Reviewing the Evidence Base for the Children and Young People Safety Thermometer (CYPST): A Mixed Studies Review
Abstract
:1. Introduction
2. Method
2.1. Data Sources
2.2. Search Strategy
| Topic | Search Terms |
|---|---|
| Pain | “pain score* OR measurement” OR pain OR “acute pain” OR “visceral pain” |
| Skin integrity | skin NEAR/3 integrity OR “pressure ulcer” |
| Extravasation | Extravasation * OR extravas * OR cannula |
| PEWS | PEWS OR neonatal OR p$ediatric early warning score* OR “rapid response system” OR “track and trigger aggregate score” OR “early warning score” OR “early warning system” OR “heart rate” OR “blood pressure” OR “blood gas result*” |
2.3. Inclusion Criteria
2.4. Critical Appraisal
2.5. Data Extraction and Synthesis
3. Results and Discussion
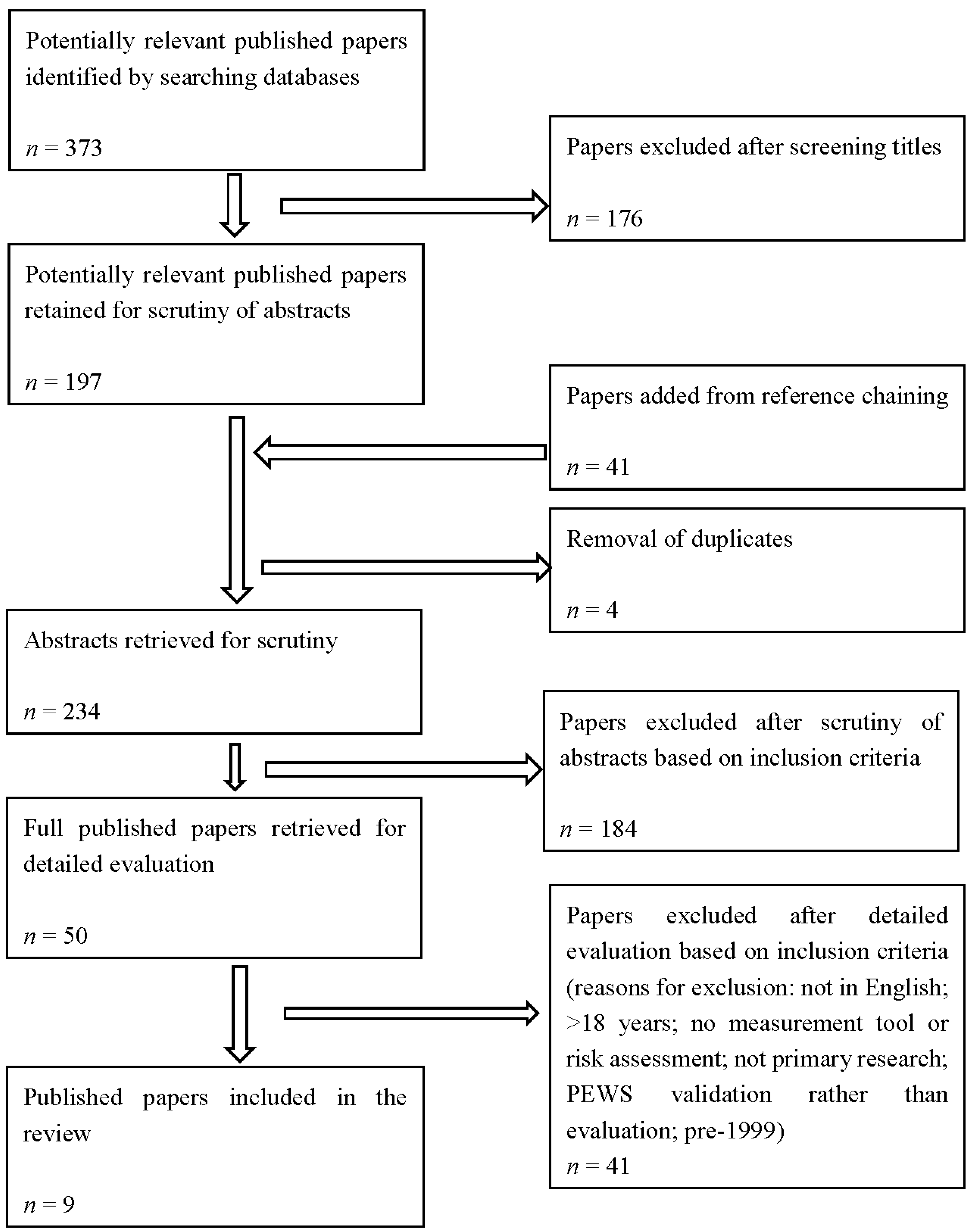
3.1. Study Selection
3.2. Study Quality
3.3. Study Characteristics
| Reference # | First Author & Date | Journal | Aim | Sampling Method | n = | Harm | Location | Data Collection Method | Analysis Method | Quality Rating |
|---|---|---|---|---|---|---|---|---|---|---|
| [17] | Anthony (2010) | Journal of Tissue Viability, 19, 98–105. | To compare three risk assessment scales with respect to predictive validity | Review | Pediatrics: 236 | Skin | England | Comparing patient data | Mann Whitney and logistic regression | 75% |
| [20] | Byrne (2001) | Journal of Psychometric Research, 50, 69–76. | (i) how pediatric nurses construed their patients’ pain; (ii) how these constructions were related to the emotional challenge of pain; and, (iii) how they influenced nurses’ communication with patients and specifically their management of pain. | Opportunistic | nurses: 13 Pediatrics: 16 | Pain | England | Observations and interviews | Grounded theory | 100% |
| [16] | Curley (2003) | Nursing Research, 52(1), 22–33. | (a) Establish the predictive validity of the Braden Q Scale in an acutely ill pediatric population; (b) determine the critical cut-off point for classifying patient risk; and (c) determine the best time to assess patient risk. | Convenience | Pediatrics: 90 | Skin | USA | Comparing patient data | Parametric and nonparametric statistics were used | 50% |
| [25] | Linhares (2012) | Brazilian Journal of Medical and Biological Research, 45, 1287–1294. | To examine the prevalence, assessment, and management of pediatric pain in a public teaching hospital. | Opportunistic | Infants: 70 Children: 36 Adolescents: 15 | Pain | Brazil | Questionnaires and interview | Systematic categorical analysis and descriptive statistics | 50% |
| [21] | Noonan (2011) | Journal of Pediatric Nursing 2006, 21(6), 445–453. | The purpose of this paper was to describe the spectrum of alterations in skin integrity and skin care needs of hospitalized infants and children. | Convenience | Pediatrics: 252 | Skin | USA | Skin integrity audit tool and the Braden Q scale | Descriptive Statistics | 100% |
| [22] | Schluer (2009) | Child and Adolescent Health, 18, 3244–3252. | The aim of the current study is to describe the frequency of pressure ulcers in a pediatric care setting and to identify the population at risk, as well as to assess the factors predisposing to the development of pressure ulcers. | Convenience | Children: 155 | Skin | Switzerland | Direct systematic inspection of the skin, and a valid risk assessment instrument: Braden Scale | Descriptive and univariate statistical methods. | 100% |
| [18] | Suddaby (2005) | Pediatric Nursing, 31(2), 132–138. | To develop a simple, single-page measurement tool that evaluates risk of skin breakdown in the pediatric population and apply it to the acutely hospitalized child | Not specified | Children: 347 | Skin | USA | Risk assessment instrument: The Starkid Skin Scale | Descriptive statistics and unconditional logistic regression | 75% |
| [23] | Taylor (2008) | Pain Research Management, 13: 25–32 | To highlight areas of good practice, identify areas for improvement, and inform development of hospital standards, education, future audits, and the research agenda. | Not specified | Children: 241 | Pain | Canada | Interviews and pain assessments. | Statistical analysis was performed, including nonparametric tests. | 100% |
| [19] | Willock (2009) | Journal of Wound Care, 18(1), 17–21 | To develop a predictive pressure ulcer risk assessment scale based on patient data. | Prospective | Children: 265 | Skin | England | Questionnaire, survey, and interview | Chi-square tests. | 75% |
3.4. Synthesis
3.4.1. Mistrust of Pain Reports
3.4.2. Validated Pain Measures and Treatment of Pain
3.4.3. Device-Related Injury
3.4.4. Vigilance and Communication
3.4.5. Tissue Viability Risk Assessment Tools
3.5. Discussion
3.6. Limitations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Health & Social Care Information Centre (HSCIC). NHS Safety Thermometer. Available online: http://www.hscic.gov.uk/thermometer (accessed on 23 July 2014).
- NHS Quality Observatory. NHS Safety Thermometer. Available online: http://www.safetythermometer.nhs.uk/index.php?option=com_content&view=article&id=1&Itemid=101 (accessed on 23 July 2014).
- Power, M.; Stewart, K.; Brotherton, A. What is the NHS Safety Thermometer? Clin. Risk 2012, 1, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.; Cooney, K.; Sills, E.; Sullivan, E. Implementing the Safety Thermometer tool in one NHS trust. Br. J. Nurs. 2014, 23, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Commissioning for Quality and Innovation (CQUIN). Harm Free Care. Available online: http://harmfreecare.org/measurement/nhs-safety-thermometer/ (accessed on 23 July 2014).
- McCabe, A.; Duncan, H.; Heward, Y. Paediatric early warning system: Where do we go from here? Paediatri. Care 2009, 21, 14–17. [Google Scholar] [CrossRef] [PubMed]
- McKay, H.; Mitchell, I.A.; Sinn, K.; Mugridge, H.; Lafferty, T.; van Leuvan, C.; Mamootil, S.; Abdel-Latif, M.E. Effect of a multifaceted intervention on documentation of vital signs and staff communication regarding deteriorating paediatric patients. J. Paediatr. Child 2013, 49, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Tume, L. The deterioration of children in ward areas in a specialist children’s hospital. BACCN 2007, 12, 12–19. [Google Scholar] [CrossRef] [PubMed]
- NHS Institute for Innovation and Improvement. Paediatric Early Warning Scores. Available online: http://www.institute.nhs.uk/safer_care/paediatric_safer_care/pews.html (accessed on 18 August 2014).
- Duncan, H.; Hutchinson, J.; Parshuram, C.S. The pediatric early warning system score: A severity of illness score to predict urgent medical need in hospitalised children. J. Crit. Care 2006, 21, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Pluye, P.; Robert, E.; Cargo, M.; Bartlett, G.; O’Cathain, A.; Griffiths, F.; Boardman, F.; Gagnon, M.P.; Rousseau, M.C. Proposal: A Mixed Methods Appraisal Tool for Systematic Mixed Studies Reviews. Available online: http://mixedmethodsappraisaltoolpublic.pbworks.com (accessed on 23 July 2014).
- Pluye, P.; Hong, Q.N. Combining the power of stories and the power of numbers: Mixed methods research and mixed studies reviews. Annu. Rev. Public Health 2014, 35, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Dixon-Woods, M.; Caver, D.; Agarwal, S.; Annadale, E.; Arthur, A.; Harvey, J.; Hsu, R.; Katbamna, S.; Olsen, R.; Smith, L.K.; et al. Conducting critical interpretative synthesis of the literature on access to healthcare by vulnerable groups. BMC Med. Res. Methodol. 2006. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.; Mays, N.; Popay, J. Synthesizing Qualitative and Quantitative Health Evidence: A Guide to Methods; Open University Press: Berkshire, UK, 2007. [Google Scholar]
- Chapman, S.M.; Grocott, M.P.W.; Franck, L.S. Systematic review of paediatric alert criteria for identifying hospitalised children at risk of critical deterioration. Intensive Care Med. 2010, 36, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Curley, M.A.Q.; Razmus, I.S.; Roberts, K.E.; Wypij, D. Predicting pressure ulcer risk in pediatric patients: The Braden Q scale. Nurs. Res. 2003, 52, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Anthony, D.; Willock, J.; Baharestani, M. A comparison of Braden Q, Garvin and Glamorgan risk assessment scales in paediatrics. J. Tissue Viability 2010, 19, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Suddaby, E.C.; Barnett, S.; Facteau, L. Skin breakdowns in acute care pediatrics. Pediatr. Nurs. 2005, 31, 132–138. [Google Scholar] [PubMed]
- Willock, J.; Baharestani, M.M.; Anthony, D. The development of the Glamorgan paediatric pressure ulcer risk assessment scale. J. Wound Care 2009, 18, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.; Morton, J.; Salmon, P. Defending against patients’ pain. A qualitative analysis of nurses’ responses to children’s postoperative pain. J. Psychosom. Res. 2001, 20, 69–76. [Google Scholar] [CrossRef]
- Noonan, C.; Quigley, S.; Curley, M.A.Q. Skin integrity in hospitalized infants and children: A prevalence survey. J. Pediatr. Nurs. 2006, 21, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Schluer, A.B.; Cignacco, E.; Muller, M.; Halfens, R.J. The prevalence of pressure ulcers in four paediatrics institutions. J. Clin. Nurs. 2009, 18, 3244–3252. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.M.; Boyer, K.; Campbell, F.A. Pain in hospitalized children: A prospective cross-sectional survey of pain prevalence, intensity, assessment and management in a Canadian pediatric teaching hospital. Pain Res. Manag. 2008, 13, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.L. Identifying and synthesising qualitative literature. In Qualitative Research Methods in Mental Health and Psychotherapy: An Introduction for Students and Practitioners; Harper, D., Thompson, A., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2011. [Google Scholar]
- Linhares, M.B.M.; Doca, F.N.P.; Martinez, F.E.; Carlotti, A.P.P.; Cassiano, R.G.M.; Pfeifer, L.I.; Funayama, C.A.; Rossi, L.R.G.; Finley, G.A. Pediatric pain: Prevalence, assessment, and management in a teaching hospital. Br. J. Med. Biol. Res. 2012, 45, 1287–1294. [Google Scholar] [CrossRef]
- European Ulcer Advisory Panel. European and US National Pressure Ulcer Advisory Panels Guidelines. Available online: http://www.epuap.org/guidelines/ (accessed on 23 July 2014).
- Ferguson, L.M.; Ward, H.; Card, S.; Sheppard, S.; McMurtry, M. Putting the “patient” back into patient-centred care: An education perspective. Nurse Edu. Pract. 2013, 13, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Draper, H.; Sorell, T. Patients’ responsibilities in medical ethics. Bioethics 2002, 16, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.; Dealey, C.; Posnett, H. The cost of pressure ulcers in the UK. Age Ageing 2004, 33, 340–235. [Google Scholar] [CrossRef] [PubMed]
- McLane, K.M.; Bookout, K.; McCord, S.; McCain, J.; Jefferson, L.S. The 2003 national paediatric pressure ulcer and skin breakdown prevalence survey. J. Wound Ostomy Continence Nurs. 2004, 31, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Cockett, A. A research review to identify the factors contributing to the development of pressure ulcers in paediatric patients. J. Tissue Viability 2001, 12, 16–23. [Google Scholar] [CrossRef]
- Andrews, T.; Waterman, H. Packaging: A grounded theory of how to report physiological deterioration effectively. J. Adv. Nurs. 2005, 52, 473–781. [Google Scholar] [CrossRef] [PubMed]
- Introducing the NHS Safety Thermometer. Available online: http://harmfreecare.org/case-studies/introducing-the-nhs-st/ (accessed on 23 July 2014).
- NHS Safety Thermometer. Mini Case Studies and Contacts. Available online: http://harmfreecare.org/wp-content/uploads/2012/05/ST-Mini-Case-Studies-May-2012-v2.pdf (accessed on 23 July 2014).
- Grol, R.; Grimshaw, J. From best evidence to best practice: Effective implementation of change in patients’ care. Lancet 2003, 362, 1225–1230. [Google Scholar] [CrossRef]
- Dixon-Woods, M.; Shaw, R.L.; Agarwal, S.; Smith, J.A. The problem of appraising qualitative research. Qual. Saf. Health Care 2004, 13, 223–225. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aston, L.; Eyre, C.; McLoughlin, M.; Shaw, R. Reviewing the Evidence Base for the Children and Young People Safety Thermometer (CYPST): A Mixed Studies Review. Healthcare 2016, 4, 8. https://doi.org/10.3390/healthcare4010008
Aston L, Eyre C, McLoughlin M, Shaw R. Reviewing the Evidence Base for the Children and Young People Safety Thermometer (CYPST): A Mixed Studies Review. Healthcare. 2016; 4(1):8. https://doi.org/10.3390/healthcare4010008
Chicago/Turabian StyleAston, Lydia, Caron Eyre, Michelle McLoughlin, and Rachel Shaw. 2016. "Reviewing the Evidence Base for the Children and Young People Safety Thermometer (CYPST): A Mixed Studies Review" Healthcare 4, no. 1: 8. https://doi.org/10.3390/healthcare4010008
APA StyleAston, L., Eyre, C., McLoughlin, M., & Shaw, R. (2016). Reviewing the Evidence Base for the Children and Young People Safety Thermometer (CYPST): A Mixed Studies Review. Healthcare, 4(1), 8. https://doi.org/10.3390/healthcare4010008






