I.; Gabler, N.B.; Kaplan, H.C.; Kravitz, R.L.; Larson, E.B.; Pace, W.D.; Schmid, C.H.; Sim, I.; Vohra, S. Design and Implementation of N-of-1 Trials: A User’s Guide; Kravitz, R., Duan, N., Eds.; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2014.
- Guyatt, G.H.; Haynes, R.B.; Jaeschke, R.Z.; Cook, D.J.; Green, L.; Naylor, C.D.; Wilson, M.C.; Richardson, W.S.; Evidence-Based Medicine Working Group. Users’ Guides to the Medical Literature: XXV. Evidence-based medicine: Principles for applying the Users’ Guides to patient care. JAMA 2000, 284, 1290–1296. [Google Scholar] [CrossRef] [PubMed]
- Lillie, E.O.; Patay, B.; Diamant, J.; Issell, B.; Topol, E.J.; Schork, N.J. The n-of-1 clinical trial: The ultimate strategy for individualizing medicine? Pers. Med. 2011, 8, 161–173. [Google Scholar] [CrossRef]
- Johnston, B.C.; Mills, E. N-of-1 randomized controlled trials: An opportunity for complementary and alternative medicine evaluation. J. Altern. Complement. Med. 2004, 10, 979–984. [Google Scholar] [CrossRef]
- Vohra, S.; Shamseer, L.; Sampson, M.; Bukutu, C.; Schmid, C.H.; Tate, R.; Nikles, J.; Zucker, D.R.; Kravitz, R.; Guyatt, G.; et al. CONSORT extension for reporting N-of-1 trials (CENT) 2015 Statement. J. Clin. Epidemiol. 2016, 76, 9–17. [Google Scholar] [CrossRef]
- Kroenke, K.; Strine, T.W.; Spitzer, R.L.; Williams, J.B.; Berry, J.T.; Mokdad, A.H. The PHQ-8 as a measure of current depression in the general population. J. Affect. Disord. 2009, 114, 163–173. [Google Scholar] [CrossRef]
- Rea, M.S.; Figueiro, M.G.; Bierman, A.; Bullough, J.D. Circadian light. J. Circadian Rhythms 2010, 8, 2. [Google Scholar] [CrossRef]
- Ahearn, E.P. The use of visual analog scales in mood disorders: A critical review. J. Psychiatr. Res. 1997, 31, 569–579. [Google Scholar] [CrossRef]
- Degner, L.F.; Sloan, J.A.; Venkatesh, P. The Control Preferences Scale. Can. J. Nurs. Res. 1997, 29, 21–43. [Google Scholar]
- Arroll, B.; Elley, C.R.; Fishman, T.; Goodyear-Smith, F.A.; Kenealy, T.; Blashki, G.; Kerse, N.; MacGillivray, S. Antidepressants versus placebo for depression in primary care. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef]
- Ostuzzi, G.; Matcham, F.; Dauchy, S.; Barbui, C.; Hotopf, M. Antidepressants for the treatment of depression in people with cancer. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Lucas, R.J.; Peirson, S.N.; Berson, D.M.; Brown, T.M.; Cooper, H.M.; Czeisler, C.A.; Figueiro, M.G.; Gamlin, P.D.; Lockley, S.W.; O’Hagan, J.B.; et al. Measuring and using light in the melanopsin age. Trends Neurosci. 2014, 37, 1–9. [Google Scholar] [CrossRef]
- Kronish, I.M.; Hampsey, M.; Falzon, L.; Konrad, B.; Davidson, K.W. Personalized (N-of-1) Trials for depression: A systematic review. J. Clin. Psychopharmacol. 2018, 38, 218–225. [Google Scholar] [CrossRef]
- Van Rijsbergen, G.D.; Burger, H.; Hollon, S.D.; Elgersma, H.J.; Kok, G.D.; Dekker, J.; de Jong, P.J.; Bockting, C.L. How do you feel? Detection of recurrent Major Depressive Disorder using a single-item screening tool. Psychiatry Res. 2014, 220, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Maruani, J.; Geoffroy, P.A. Bright light as a personalized precision treatment of mood disorders. Front. Psychiatry 2019, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.; López, G.; Lopez, M.H. Digital Divide Narrows for Latinos as More Spanish-Speakers and Immigrants Go On-Line; Pew Research Center: Washington, WA, USA, 2016. [Google Scholar]
- Molenaar, P. A manifesto on psychology as idiographic science: Bringing the person back into scientific psychology, this time forever. Measurement 2004, 2, 201–218. [Google Scholar] [CrossRef]
| Characteristic | Mean (SD) or n (Percentage) |
|---|---|
| Age in years, mean (SD) | 54.2 (17.7) |
| Female | 7 (78%) |
| Hispanic | 3 (33%) |
| Race: | |
| White | 7 (78%) |
| Black | 1 (11%) |
| Other | 1 (11%) |
| Cancer type: | |
| Breast | 5 (56%) |
| Thyroid | 2 (22%) |
| Bone | 1 (11%) |
| Skin | 1 (11%) |
| Prior cancer treatment: | |
| Surgery | 7 (78%) |
| Chemotherapy | 4 (44%) |
| Radiation | 3 (33%) |
| Hormonal | 4 (44%) |
| Depressed (PHQ8 ≥ 10) | 6 (67%) |
| PHQ-8 score, mean (SD) | 9.7 (2.2) |
| Current depression treatment: | |
| Receiving any depression treatment | 7 (78%) |
| Prescribed antidepressants | 4 (44%) |
| Receiving psychotherapy | 6 (67%) |
| Complementary and alternative medicine | 3 (33%) |
| Decision-making preference: | |
| I want doctor to decide | 0 (0%) |
| I want doctor to consider my ideas | 1 (11%) |
| I want doctor and I to make decisions together | 6 (67%) |
| I want to decide | 2 (22%) |
| Patient | Season (Months) | Treatment Sequence | Percent of Days with Symptom Assessments | Mean Depressive Symptoms (0, None to 10, Severe) | Mean Fatigue Symptoms (0, None to 10, Severe) | Treatment Preference at end of Trial | Likelihood of Continuing Light Therapy (Not at All to Very Much) | Helpfulness of Participation (Not at All to Very Much) | Recommend to Others (Not at All to Very Much) |
|---|---|---|---|---|---|---|---|---|---|
| 1 (7702) | Fall (September-December) | W-R-R-W | 19% | Insufficient | Insufficient | Neither | Not at all likely to continue | Not at all helpful | Recommend a little bit |
| 2 (7703) | Fall (September-December) | R-W-W-R | 62% † | R: 5.9 W: 6.7 Difference: 0.7 SE 0.8, p = 0.40 | R: 7.0 W: 8.2 Difference: 1.3 SE 0.5, p = 0.001 | R | Somewhat likely to continue | Somewhat helpful | Recommend a little bit |
| 3 (7705) | Fall (October–January) | W-R-R-W | 80% | R: 3.6 W: 5.1 Difference: 1.3 SE 0.6, p = 0.04 | R: 4.7 W: 5.7 Difference: 0.9 SE 0.7, p = 0.17 | R | Somewhat likely to continue | Very much helpful | Recommend strongly |
| 4 (7706) | Fall (November–January) | W-R-R-W | 64% | R: 2.6 W: 2.8 Difference: −0.01 SE 0.3, p = 0.98 | R: 2.6 W: 2.6 Difference: −0.2 SE 0.2, p = 0.43 | Neither | Not at all likely to continue | Not at all helpful | Recommend not at all |
| 5 7707 | Fall (November–January) | R-W-W-R | 66% | R: 3.0 W: 1.8 Difference: −1.3 SE 0.5, p = 0.005 | R: 3.8 W: 3.7 Difference: −0.3 SE 0.5, p = 0.59 | W | Very likely to continue | Very much helpful | Recommend strongly |
| 6 7708 | Winter (November–February) | W-R-R-W | 57% | R: 3.0 W: 1.3 Difference: −1.3 SE 0.9, p = 0.17 | R: 4.6 W: 2.9 Difference: −1.7 SE 0.7, p = 0.03 | W | Very likely to continue | Very much helpful | Recommend strongly |
| 7 7709 | Winter (December–March) | W-R-R-W | 64% | R: 3.6 W: 4.0 Difference: 0.3 SE 1.0, p = 0.74 | R: 4.8 W: 6.0 Difference: 0.6 SE 1.0, p = 0.55 | W | Very likely to continue | Very much helpful | Recommend strongly |
| 8 7710 | Spring (March–June) | W-R-R-W | 77% | R: 2.2 W: 3.0 Difference: 0.7 SE 0.7, p = 0.32 | R: 3.1 W: 3.4 Difference: 0.3 SE 0.6, p = 0.60 | Either | Unlikely to continue | Somewhat helpful | Recommend strongly |
| 9 7713 | Spring (April–June) | R-W-W-R | 89% | R: 1.1 W: 1.0 Difference: −0.1 SE 0.1, p = 0.51 | R: 7.3 W: 7.1 Difference: −0.2 SE 0.4, p = 0.65 | Either | Unlikely to continue | A little bit helpful | Recommend strongly |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kronish, I.M.; Cheung, Y.K.; Julian, J.; Parsons, F.; Lee, J.; Yoon, S.; Valdimarsdottir, H.; Green, P.; Suls, J.; Hershman, D.L.; et al. Clinical Usefulness of Bright White Light Therapy for Depressive Symptoms in Cancer Survivors: Results from a Series of Personalized (N-of-1) Trials. Healthcare 2020, 8, 10. https://doi.org/10.3390/healthcare8010010
Kronish IM, Cheung YK, Julian J, Parsons F, Lee J, Yoon S, Valdimarsdottir H, Green P, Suls J, Hershman DL, et al. Clinical Usefulness of Bright White Light Therapy for Depressive Symptoms in Cancer Survivors: Results from a Series of Personalized (N-of-1) Trials. Healthcare. 2020; 8(1):10. https://doi.org/10.3390/healthcare8010010
Chicago/Turabian StyleKronish, Ian M., Ying Kuen Cheung, Jacob Julian, Faith Parsons, Jenny Lee, Sunmoo Yoon, Heidis Valdimarsdottir, Paige Green, Jerry Suls, Dawn L. Hershman, and et al. 2020. "Clinical Usefulness of Bright White Light Therapy for Depressive Symptoms in Cancer Survivors: Results from a Series of Personalized (N-of-1) Trials" Healthcare 8, no. 1: 10. https://doi.org/10.3390/healthcare8010010
APA StyleKronish, I. M., Cheung, Y. K., Julian, J., Parsons, F., Lee, J., Yoon, S., Valdimarsdottir, H., Green, P., Suls, J., Hershman, D. L., & Davidson, K. W. (2020). Clinical Usefulness of Bright White Light Therapy for Depressive Symptoms in Cancer Survivors: Results from a Series of Personalized (N-of-1) Trials. Healthcare, 8(1), 10. https://doi.org/10.3390/healthcare8010010





