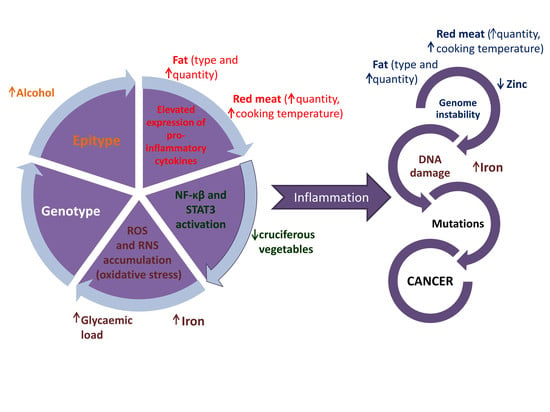
The Mediterranean Diet and Breast Cancer: A Personalised Approach
Abstract
1. Introduction
2. Materials and Methods
3. Mediterranean Diet and Breast Cancer
3.1. Fatty Acids Commonly Consumed as Part of a Mediterranean-Style Diet
3.2. Mediterranean Polyphenols
3.2.1. Epigallocatechin-3-Gallate (EGCG)
3.2.2. Resveratrol
3.2.3. Organosulfur Compounds
3.2.4. Quercetin
3.2.5. Kaempferol
3.2.6. Apigenin
3.3. Micronutrients Commonly Found in a Mediterranean-Style Diet
3.3.1. Zinc
3.3.2. Selenium (Se)
4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eyre, H.; Kahn, R.; Robertson, R.M.; Clark, N.G.; Doyle, C.; Hong, Y.; Gansler, T.; Glynn, T.; Smith, R.A.; Taubert, K.; et al. Preventing Cancer, Cardiovascular Disease, and Diabetes. Circulation 2004, 109, 3244–3255. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Theodoratou, E.; Timofeeva, M.; Li, X.; Meng, X.; Ioannidis, J.P. Nature, nurture, and cancer risks: Genetic and nutritional contributions to cancer. Annu. Rev. Nutr. 2017, 37, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in Globocan 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- El Saghir, N.S.; Khalil, M.K.; Eid, T.; El Kinge, A.R.; Charafeddine, M.; Geara, F.; Seoud, M.; Shamseddine, A.I. Trends in epidemiology and management of breast cancer in developing Arab countries: A literature and registry analysis. Int. J. Surg. 2007, 5, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, S.N. Temporal trends in breast cancer presentation in the third world. J. Exp. Clin. Cancer Res. 2008, 27, 17. [Google Scholar] [CrossRef] [PubMed]
- Porter, P. “Westernizing” women’s risks? Breast cancer in lower-income countries. N. Engl. J. Med. 2008, 358, 213–216. [Google Scholar] [CrossRef]
- Castelló, A.; Pollán, M.; Buijsse, B.; Ruiz, A.; Casas, A.; Baena-Cañada, J.; Lope, V.; Antolín, S.; Ramos, M.; Muñoz, M. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: Case–control EpiGEICAM study. Br. J. Cancer 2014, 111, 1454–1462. [Google Scholar] [CrossRef]
- Glade, M.J. Food, nutrition, and the prevention of cancer: A global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition 1999, 15, 523. [Google Scholar]
- Kilpeläinen, T.O.; Carli, J.F.M.; Skowronski, A.A.; Sun, Q.; Kriebel, J.; Feitosa, M.F.; Hedman, Å.K.; Drong, A.W.; Hayes, J.E.; Zhao, J. Genome-wide meta-analysis uncovers novel loci influencing circulating leptin levels. Nat. Commun. 2016, 7, 10494. [Google Scholar] [CrossRef]
- Treur, J.L.; Boomsma, D.I.; Ligthart, L.; Willemsen, G.; Vink, J.M. Heritability of high sugar consumption through drinks and the genetic correlation with substance use 2. Am. J. Clin. Nutr. 2016, 104, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Pallister, T.; Sharafi, M.; Lachance, G.; Pirastu, N.; Mohney, R.P.; MacGregor, A.; Feskens, E.J.; Duffy, V.; Spector, T.D.; Menni, C. Food preference patterns in a UK twin cohort. Twin Res. Hum. Genet. 2015, 18, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Gammon, M.D.; Santella, R.M.; Gaudet, M.M.; Britton, J.A.; Teitelbaum, S.L.; Terry, M.B.; Nowell, S.; Davis, W.; Garza, C.; et al. Associations between Breast Cancer Risk and the Catalase Genotype, Fruit and Vegetable Consumption, and Supplement Use. Am. J. Epidemiol. 2005, 162, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gammon, M.D.; Chan, W.; Palomeque, C.; Wetmur, J.G.; Kabat, G.C.; Teitelbaum, S.L.; Britton, J.A.; Terry, M.B.; Neugut, A.I.; et al. One-carbon metabolism, MTHFR polymorphisms, and risk of breast cancer. Cancer Res. 2005, 65, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, K.; Jakubowska, A.; Sukiennicki, G.; Muszynska, M.; Jaworska-Bieniek, K.; Durda, K.; Huzarski, T.; Serrano-Fernandez, P.; Byrski, T.; Gronwald, J.; et al. Zinc and breast cancer risk. Hered. Cancer Clin. Pract. 2012, 10. [Google Scholar] [CrossRef]
- Xu, X.; Gammon, M.D.; Wetmur, J.G.; Bradshaw, P.T.; Teitelbaum, S.L.; Neugut, A.I.; Santella, R.M.; Chen, J. B-vitamin intake, one-carbon metabolism, and survival in a population-based study of women with breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2109–2116. [Google Scholar] [CrossRef] [PubMed]
- Lenihan-Geels, G.; Bishop, K.S.; Ferguson, L.R. Cancer Risk and Eicosanoid Production: Interaction between the Protective Effect of Long Chain Omega-3 Polyunsaturated Fatty Acid Intake and Genotype. J. Clin. Med. 2016, 5. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Flatt, S.W.; Byers, T.E.; Colditz, G.A.; Demark-Wahnefried, W.; Ganz, P.A.; Wolin, K.Y.; Elias, A.; Krontiras, H.; Liu, J. Results of the exercise and nutrition to enhance recovery and good health for you (ENERGY) trial: A behavioral weight loss intervention in overweight or obese breast cancer survivors. J. Clin. Oncol. 2015, 33, 3169–3176. [Google Scholar] [CrossRef]
- Wang, T.; Heianza, Y.; Sun, D.; Huang, T.; Ma, W.; Rimm, E.B.; Manson, J.E.; Hu, F.B.; Willett, W.C.; Qi, L. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: Gene-diet interaction analysis in two prospective cohort studies. BMJ 2018, 360, 5644. [Google Scholar] [CrossRef]
- Escrich, E.; Solanas, M.; Moral, R.; Escrich, R. Modulatory effects and molecular mechanisms of olive oil and other dietary lipids in breast cancer. Curr. Pharm. Des. 2011, 17, 813–830. [Google Scholar] [CrossRef]
- Tillotson, J.; Darzynkiewicz, Z.; Cohen, L.A.; Ronai, Z. Effects of linoleic acid on mammary tumor cell proliferation are associated with changes in p53 protein expression. Int. J. Oncol. 1993, 3, 81. [Google Scholar] [CrossRef] [PubMed]
- Pirouzpanah, S.; Taleban, F.-A.; Mehdipour, P.; Atri, M. Association of folate and other one-carbon related nutrients with hypermethylation status and expression of RARB. J. Mol. Med. 2015, 93, 917–934. [Google Scholar] [CrossRef] [PubMed]
- Shayoun, N.R.; Sankavaram, K. Historical origins of the Mediterranean Diet, Regional Dietary Profiles, and the Development of the Dietary Guidelines. In Mediterranean Diet, Nutrition and Health; Donato, R., Ornella, S., Eds.; Humana Press: Cham, Germany, 2016; pp. 43–56. [Google Scholar]
- van den Brandt, P.A.; Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Andreescu, N.; Puiu, M.; Niculescu, M. Effects of Dietary Nutrients on Epigenetic Changes in Cancer. In Cancer Epigenetics for Precision Medicine; Ramona, D., Mukesh, V., Eds.; Springer Nature, Humana Press: New York, NY, USA, 2018; Volume 1856, pp. 121–139. [Google Scholar]
- World Cancer Research Fund. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective; American Institute for Cancer Research: Washington, DC, USA, 2007. [Google Scholar]
- Chlebowski, R.; Aragaki, A.; Thomson, C.; Anderson, G.; Manson, J.; Simon, M.; Rohan, T.; Snetselar, L.; Lane, D.; Barrington, W. Abstract S5–04: Low-fat dietary pattern and breast cancer overall survival in the women’s health initiative dietary modification randomized controlled trial. Cancer Res. 2017, 77. [Google Scholar] [CrossRef]
- Xing, M.-Y.; Xu, S.-Z.; Shen, P. Effect of low-fat diet on breast cancer survival: A meta-analysis. Asian Pac. J. Cancer Preven. 2014, 15, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- Mourouti, N.; Kontogianni, M.D.; Papavagelis, C.; Plytzanopoulou, P.; Vassilakou, T.; Malamos, N.; Linos, A.; Panagiotakos, D.B. Adherence to the Mediterranean diet is associated with lower likelihood of breast cancer: A case-control study. Nutr. Cancer 2014, 66, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.L.; Weltzien, E.; Kushi, L.H.; Castillo, A.; Slattery, M.L.; Caan, B.J. Dietary patterns and breast cancer recurrence and survival among women with early-stage breast cancer. J. Clin. Oncol. 2008, 27, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Willett, W.C.; Fung, T.; Rosner, B.; Holmes, M.D. Diet quality indices and postmenopausal breast cancer survival. Nutr. Cancer 2011, 63, 381–388. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Olopade, O.I.; Ghadirian, P.; Lubinski, J.; Lynch, H.T.; Isaacs, C.; Weber, B.; Kim-Sing, C.; Ainsworth, P.; Foulkes, W.D. Changes in body weight and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2005, 7, R833. [Google Scholar] [CrossRef]
- Thomson, C.A.; Thompson, P.A. Dietary patterns, risk and prognosis of breast cancer. Future Oncol. 2009, 5, 1257–1269. [Google Scholar] [CrossRef]
- Grosso, G.; Marventano, S.; D’Urso, M.; Mistretta, A.; Galvano, F. The Mediterranean healthy eating, ageing, and lifestyle (MEAL) study: Rationale and study design. Int. J. Food Sci. Nutr. 2017, 68, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Drabsch, T.; Holzapfel, C. A Scientific Perspective of Personalised Gene-Based Dietary Recommendations for Weight Management. Nutrients 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Rush, E.C. Water: Neglected, unappreciated and under researched. Eur. J. Clin. Nutr. 2013, 67, 492–495. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.; White, E.; Shattuck, A.L.; Potter, J.D. Relationship of food groups and water intake to colon cancer risk. Cancer Epidemiol. Biomark. Prev. 1996, 5, 495–502. [Google Scholar]
- Stookey, J.D.; Belderson, P.E.; Russell, J.M.; Barker, M.E. Correspondence to: Shannon, J.; White, E.; Shattuck, A.L.; Potter, J.D. Relationship of food groups and water intake to colon cancer risk. Cancer Epidemiol. Cancer Epidemiol. Biomark. Prev. 1997, 6, 657–658. [Google Scholar]
- Beasley, J.M.; Newcomb, P.A.; Trentham-Dietz, A.; Hampton, J.M.; Bersch, A.J.; Passarelli, M.N.; Holick, C.N.; Titus-Ernstoff, L.; Egan, K.M.; Holmes, M.D. Post-diagnosis dietary factors and survival after invasive breast cancer. Breast Cancer Res. Treat. 2011, 128, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Travier, N.; Agudo, A.; Fonseca-Nunes, A.; Navarro, C.; Lagiou, P.; Demetriou, C.; Amiano, P.; Dorronsoro, M.; Chirlaque, M.D. Olive oil intake and breast cancer risk in the Mediterranean countries of the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2012, 131, 2465–2469. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Carrasco-Pancorbo, A.; Simal-Gándara, J.; Giampieri, F. Characterization of phenolic extracts from Brava extra virgin olive oils and their cytotoxic effects on MCF-7 breast cancer cells. Food Chem. Toxicol. 2018, 119, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Patterson, R.E.; Flatt, S.W.; Newman, V.A.; Natarajan, L.; Rock, C.L.; Thomson, C.A.; Caan, B.J.; Parker, B.A.; Pierce, J.P. Marine fatty acid intake is associated with breast cancer prognosis. J. Nutr. 2011, 141, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Biondo, P.D.; Brindley, D.N.; Sawyer, M.B.; Field, C.J. The potential for treatment with dietary long-chain polyunsaturated n-3 fatty acids during chemotherapy. J. Nutr. Biochem. 2008, 19, 787–796. [Google Scholar] [CrossRef]
- Saedisomeolia, A.; Wood, L.G.; Garg, M.L.; Gibson, P.G.; Wark, P.A. Anti-inflammatory effects of long-chain n-3 PUFA in rhinovirus-infected cultured airway epithelial cells. Br. J. Nutr. 2009, 101, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Bougnoux, P.; Germain, E.; Chajes, V.; Hubert, B.; Lhuillery, C.; Le Floch, O.; Body, G.; Calais, G. Cytotoxic drugs efficacy correlates with adipose tissue docosahexaenoic acid level in locally advanced breast carcinoma. Br. J. Cancer 1999, 79, 1765. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Connolly, J.M.; Rayburn, J.; Coleman, M. Influence of diets containing eicosapentaenoic or docosahexaenoic acid on growth and metastasis of breast cancer cells in nude mice. J. Natl. Cancer Inst. 1995, 87, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E.; Ion, G.; Akinsete, J.A.; Witte, T.R. Dietary walnut suppressed mammary gland tumorigenesis in the C (3) 1 TAg mouse. Nutr. Cancer 2011, 63, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Escrich, E.; Moral, R.; Grau, L.; Costa, I.; Solanas, M. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol. Nutr. Food Res. 2007, 51, 1279–1292. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Gallon, D.J.; Vissac-Sabatier, C.; Antoine-Vincent, D.; Rio, P.G.; Maurizis, J.-C.; Fustier, P.; Bignon, Y.-J. Differential effects of n-3 and n-6 polyunsaturated fatty acids on BRCA1 and BRCA2 gene expression in breast cell lines. Br. J. Nutr. 2002, 87, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.; Vellon, L.; Colomer, R.; Lupu, R. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses Her-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin™) in breast cancer cells with Her-2/neu oncogene amplification. Ann. Oncol. 2005, 16, 359–371. [Google Scholar] [CrossRef]
- Gago-Dominguez, M.; Castelao, J.E.; Sun, C.L.; Van Den Berg, D.; Koh, W.P.; Lee, H.P.; Yu, M.C. Marine n-3 fatty acid intake, glutathione S-transferase polymorphisms and breast cancer risk in post-menopausal Chinese women in Singapore. Carcinogenesis 2004, 25, 2143–2147. [Google Scholar] [CrossRef] [PubMed]
- Ceschi, M.; Sun, C.L.; Van Den Berg, D.; Koh, W.P.; Yu, M.C.; Probst-Hensch, N. The effect of cyclin D1 (CCND1) G870A-polymorphism on breast cancer risk is modified by oxidative stress among Chinese women in Singapore. Carcinogenesis 2005, 26, 1457–1464. [Google Scholar] [CrossRef]
- Prasad, V.V.T.S.; Kolli, P.; Moganti, D. Association of a functional polymorphism (Gln261Arg) in 12-lipoxygenase with breast cancer. Exp. Ther. Med. 2011, 2, 317–323. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary Polyphenols and the Prevention of Diseases. Crit. Rev. Food. Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Tsao, R.; Yang, R.; Cui, S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006, 95, 466–473. [Google Scholar] [CrossRef]
- Adom, K.K.; Liu, R.H. Antioxidant activity of grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, A.; Szostak-Wegierek, D. Flavonoids-food sources and health benefits. Rocz. Państwowego Zakładu Hig. 2014, 65, 79–85. [Google Scholar]
- Seo, H.-S.; Ku, J.M.; Hong, S.H.; Choi, H.S.; Woo, J.-K.; Jang, B.-H.; Shin, Y.C.; Ko, S.-G. Apigenin overcomes drug resistance by blocking signal transducer and activator of transcription 3 (STAT3) signaling in breast cancer. Oncol. Rep. 2017, 38, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Lakhanpal, P.; Rai, D.K. Quercetin: A versatile flavonoid. Internet J. Med. Update 2007, 2, 22–37. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Cancer and metastasis: Prevention and treatment by green tea. Cancer Metastasis Rev. 2010, 29, 435–445. [Google Scholar] [CrossRef]
- Yang, C.S.; Landau, J.M.; Huang, M.-T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Berner, C.; Aumüller, E.; Gnauck, A.; Nestelberger, M.; Just, A.; Haslberger, A.G. Epigenetic control of estrogen receptor expression and tumor suppressor genes is modulated by bioactive food compounds. Ann. Nutr. Metab. 2010, 57, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.Z.; Wang, Y.; Ai, N.; Hou, Z.; Sun, Y.; Lu, H.; Welsh, W.; Yang, C.S. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003, 63, 7563–7570. [Google Scholar] [PubMed]
- Lu, H.; Meng, X.; Li, C.; Sang, S.; Patten, C.; Sheng, S.; Hong, J.; Bai, N.; Winnik, B.; Ho, C.-T. Glucuronides of tea catechins: Enzymology of biosynthesis and biological activities. Drug Metab. Dispos. 2003, 31, 452–461. [Google Scholar] [CrossRef]
- Berletch, J.B.; Liu, C.; Love, W.K.; Andrews, L.G.; Katiyar, S.K.; Tollefsbol, T.O. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J. Cell Biochem. 2008, 103, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Deb, G.; Thakur, V.S.; Limaye, A.M.; Gupta, S. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol. Carcinog. 2015, 54, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; George, J.; Srivastava, S.; Tyagi, S.; Shukla, Y. Inhibitory effects of tea polyphenols by targeting cyclooxygenase-2 through regulation of nuclear factor kappa B, Akt and p53 in rat mammary tumors. Investig. New Drugs 2011, 29, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Alotaibi, A.; Bhatnagar, P.; Najafzadeh, M.; Gupta, K.C.; Anderson, D. Tea phenols in bulk and nanoparticle form modify DNA damage in human lymphocytes from colon cancer patients and healthy individuals treated in vitro with platinum-based chemotherapeutic drugs. Nanomedicine 2013, 8, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Glei, M.; Pool-Zobel, B. The main catechin of green tea,(−)-epigallocatechin-3-gallate (EGCG), reduces bleomycin-induced DNA damage in human leucocytes. Toxicol. In Vitro 2006, 20, 295–300. [Google Scholar] [CrossRef]
- Ogunleye, A.A.; Xue, F.; Michels, K.B. Green tea consumption and breast cancer risk or recurrence: A meta-analysis. Breast Cancer Res. Ther. 2009, 119, 477. [Google Scholar] [CrossRef]
- Bao, P.-P.; Zhao, G.-M.; Shu, X.-O.; Peng, P.; Cai, H.; Lu, W.; Zheng, Y. Modifiable Lifestyle Factors and Triple-negative Breast Cancer Survival: A Population-based Prospective Study. Epidemiology 2015, 26, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Chachay, V.S.; Kirkpatrick, C.M.; Hickman, I.J.; Ferguson, M.; Prins, J.B.; Martin, J.H. Resveratrol–pills to replace a healthy diet? Br. J. Clin. Pharmacol. 2011, 72, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Vilain, A.; Vogt, N.; Dutrillaux, B.; Malfoy, B. DNA methylation and chromosome instability in breast cancer cell lines. FEBS Lett. 1999, 460, 231–234. [Google Scholar] [CrossRef][Green Version]
- Baylin, S.B.; Esteller, M.; Rountree, M.R.; Bachman, K.E.; Schuebel, K.; Herman, J.G. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum. Mol. Genet. 2001, 10, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Girault, I.; Tozlu, S.; Lidereau, R.; Bièche, I. Expression analysis of DNA methyltransferases 1, 3A, and 3B in sporadic breast carcinomas. Clin. Cancer Res. 2003, 9, 4415–4422. [Google Scholar] [PubMed]
- Qin, W.; Zhang, K.; Clarke, K.; Weiland, T.; Sauter, E.R. Methylation and miRNA Effects of Resveratrol on Mammary Tumors vs. Normal Tissue. Nutr. Cancer 2014, 66, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Fustier, P.; Le Corre, L.; Chalabi, N.; Vissac-Sabatier, C.; Communal, Y.; Bignon, Y.; Bernard-Gallon, D. Resveratrol increases BRCA1 and BRCA2 mRNA expression in breast tumour cell lines. Br. J. Cancer 2003, 89, 168–172. [Google Scholar] [CrossRef]
- Papoutsis, A.J.; Lamore, S.D.; Wondrak, G.T.; Selmin, O.I.; Romagnolo, D.F. Resveratrol prevents epigenetic silencing of BRCA-1 by the aromatic hydrocarbon receptor in human breast cancer cells. J. Nutr. 2010, 140, 1607–1614. [Google Scholar] [CrossRef]
- Kala, R.; Shah, H.N.; Martin, S.L.; Tollefsbol, T.O. Epigenetic-based combinatorial resveratrol and pterostilbene alters DNA damage response by affecting SIRT1 and DNMT enzyme expression, including SIRT1-dependent γ-H2AX and telomerase regulation in triple-negative breast cancer. BMC Cancer 2015, 15, 672. [Google Scholar] [CrossRef]
- Banerjee, S.; Bueso-Ramos, C.; Aggarwal, B.B. Suppression of 7, 12-dimethylbenz (a) anthracene-induced mammary carcinogenesis in rats by resveratrol. Cancer Res. 2002, 62, 4945–4954. [Google Scholar]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.; Ghadirian, P.; Little, J.; Lubinski, J.; Gronwald, J.; Kim-Sing, C.; Foulkes, W.; Moller, P.; Lynch, H.T.; Neuhausen, S.L. Alcohol consumption and the risk of breast cancer among BRCA1 and BRCA2 mutation carriers. Breast 2010, 19, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Vainio, H. Wine and resveratrol: Mechanisms of cancer prevention? Eur. J. Cancer Prev. 2003, 12, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.H.; Marian, C.; Shields, P.G.; Nie, J.; McCann, S.E.; Millen, A.; Ambrosone, C.; Hutson, A.; Edge, S.B.; Krishnan, S.S. Alcohol consumption in relation to aberrant DNA methylation in breast tumors. Alcohol 2011, 45, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Purohit, V. Moderate alcohol consumption and estrogen levels in postmenopausal women: A review. Alcohol. Clin. Exp. Res. 1998, 22, 994–997. [Google Scholar] [CrossRef]
- Le Corre, L.; Fustier, P.; Chalabi, N.; Bignon, Y.-J.; Bernard-Gallon, D. Effects of resveratrol on the expression of a panel of genes interacting with the BRCA1 oncosuppressor in human breast cell lines. Clin. Chim. Acta 2004, 344, 115–121. [Google Scholar] [CrossRef]
- Flatt, S.W.; Thomson, C.A.; Gold, E.B.; Natarajan, L.; Rock, C.L.; Al-Delaimy, W.K.; Patterson, R.E.; Saquib, N.; Caan, B.J.; Pierce, J.P. Low to moderate alcohol intake is not associated with increased mortality after breast cancer. Cancer Epidemiol. Prev. Biomark. 2010, 19, 681–688. [Google Scholar] [CrossRef]
- Dumitrescu, R.G. Alcohol-Induced Epigenetic Changes in Cancer. In Cancer Epigenetics for Precision Medicine. Methods in Molecular Biology; Springer Nature, Humana Press: New York, NY, USA, 2018; Volume 1856. [Google Scholar]
- Hankinson, S.E.; Willett, W.C.; Manson, J.E.; Colditz, G.A.; Hunter, D.J.; Spiegelman, D.; Barbieri, R.L.; Speizer, F.E. Plasma sex steroid hormone levels and risk of breast cancer in postmenopausal women. J. Natl. Cancer Inst. 1998, 90, 1292–1299. [Google Scholar] [CrossRef]
- Rose, D.P.; Vona-Davis, L. The cellular and molecular mechanisms by which insulin influences breast cancer risk and progression. Endocr. Relat. Cancer 2012, 19, 225–241. [Google Scholar] [CrossRef]
- Muendlein, A.; Lang, A.H.; Geller-Rhomberg, S.; Winder, T.; Gasser, K.; Drexel, H.; Decker, T.; Mueller-Holzner, E.; Chamson, M.; Marth, C. Association of a common genetic variant of the IGF-1 gene with event-free survival in patients with HER2-positive breast cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 491–498. [Google Scholar] [CrossRef]
- Jung, S.Y.; Papp, J.C.; Sobel, E.M.; Zhang, Z.-F. Genetic variants in metabolic signaling pathways and their interaction with lifestyle factors on breast cancer risk: A random survival forest analysis. Cancer Prev. Res 2018, 11, 44–51. [Google Scholar] [CrossRef]
- Zhang, S.; Hunter, D.J.; Hankinson, S.E.; Giovannucci, E.L.; Rosner, B.A.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. A prospective study of folate intake and the risk of breast cancer. J. Am. Med Assoc. 1999, 281, 1632–1637. [Google Scholar] [CrossRef]
- Hillman, R.; Steinberg, S. The effects of alcohol on folate metabolism. Annu. Rev. Med. 1982, 33, 345–354. [Google Scholar] [CrossRef]
- Meeran, S.M.; Patel, S.N.; Li, Y.; Shukla, S.; Tollefsbol, T.O. Bioactive dietary supplements reactivate ER expression in ER-negative breast cancer cells by active chromatin modifications. PLoS ONE 2012, 7, e37748. [Google Scholar] [CrossRef] [PubMed]
- Myzak, M.C.; Dashwood, R.H. Histone deacetylases as targets for dietary cancer preventive agents: Lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr. Drug Targets 2006, 7, 443–452. [Google Scholar] [CrossRef]
- Nian, H.; Delage, B.; Ho, E.; Dashwood, R.H. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: Studies with sulforaphane and garlic organosulfur compounds. Environ. Mol. Mutagen. 2009, 50, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Dashwood, R.H.; Ho, E. Dietary histone deacetylase inhibitors: From cells to mice to man. Semin. Cancer Biol. 2007, 17, 363–369. [Google Scholar] [CrossRef]
- Riso, P.; Martini, D.; Visioli, F.; Martinetti, A.; Porrini, M. Effect of broccoli intake on markers related to oxidative stress and cancer risk in healthy smokers and nonsmokers. Nutr. Cancer 2009, 61, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Kyo, E.; Uda, N.; Suzuki, A.; Kakimoto, M.; Ushijima, M.; Kasuga, S.; Itakura, Y. Immunomodulation and antitumor activities of aged garlic extract. Phytomedicine 1998, 5, 259–267. [Google Scholar] [CrossRef]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Recent advances on the nutritional effects associated with the use of garlic as a supplement. J. Nutr. 2001, 131, 955–962. [Google Scholar] [CrossRef]
- Ishikawa, H.; Saeki, T.; Otani, T.; Suzuki, T.; Shimozuma, K.; Nishino, H.; Fukuda, S.; Morimoto, K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006, 136, 816–820. [Google Scholar] [CrossRef]
- Kuttan, G. Immunomodulatory effect of some naturally occuring sulphur-containing compounds. J Ethnopharmacol. 2000, 72, 93–99. [Google Scholar] [CrossRef]
- Murakami, A.; Ashida, H.; Terao, J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008, 269, 315–325. [Google Scholar] [CrossRef]
- O’Leary, K.A.; de Pascual-Tereasa, S.; Needs, P.W.; Bao, Y.-P.; O’Brien, N.M.; Williamson, G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 551, 245–254. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kim, J.H.; Prasad, S.; Aggarwal, B.B. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010, 29, 405–434. [Google Scholar] [CrossRef]
- Ranganathan, S.; Halagowder, D.; Sivasithambaram, N.D. Quercetin Suppresses Twist to Induce Apoptosis in MCF-7 Breast Cancer Cells. PLoS ONE 2015, 10, e0141370. [Google Scholar] [CrossRef] [PubMed]
- Duo, J.; Ying, G.; Wang, G.; Zhang, L. Quercetin inhibits human breast cancer cell proliferation and induces apoptosis via Bcl-2 and Bax regulation. Mol. Med. Rep. 2012, 5, 1453–1456. [Google Scholar] [CrossRef] [PubMed]
- Roslan, J.; Giribabu, N.; Karim, K.; Salleh, N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed. Pharmacother. 2017, 86, 570–582. [Google Scholar] [CrossRef]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of Quercetin-Loaded Nanoparticles on MCF-7 Human Breast Cancer Cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef]
- Li, S.; Yuan, S.; Zhao, Q.; Wang, B.; Wang, X.; Li, K. Quercetin enhances chemotherapeutic effect of doxorubicin against human breast cancer cells while reducing toxic side effects of it. Biomedicine & Pharmacotherapy 2018, 100, 441–447. [Google Scholar] [CrossRef]
- Kowalski, J.; Samojedny, A.; Paul, M.; Pietsz, G.; Wilczok, T. Effect of apigenin, kaempferol and resveratrol on the expression of interleukin-1beta and tumor necrosis factor-alpha genes in J774. 2 macrophages. Pharmacol. Rep. 2004, 57, 390–394. [Google Scholar]
- Lee, S.-H.; Kim, Y.-J.; Kwon, S.-H.; Lee, Y.-H.; Choi, S.-Y.; Park, J.-S.; Kwon, H.-J. Inhibitory effects of flavonoids on TNF-α-induced IL-8 gene expression in HEK 293 cells. BMB Rep. 2009, 42, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.J.; Ahn, W.S. Kaempferol induced the apoptosis via cell cycle arrest in human breast cancer MDA-MB-453 cells. Nutr. Res. Pract. 2008, 2, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, T.; Deng, R.; Jiang, X.; Xiong, H.; Wang, Y.; Yu, Q.; Wang, X.; Chen, C.; Zhu, Y. Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. Oncotargets Ther. 2017, 10, 4809–4819. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, C.; Correia-Branco, A.; Araújo, J.R.; Guimarães, J.T.; Keating, E.; Martel, F. The Chemopreventive Effect of the Dietary Compound Kaempferol on the MCF-7 Human Breast Cancer Cell Line Is Dependent on Inhibition of Glucose Cellular Uptake. Nutr. Cancer 2015, 67, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.E.; Coombs, M.R.P.; Delaney, L.M.; Hoskin, D.W. Exposure of breast cancer cells to a subcytotoxic dose of apigenin causes growth inhibition, oxidative stress, and hypophosphorylation of Akt. Exp. Mol. Pathol. 2014, 97, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Zhou, H.Y.; Cho, S.Y.; Kim, Y.S.; Lee, Y.S.; Jeong, C.S. Anti-inflammatory mechanisms of apigenin: Inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch. Pharmacal Res. 2007, 30, 1318–1327. [Google Scholar] [CrossRef]
- Maret, W.; Li, Y. Coordination dynamics of zinc in proteins. Chem. Rev. 2009, 109, 4682–4707. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Imanishi, M.; Futaki, S.; Sugiura, Y. α-helical linker of an artificial 6-zinc finger peptide contributes to selective DNA binding to a discontinuous recognition sequence. Biochemistry 2007, 46, 8517–8524. [Google Scholar] [CrossRef] [PubMed]
- Sri Krishna, S.; Majumdar, I.; Grishin, N.V. Structural classification of zinc fingers. Nucleic Acids Res. 2003, 31, 532–550. [Google Scholar] [CrossRef]
- Murphy, E.; Willis, B.W.; Watt, B. Provisional tables on the zinc content of foods. J. Am. Diet. Assoc. 1975, 66, 345–355. [Google Scholar] [PubMed]
- Al-saran, N.; Subash-Babu, P.; Al-Nouri, D.M.; Alfawaz, H.A.; Alshatwi, A.A. Zinc enhances CDKN2A, pRb1 expression and regulates functional apoptosis via upregulation of p53 and p21 expression in human breast cancer MCF-7 cell. Environ. Toxicol. Pharmacol. 2016, 47, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Pomerantz, J.; Schreiber-Agus, N.; Liégeois, N.J.; Silverman, A.; Alland, L.; Chin, L.; Potes, J.; Chen, K.; Orlow, I.; Lee, H.-W. The Ink4a tumor suppressor gene product, p19 Arf, interacts with MDM2 and neutralizes MDM2’s inhibition of p53. Cell 1998, 92, 713–723. [Google Scholar] [CrossRef]
- Ho, E.; Ames, B.N. Low intracellular zinc induces oxidative DNA damage, disrupts p53, NFκB, and AP1 DNA binding, and affects DNA repair in a rat glioma cell line. Proc. Natl. Acad. Sci. USA 2002, 99, 16770–16775. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.Y.; Zhou, J.; Gibbons, L.; Morrison, H.; Wen, S.W.; Canadian Cancer Registries Epidemiology Research Group. Antioxidants and breast cancer risk—A population-based case-control study in Canada. BMC Cancer 2011, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, S.L.; Seo, Y.A.; Lopez, V. Mammary gland zinc metabolism: Regulation and dysregulation. Genes Nutr. 2009, 4, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Karunasinghe, N. Nutrigenetics, nutrigenomics and selenium. Front. Genet. 2011, 2. [Google Scholar] [CrossRef]
- Guo, C.-H.; Hsia, S.; Shih, M.-Y.; Hsieh, F.-C.; Chen, P.-C. Effects of Selenium Yeast on Oxidative Stress, Growth Inhibition, and Apoptosis in Human Breast Cancer Cells. Int. J. Med Sci. 2015, 12, 748. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). J. Am. Med Assoc. 2009, 301, 39–51. [Google Scholar] [CrossRef]
- Tsavachidou, D.; McDonnell, T.J.; Wen, S.; Wang, X.; Vakar-Lopez, F.; Pisters, L.L.; Pettaway, C.A.; Wood, C.G.; Do, K.-A.; Thall, P.F. Selenium and Vitamin E: Cell Type–and Intervention-Specific Tissue Effects in Prostate Cancer. J. Natl. Cancer Inst. 2009, 101, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Kahya, M.C.; Nazıroğlu, M.; Çiğ, B. Selenium reduces mobile phone (900 MHz)-induced oxidative stress, mitochondrial function, and apoptosis in breast cancer cells. Biol. Trace Elem. Res. 2014, 160, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Lubinski, J.; Marciniak, W.; Muszynska, M.; Huzarski, T.; Gronwald, J.; Cybulski, C.; Jakubowska, A.; Debniak, T.; Falco, M.; Kladny, J. Serum selenium levels predict survival after breast cancer. Breast Cancer Res. Treat. 2018, 167, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.A.; Flatt, S.W.; Rock, C.L.; Ritenbaugh, C.; Newman, V.; Pierce, J.P. Increased fruit, vegetable and fiber intake and lower fat intake reported among women previously treated for invasive breast cancer. J. Am. Diet. Assoc. 2002, 102, 801–808. [Google Scholar] [CrossRef]
- De Assis, S.; Khan, G.; Hilakivi-Clarke, L. High birth weight increases mammary tumorigenesis in rats. Int. J. Cancer 2006, 119, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Ion, G.; Akinsete, J.A.; Hardman, W.E. Maternal consumption of canola oil suppressed mammary gland tumorigenesis in C3 (1) TAg mice offspring. BMC Cancer 2010, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Andrade, F.; de Assis, S.; Jin, L.; Fontelles, C.C.; Barbisan, L.F.; Purgatto, E.; Hilakivi-Clarke, L.; Ong, T.P. Lipidomic fatty acid profile and global gene expression pattern in mammary gland of rats that were exposed to lard-based high fat diet during fetal and lactation periods associated to breast cancer risk in adulthood. Chem. Biol. Interact. 2015, 239, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Castro-Quezada, I.; Román-Viñas, B.; Serra-Majem, L. The Mediterranean diet and nutritional adequacy: A review. Nutrients 2014, 6, 231–248. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Bamia, C.; Trichopoulos, D. Anatomy of health effects of Mediterranean diet: Greek EPIC prospective cohort study. BMJ 2009, 338, 2337. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.A.; García-López, M.; Bes-Rastrollo, M.; Toledo, E.; Martínez-Lapiscina, E.H.; Delgado-Rodriguez, M.; Vazquez, Z.; Benito, S.; Beunza, J.J. Mediterranean diet and the incidence of cardiovascular disease: A Spanish cohort. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 237–244. [Google Scholar] [CrossRef] [PubMed]
- León-Muñoz, L.; Galán, I.; Valencia-Martín, J.; López-García, E.; Guallar-Castillón, P.; Rodríguez-Artalejo, F. Is a specific drinking pattern a consistent feature of the Mediterranean diet in Spain in the XXI century? Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
| Polyphenol | Sub-Groups | Food Source |
|---|---|---|
| Phenolic acid | Benzoic acid Cinnamic acid | Mostly in grains [55,56,57]. |
| Flavonoids | Anthocyanins Flavan-3-ols Flavones Flavanones and Flavonols | Vegetables, fruits, seeds, some cereals, together with wine, tea and certain spices [58]. |
| Flavonoids (Flavones) | Apigenin | Parsley, grapefruit, chamomile tea, oranges, and grapefruit [59]. |
| Flavonoids (Flavonols) | Quercetin Kaempferol | Onions, broccoli, apples and berries [60]. |
| Flavonoids (Flavanols) | Catechin Epicatechin Epigallocatechin Epigallocatechin gallate | Many types of fruits, red wine, green and red tea [61]. |
| Polyphenolic amides | Capsaicinoids and avenanthramdes Resveratrol (a stilbene) | Chilli peppers and oats (respectively) grapes and red wine [62]. |
| Other Polyphenols | Ellagic acid and gallic acid | Berry fruits, e.g., strawberries and raspberries, and in the skins of different tree nuts [62]. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, A.A.; Braakhuis, A.J.; Bishop, K.S. The Mediterranean Diet and Breast Cancer: A Personalised Approach. Healthcare 2019, 7, 104. https://doi.org/10.3390/healthcare7030104
Shaikh AA, Braakhuis AJ, Bishop KS. The Mediterranean Diet and Breast Cancer: A Personalised Approach. Healthcare. 2019; 7(3):104. https://doi.org/10.3390/healthcare7030104
Chicago/Turabian StyleShaikh, Amani Al, Andrea J. Braakhuis, and Karen S. Bishop. 2019. "The Mediterranean Diet and Breast Cancer: A Personalised Approach" Healthcare 7, no. 3: 104. https://doi.org/10.3390/healthcare7030104
APA StyleShaikh, A. A., Braakhuis, A. J., & Bishop, K. S. (2019). The Mediterranean Diet and Breast Cancer: A Personalised Approach. Healthcare, 7(3), 104. https://doi.org/10.3390/healthcare7030104