Filgotinib in Moderate-to-Severe Crohn’s Disease: A Network Meta-Analysis of Efficacy and Adverse Events
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
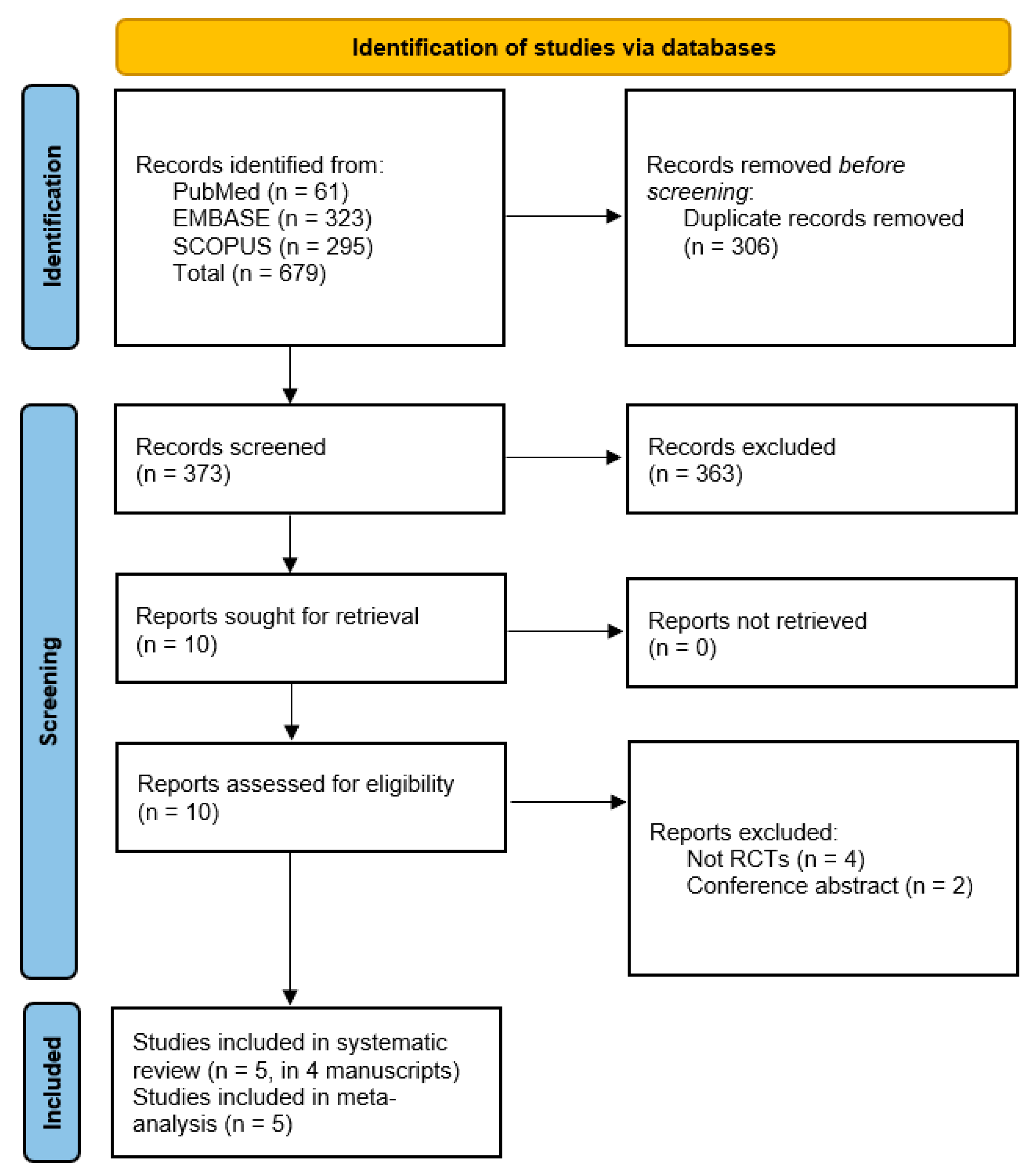
2.2. Information Sources, Search Strategy, and Study Selection
2.3. Data Collection Process
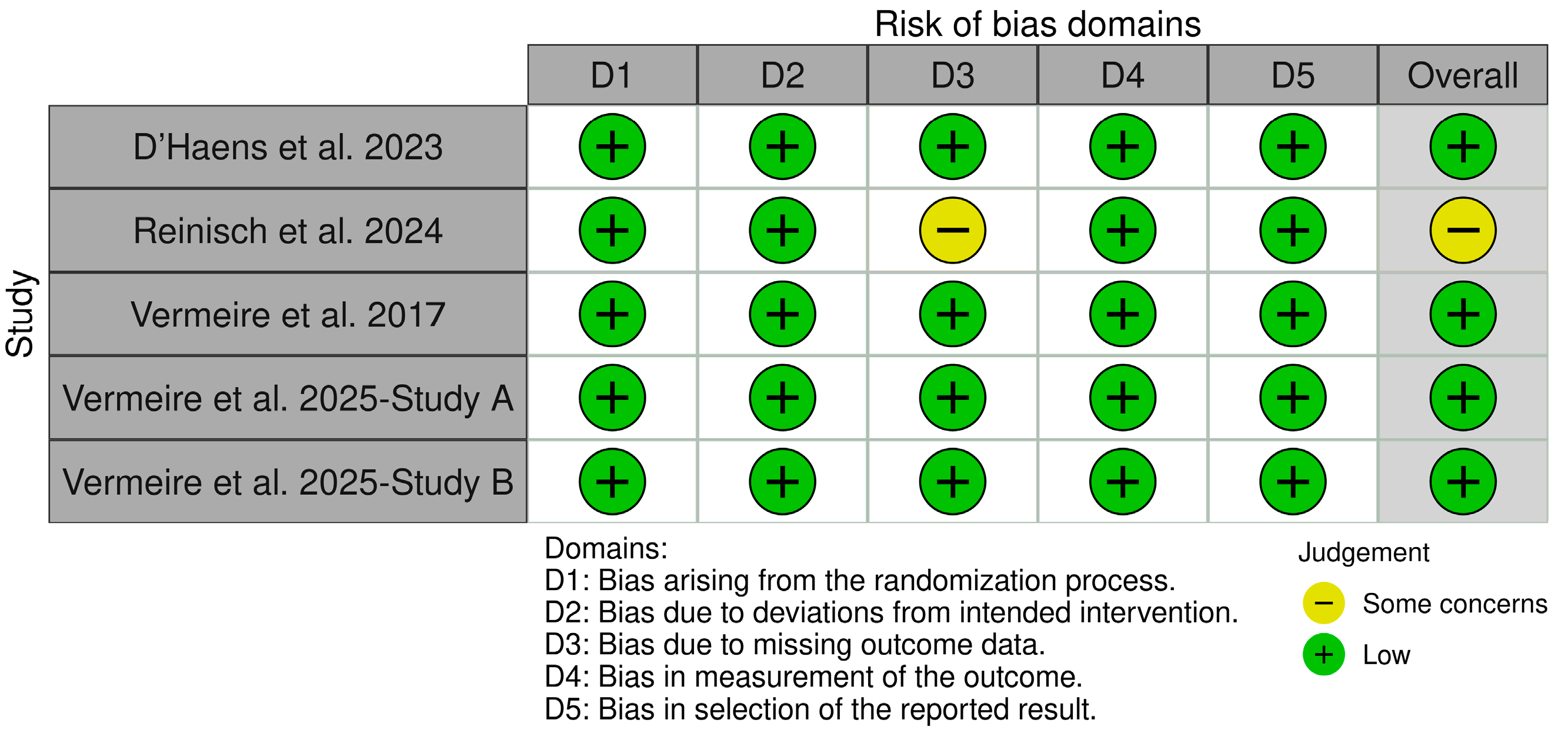
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
2.6. Certainty of Evidence
3. Results
3.1. Baseline Characteristics and Summary of the Included Studies
3.2. Quality Assessment
3.3. Outcomes
3.3.1. Efficacy Outcomes
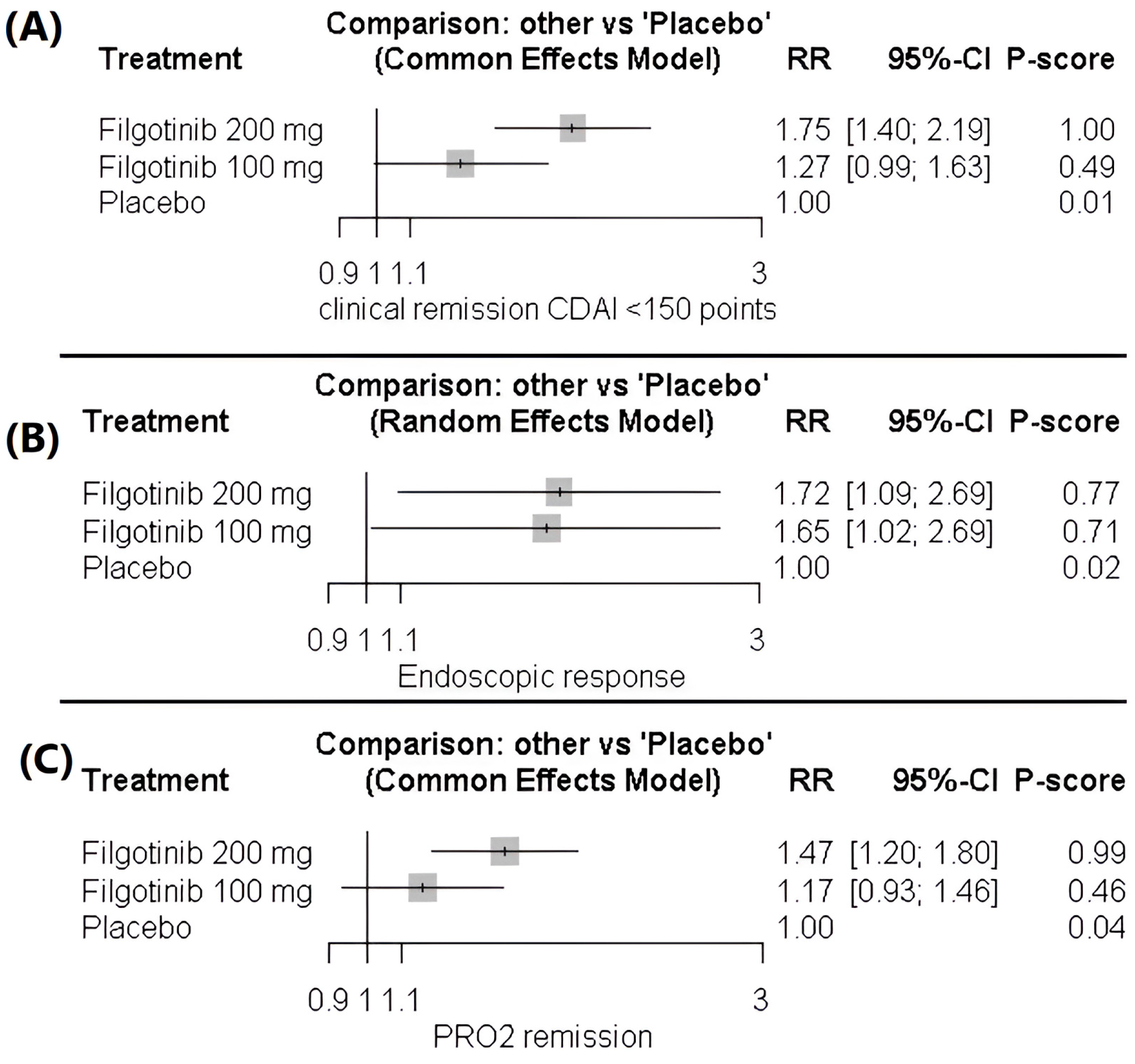
Clinical Remission CDAI < 150 Points
Endoscopic Response (Reduction of at Least 50% in Centrally Read SES-CD)
Two-Item Patient-Reported Outcome (PRO2)
3.3.2. Safety Outcomes
Treatment-Emergent Adverse Events (TEAEs)
Serious Treatment-Emergent Adverse Events
Occurrence of Any Infection
3.3.3. Qualitative Synthesis
Role of Filgotinib in Perianal Fistulizing CD (PFCD)
Difference Between Prior Treatments and Naive Patients
Maintenance Role of Filgotinib
3.4. GRADE Assessment
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CD | Crohn’s Disease |
| CDAI | Crohn’s Disease Activity Index |
| SES-CD | Simple Endoscopic Score for Crohn’s Disease |
| JAK1 | Janus Kinase 1 |
| RR | Risk Ratios |
| CI | Confidence Intervals |
| SD | Standard Deviation |
| NMA | Network Meta-Analysis |
| PRISMA | The Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RCT | Randomized Controlled Trials |
| PRO2 | Two-Item Patient-Reported Outcome |
| TEAEs | Treatment-Emergent Adverse Events |
| PFCD | Perianal Fistulizing Crohn’s Disease |
References
- Feuerstein, J.D.; Cheifetz, A.S. Crohn Disease: Epidemiology, Diagnosis, and Management. Mayo Clin. Proc. 2017, 92, 1088–1103. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54.e42, quiz-e30. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; George, J.; Boland, B.S.; Vande Casteele, N.; Sandborn, W.J. Primary Non-Response to Tumor Necrosis Factor Antagonists is Associated with Inferior Response to Second-line Biologics in Patients with Inflammatory Bowel Diseases: A Systematic Review and Meta-analysis. J. Crohns Colitis 2018, 12, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Gils, A.; Rutgeerts, P.; Levesque, B.G.; Vermeire, S.; Sandborn, W.J.; Vande Casteele, N. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: Evolution in the definition and management of primary nonresponse. Inflamm. Bowel Dis. 2015, 21, 182–197. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Benucci, M.; Bardelli, M.; Cazzato, M.; Laurino, E.; Bartoli, F.; Damiani, A.; Li Gobbi, F.; Panaccione, A.; Di Cato, L.; Niccoli, L.; et al. ReLiFiRa (Real Life Filgotinib in Rheumatoid Arthritis): Retrospective Study of Efficacy and Safety in Common Clinical Practice. J. Pers. Med. 2023, 13, 1303. [Google Scholar] [CrossRef]
- Dudek, P.; Fabisiak, A.; Zatorski, H.; Malecka-Wojciesko, E.; Talar-Wojnarowska, R. Efficacy, Safety and Future Perspectives of JAK Inhibitors in the IBD Treatment. J. Clin. Med. 2021, 10, 5660. [Google Scholar] [CrossRef]
- Vermeire, S.; Schreiber, S.; Petryka, R.; Kuehbacher, T.; Hebuterne, X.; Roblin, X.; Klopocka, M.; Goldis, A.; Wisniewska-Jarosinska, M.; Baranovsky, A.; et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): Results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 2017, 389, 266–275. [Google Scholar] [CrossRef]
- Vermeire, S.; Schreiber, S.; Rubin, D.T.; D’Haens, G.; Reinisch, W.; Watanabe, M.; Mehta, R.; Roblin, X.; Beales, I.; Gietka, P.; et al. Efficacy and safety of filgotinib as induction and maintenance therapy for Crohn’s disease (DIVERSITY): A phase 3, double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2025, 10, 138–153. [Google Scholar] [CrossRef]
- Elgendy, M.S.; Raza, A.; Rifai, M.; Rehman, W.U.; Emara, A.; Khan, M.H.; Jan, A.; Younas, M.; Nawaz, A.; Khan, U. Dose-dependent efficacy and safety of Filgotinib in moderate to severe Crohn’s disease: A grade-assessed systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Pharmacol. 2025, 81, 1517–1531. [Google Scholar] [CrossRef]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.T.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, Ed000142. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef]
- Reinisch, W.; Colombel, J.F.; D’Haens, G.R.; Rimola, J.; Masior, T.; McKevitt, M.; Ren, X.; Serone, A.; Schwartz, D.A.; Gecse, K.B. Efficacy and Safety of Filgotinib for the Treatment of Perianal Fistulising Crohn’s Disease [DIVERGENCE 2]: A Phase 2, Randomised, Placebo-controlled Trial. J. Crohns Colitis 2024, 18, 864–874. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Lee, S.; Taylor, S.A.; Serone, A.; Rimola, J.; Colombel, J.F. Filgotinib for the Treatment of Small Bowel Crohn’s Disease: The DIVERGENCE 1 Trial. Gastroenterology 2023, 165, 289–292.e283. [Google Scholar] [CrossRef]
- Ma, C.; Solitano, V.; Danese, S.; Jairath, V. The Future of Clinical Trials in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2025, 23, 480–489. [Google Scholar] [CrossRef]
- Moss, A.C. Approach to Treatment Failure in Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2022, 18, 360–363. [Google Scholar]
- Marsal, J.; Barreiro-de Acosta, M.; Blumenstein, I.; Cappello, M.; Bazin, T.; Sebastian, S. Management of Non-response and Loss of Response to Anti-tumor Necrosis Factor Therapy in Inflammatory Bowel Disease. Front. Med. 2022, 9, 897936. [Google Scholar] [CrossRef]
- Lopez, N.; Ramamoorthy, S.; Sandborn, W.J. Recent advances in the management of perianal fistulizing Crohn’s disease: Lessons for the clinic. Expert. Rev. Gastroenterol. Hepatol. 2019, 13, 563–577. [Google Scholar] [CrossRef]
- Berg, D.R.; Colombel, J.F.; Ungaro, R. The Role of Early Biologic Therapy in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2019, 25, 1896–1905. [Google Scholar] [CrossRef]
- Srinivasan, A.R. Treat to target in Crohn’s disease: A practical guide for clinicians. World J. Gastroenterol. 2024, 30, 50–69. [Google Scholar] [CrossRef]

| Study | Treatment Arm | Age, Years, Mean (SD) | Gender (Female %) | Duration of Disease, Years, Mean (SD) | CDAI Score, Mean (SD) | SES-CD Score, Mean (SD) |
|---|---|---|---|---|---|---|
| D’Haens et al. 2023 [16] | Placebo (n = 18) | 45 (12.9) | 50 | 11.2 (9.1) | 300 (63.7) | NR |
| Filgotinib 100 mg (n = 32) | 42 (12.9) | 71.9 | 14.6 (13.7) | 297 (64.9) | NR | |
| Filgotinib 200 mg (n = 28) | 46 (16.3) | 67.9 | 10.6 (8.4) | 309 (55.7) | NR | |
| Reinisch et al. 2024 [15] | Placebo (n = 15) | 39 (11.8) | 26.7 | 7.5 (7.9) (fistula) | 190 (57.9) | NR |
| Filgotinib 100 mg (n = 25) | 41 (14.0) | 40 | 11.9 (11.1) (fistula) | 194 (67.1) | NR | |
| Filgotinib 200 mg (n = 17) | 39 (11.2) | 52.9 | 10.3 (8.3) (fistula) | 190 (62.4) | NR | |
| Vermeire et al. 2017 [8] | Placebo (n = 44) | 35.1 (11.8) | 59 | 6.8 (5.7) | 298.6 (56.8) | 15.8 (7.2) |
| Filgotinib 200 mg (n = 130) | 37.4 (11.6) | 55 | 8.8 (8.5) | 291.3 (53.8) | 14.2 (6.8) | |
| Vermeire et al. 2025 [9] (Induction Study A) | Placebo (n = 237) | 38 (14) | 55 | 9.3 (8.4) | 320 (59.4) | 13 (7.2) |
| Filgotinib 100 mg (n = 245) | 39 (14.1) | 43 | 9.9 (10.0) | 322 (55.5) | 14 (7.9) | |
| Filgotinib 200 mg (n = 222) | 39 (13.8) | 50 | 9.2 (8.4) | 323 (55.6) | 13 (7.1) | |
| Vermeire et al. 2025 [9] (Induction Study B) | Placebo (n = 229) | 39 (12.5) | 50 | 13.0 (9.5) | 322 (57.5) | 15 (7.8) |
| Filgotinib 100 mg (n = 228) | 42 (13.5) | 56 | 13.3 (9.7) | 321 (55.7) | 15 (8.2) | |
| Filgotinib 200 mg (n = 202) | 39 (14.2) | 56 | 11.5 (8.0) | 306 (54.0) | 15 (7.9) |
| ID | Study Design | NCT | Patient Details | Previous Interventions | Filgotinib Doses and Duration | Primary Outcomes | Follow-Up | Conclusion |
|---|---|---|---|---|---|---|---|---|
| D’Haens et al. 2023 [16] | Multicenter, double-blinded, RCT | NCT03046056 | Patients aged 18–75 years with a confirmed diagnosis of CD for ≥6 months (by imaging, histopathology, or ileoscopy) | Corticosteroids, immunomodulators, TNF inhibitors, vedolizumab, or ustekinumab. | Filgotinib 200 mg or 100 mg once daily for 24 weeks. | Clinical remission (CDAI < 150) at Week 24 | 24 weeks | This study showed that 24 weeks of filgotinib did not significantly surpass placebo in attaining clinical or MaRIA remission. Nevertheless, biomarker responses indicated a pharmacodynamic effect, and both doses were well tolerated. |
| Reinisch et al. 2024 [15] | Phase 2, Randomized, Placebo-controlled | NCT03077412 | Adults with perianal fistulising Crohn’s disease and prior treatment failure. | Prior treatment failure with TNF inhibitors, antibiotics, or immunomodulators. | Filgotinib 200 mg or 100 mg once daily for 24 weeks. | Combined fistula response at Week 24. | 24 weeks | Filgotinib 200 mg resulted in numerical decreases in the number of draining perianal fistulas compared to placebo and was generally well tolerated. |
| Vermeire et al. 2017 [8] | Phase 2, Randomized, Placebo-controlled | NCT02048618 | Patients aged 18–75 years with moderate-to-severe Crohn’s disease confirmed by endoscopy (CDAI) score during screening between 220 and 450 inclusive. | Previous exposure to anti-TNF agents. | Filgotinib 200 mg once daily for 10 weeks, followed by 100 mg or placebo for an additional 10 weeks. | Clinical remission (CDAI < 150) at Week 10. | 20 weeks | Filgotinib led to clinical remission in a significantly higher number of patients with active Crohn’s disease compared to placebo, while maintaining an acceptable safety profile. |
| Vermeire et al. 2025 [9] | Phase 3, Randomized, Placebo-controlled | NCT02914561 | Adults aged 18–75 years with moderately to severely active Crohn’s disease (CDAI) score during screening between 220 and 450 inclusive). | Induction Study A includes both biologic-naive and biologic-experienced patients, while Induction Study B focuses exclusively on biologic-experienced patients. | Filgotinib 200 mg or 100 mg once daily for 11 weeks. | PRO2 clinical remission and endoscopic response at Week 10. | 58 weeks | Filgotinib 200 mg did not achieve the co-primary endpoints by Week 10, but it proved effective in inducing clinical remission and endoscopic response by Week 58. |
| Outcome | No. of Studies (Patients) | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias (Egger Test p Value) | Quality of Evidence | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Clinical Remission (CDAI < 150) | 4 RCTs | RCTs | Low | None | None | Some | 0.94 | Moderate | 200 mg dose showed significant benefit; 100 mg dose no significant difference vs. placebo |
| Endoscopic Response (≥50% SES-CD reduction) | 3 RCTs | RCTs | Low | Moderate (I2 = 57%) | None | Some | Not significant | Moderate | Both doses effective vs. placebo; heterogeneity limits confidence in direct dose comparison |
| Patient-Reported Outcomes (PRO2) | 3 RCTs | RCTs | Low | None | None | Some | Not significant | Moderate | 200 mg dose significantly better; 100 mg dose not significantly different from placebo |
| Treatment-Emergent Adverse Events (TEAEs) | 5 RCTs | RCTs | Low | None | None | None | 0.36 | High | No increase in adverse events for either dose vs. placebo |
| Serious Adverse Events | 4 RCTs | RCTs | Low | None | None | None | 0.647 | High | No significant differences between active doses and placebo |
| Infection Risk | 4 RCTs | RCTs | Low | None | None | None | 0.36 | High | No increased infection risk with filgotinib vs. placebo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Khoshaim, Y.A.; Habis, Y.Z.; Daqnah, A.G.; Alqurashi, R.K.; Abdulrahim, Y.S.; Sakkat, A.; Alsubhi, S.A.; Almuwlad, D.T.; Bukhari, H.S.; Shogdar, A.J.; et al. Filgotinib in Moderate-to-Severe Crohn’s Disease: A Network Meta-Analysis of Efficacy and Adverse Events. Healthcare 2026, 14, 5. https://doi.org/10.3390/healthcare14010005
Khoshaim YA, Habis YZ, Daqnah AG, Alqurashi RK, Abdulrahim YS, Sakkat A, Alsubhi SA, Almuwlad DT, Bukhari HS, Shogdar AJ, et al. Filgotinib in Moderate-to-Severe Crohn’s Disease: A Network Meta-Analysis of Efficacy and Adverse Events. Healthcare. 2026; 14(1):5. https://doi.org/10.3390/healthcare14010005
Chicago/Turabian StyleKhoshaim, Yasser Ali, Yahya Z. Habis, Afnan Ghazi Daqnah, Razan Khalid Alqurashi, Yazeed Shaker Abdulrahim, Abdullah Sakkat, Sultan Ali Alsubhi, Deema Tawfeq Almuwlad, Halah Samer Bukhari, Abdulrhman J. Shogdar, and et al. 2026. "Filgotinib in Moderate-to-Severe Crohn’s Disease: A Network Meta-Analysis of Efficacy and Adverse Events" Healthcare 14, no. 1: 5. https://doi.org/10.3390/healthcare14010005
APA StyleKhoshaim, Y. A., Habis, Y. Z., Daqnah, A. G., Alqurashi, R. K., Abdulrahim, Y. S., Sakkat, A., Alsubhi, S. A., Almuwlad, D. T., Bukhari, H. S., Shogdar, A. J., Amir, O. A., & Zaazouee, M. S. (2026). Filgotinib in Moderate-to-Severe Crohn’s Disease: A Network Meta-Analysis of Efficacy and Adverse Events. Healthcare, 14(1), 5. https://doi.org/10.3390/healthcare14010005