Prescribing Patterns and Adverse Effects of Semaglutide: A Real-World Comparative Evaluation
Abstract
1. Introduction
Specific Objectives
- 1.
- Identify potential correlations in patient characteristics and incidence of ADRs experienced associated with the use of semaglutide.
- 2.
- Compare local prescribing patterns of semaglutide in the West Midlands/England area studied with national trends.
- 3.
- Assess ADR incidence locally versus nationally.
- 4.
- Examine associations between semaglutide prescribing and socioeconomic deprivation.
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Real-World Patient Data
2.3. National Prescribing Data
2.4. Adverse Drug Reaction Data
2.5. Effectiveness Outcomes
2.6. Identification of Socioeconomic Impact
3. Results
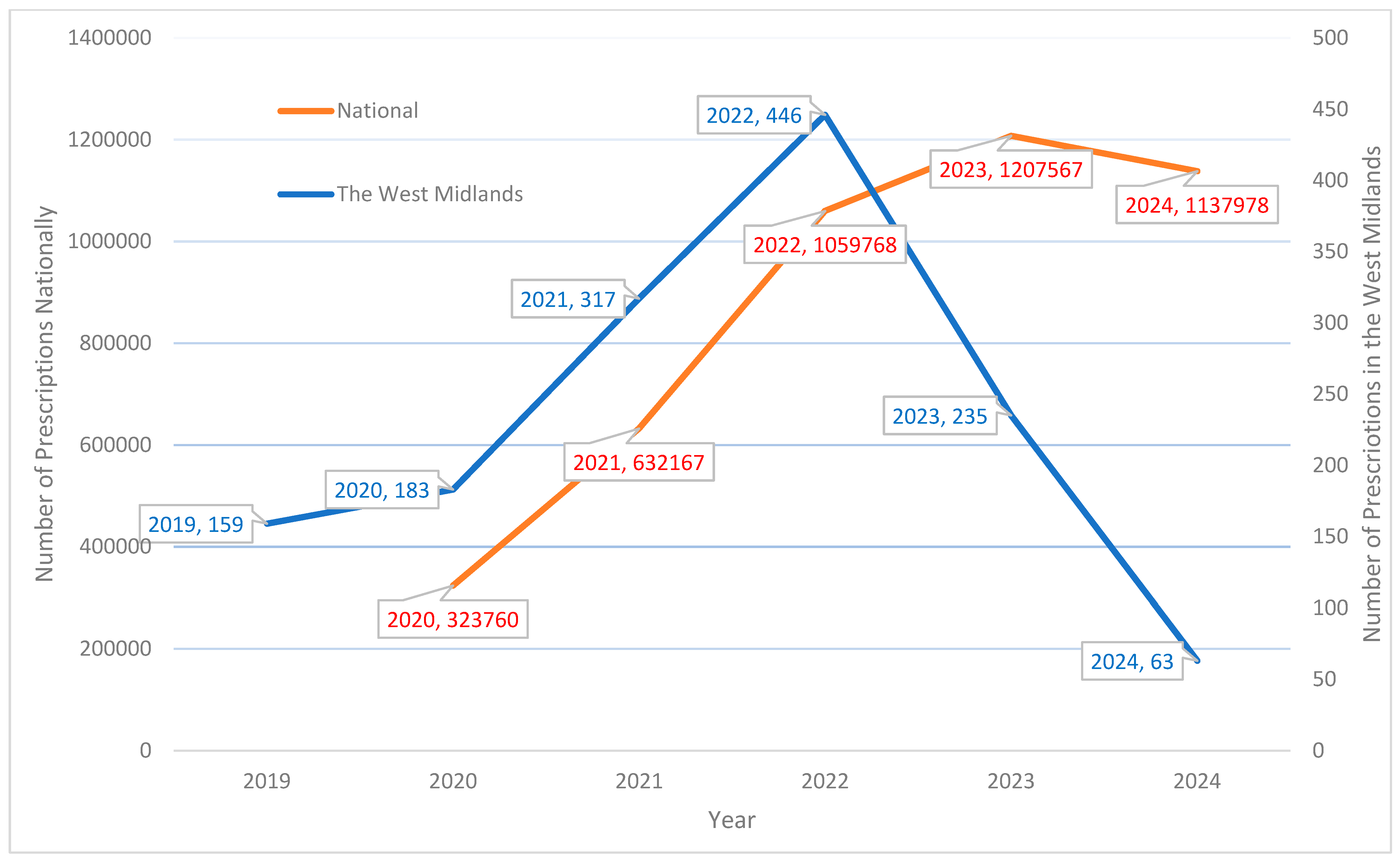
3.1. Prescribing Patterns
3.2. Ethnicity of Patients Prescribed Semaglutide
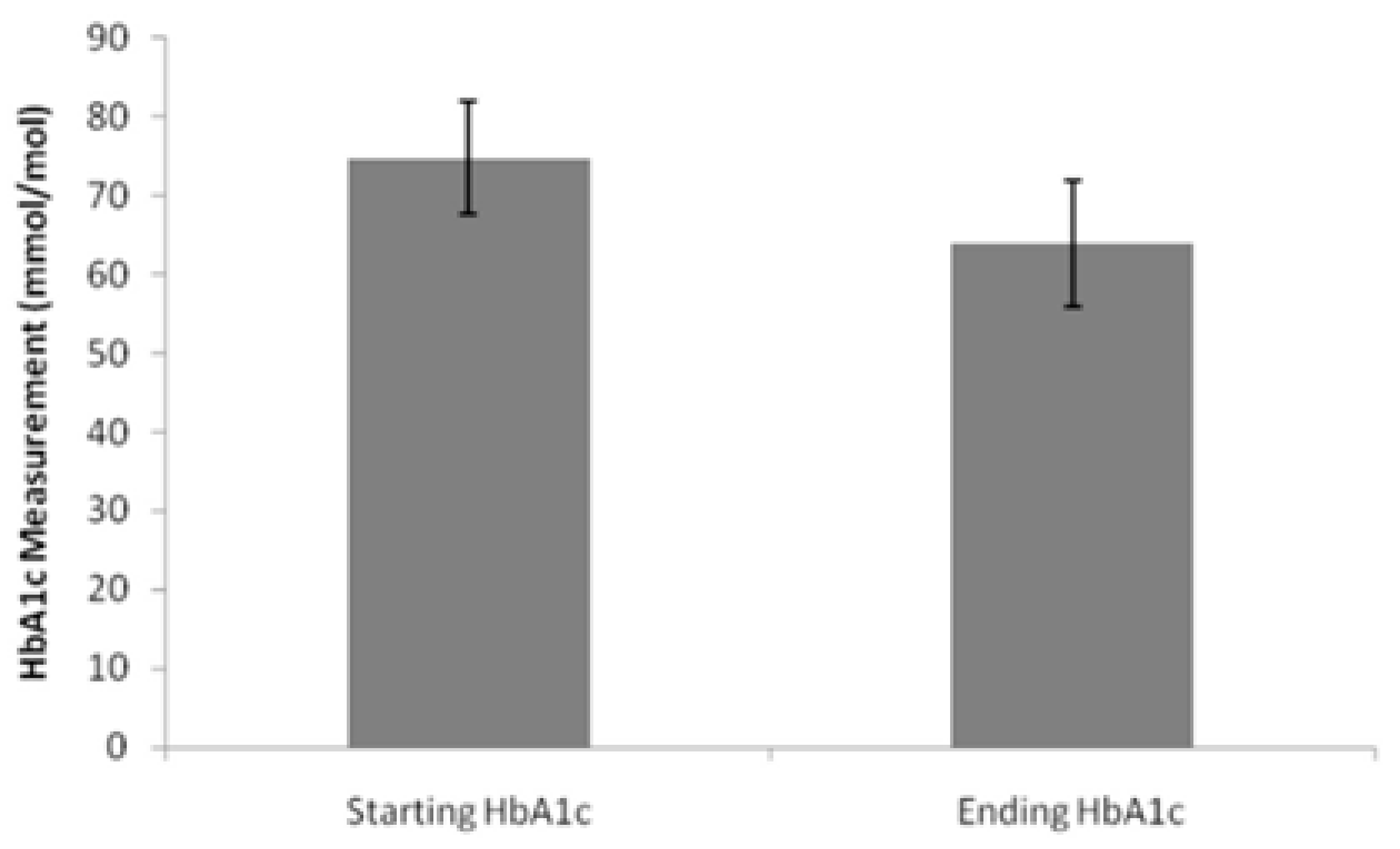
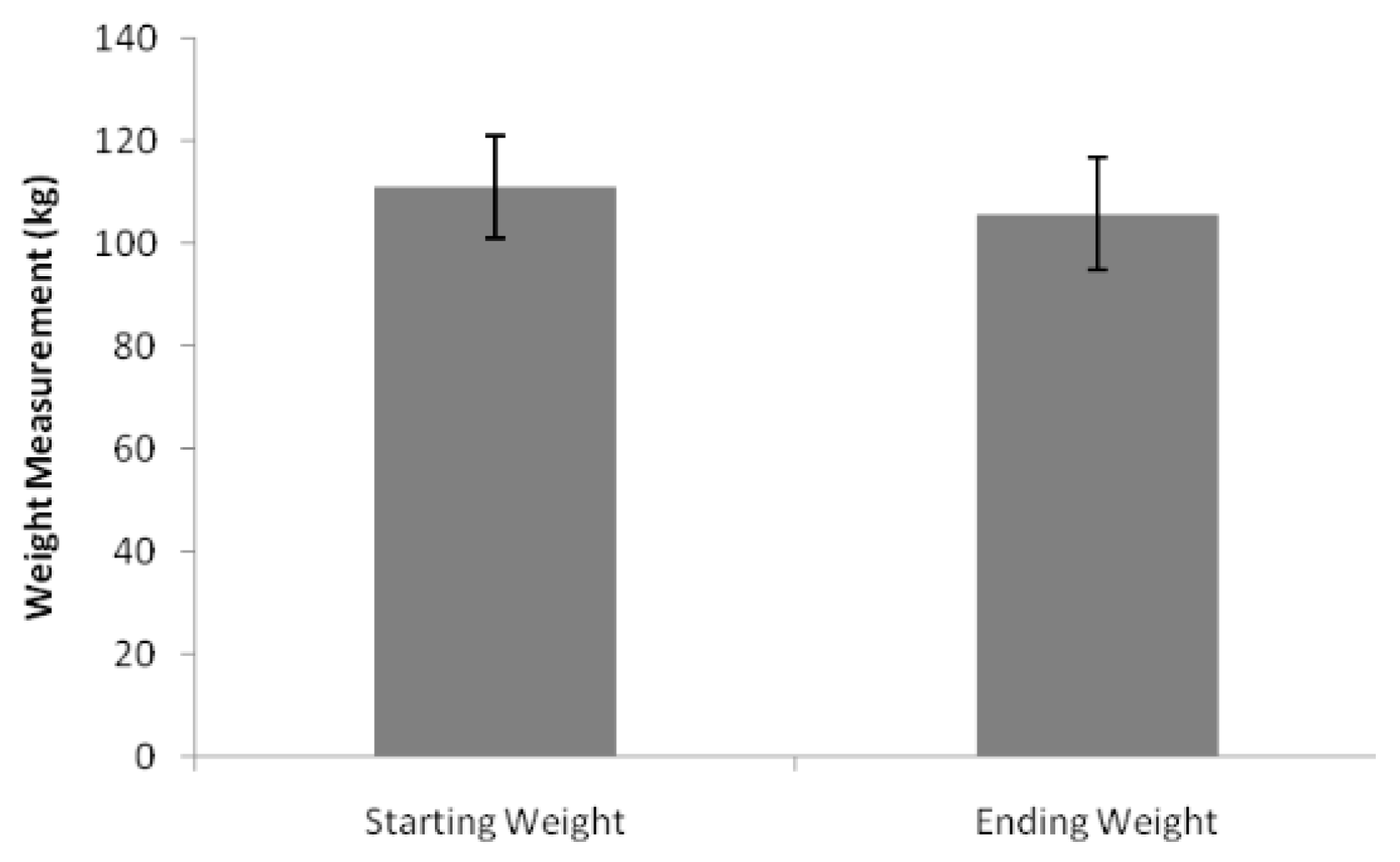
3.3. Treatment Efficacy
3.4. ADRs
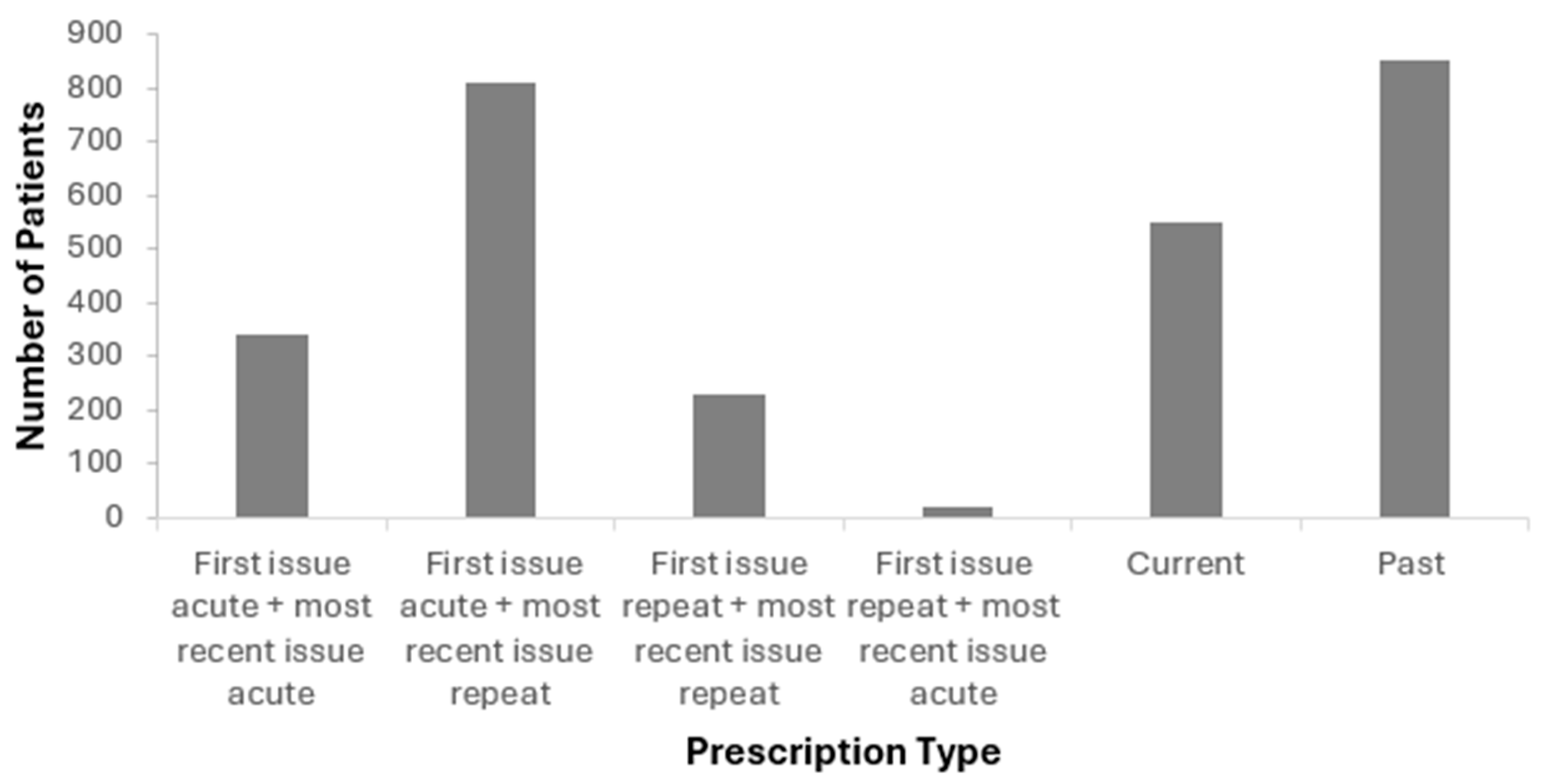
3.5. Prescription Type
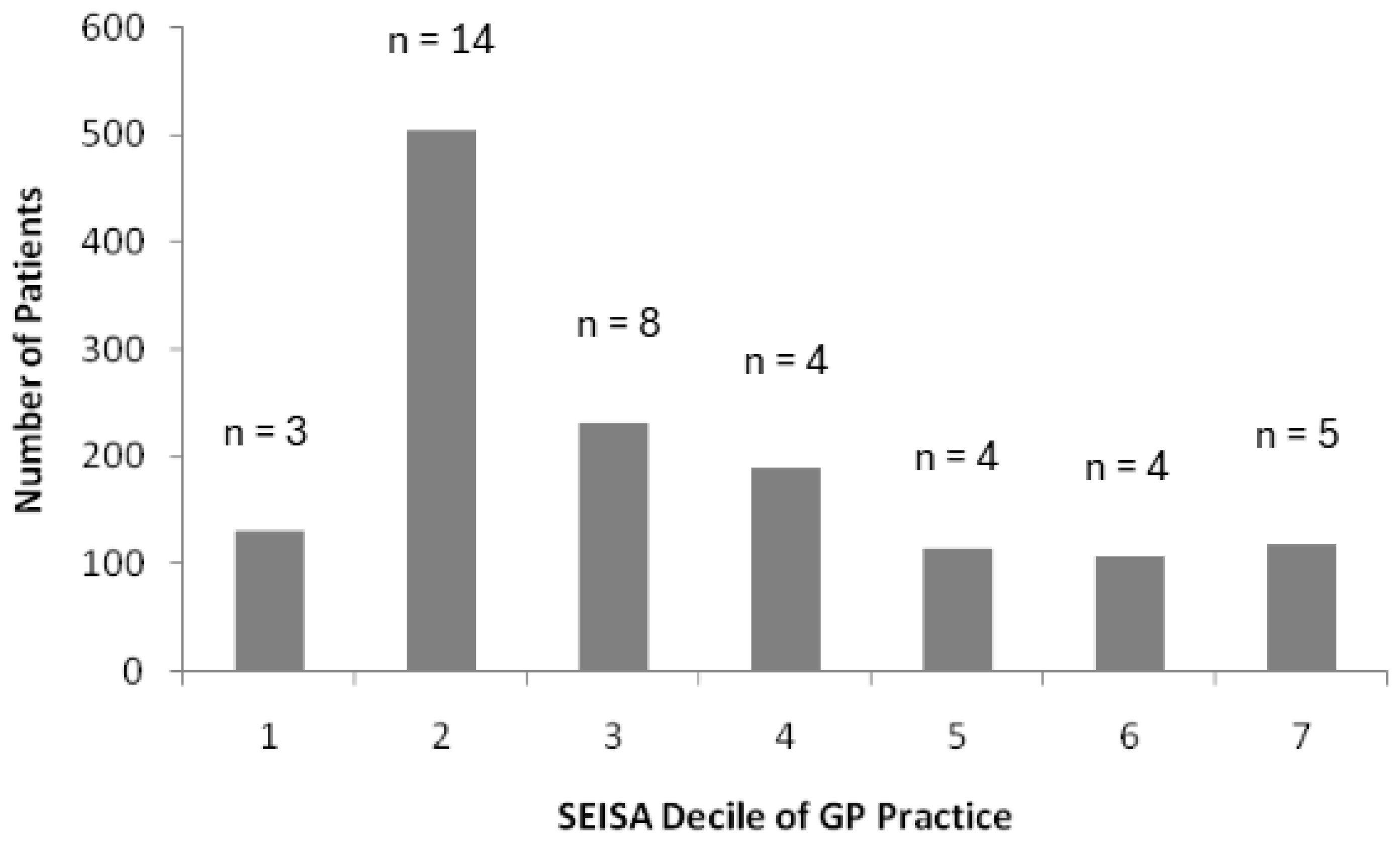
3.6. Socioeconomic Impact
4. Discussion
4.1. Prescribing Patterns and Supply Constraints
4.2. Ethnic Disparities in Prescribing
4.3. Clinical Effectiveness in Practice
4.4. Underreporting of ADRs
4.5. Treatment Continuity and Medication Switching
4.6. Deprivation and Prescribing Volume
4.7. Recommendations to Practice
4.8. Strengths and Limitations
4.9. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Yi, Z. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 721135. [Google Scholar] [CrossRef] [PubMed]
- Cornell, S. A review of GLP-1 receptor agonists in type 2 diabetes: A focus on the mechanism of action of once-weekly agents. J. Clin. Pharm. Ther. 2020, 45, 17–27. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Clinical Knowledge Summaries (CKS): Diabetes–Type 2: GLP-1 Receptor Agonists. Available online: https://cks.nice.org.uk/topics/diabetes-type-2/prescribing-information/glp-1-receptor-agonists/ (accessed on 26 October 2024).
- Miles, K.E.; Kerr, J.L. Semaglutide for the Treatment of Type 2 Diabetes Mellitus. J. Pharm. Technol. 2018, 34, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Electronic Medicines Compendium. Ozempic 0.25 mg Solution for Injection in Pre-Filled Pen. Available online: https://www.medicines.org.uk/emc/product/9748/smpc (accessed on 3 November 2024).
- Electronic Medicines Compendium. Wegovy 0.25 mg, FlexTouch Solution for Injection in Pe-Filled Pen. Available online: https://www.medicines.org.uk/emc/product/13799/smpc (accessed on 3 November 2024).
- Sorli, C.; Harashima, S.I.; Tsoukas, G.M.; Unger, J.; Karsbol, J.D.; Hansen, T.; Bain, S.C. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): A double blind, randomized, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017, 5, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Wilding, J.P.H.; Batterham, R.L.; Calanna, S.; Davies, M.; Van Gaal, L.F.; Lingvay, I.; McGowan, B.M.; Rosenstock, J.; Tran, M.T.D.; Wadden, T.A.; et al. Once-weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021, 384, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Diabetes UK. Our Response to Serious Supply Issues of Drugs for People with Type 2 Diabetes. Available online: https://www.diabetes.org.uk/about-us/news-and-views/our-response-serious-supply-issues-drugs-people-living-type-2-diabetes (accessed on 3 November 2024).
- BBC News. Weight Loss Injection Hype Fuels Online Black Market. Available online: https://www.bbc.co.uk/news/health-67414203 (accessed on 22 December 2024).
- The Pharmaceutical Journal. The Unregulated Semaglutide Prescribing Circus. Available online: https://pharmaceutical-journal.com/article/opinion/the-unregulated-semaglutide-prescribing-circus (accessed on 3 November 2024).
- Gov.UK. MHRA Warns of Unsafe Fake Weight Loss Pens. Available online: https://www.gov.uk/government/news/mhra-warns-of-unsafe-fake-weight-loss-pens (accessed on 1 January 2025).
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef] [PubMed]
- Gov.UK. MHRA Reminds Healthcare Professionals to Advise Patients of the Side Effects of GLP-1 Agonists and to Report Misuse. Available online: https://www.gov.uk/government/news/mhra-reminds-healthcare-professionals-to-advise-patients-of-the-side-effects-of-glp-1-agonists-and-to-report-misuse (accessed on 3 November 2024).
- National Institute for Health and Care Excellence. British National Formulary: Semaglutide–Side-effects. Available online: https://bnf.nice.org.uk/drugs/semaglutide/#side-effects (accessed on 3 November 2024).
- Zhou, J.; Zheng, Y.; Xu, B.; Long, S.; Zhu, L.; Liu, Y.; Li, C.; Zhang, Y.; Liu, M.; Wu, X. Exploration of the potential association between GLP-1 receptor agonists and suicidal or self-injurious behaviours: A pharmacovigilance study based on the FDA Adverse Event Reporting System database. BMC Med. 2024, 22, 65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Huang, W.; Xie, Y.; Shen, H.; Liu, M.; Wu, X. Risk of ophthalmic adverse drug reactions in patients prescribed glucagon-like peptide 1 receptor agonists: A pharmacovigilance study based on the FDA adverse event reporting system database. Endocrine 2024, 88, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; He, X.; Wu, P.; Liu, Y.; Ding, Y.; Zhang, Q. Gastrointestinal adverse events associated with semaglutide: A pharmacovigilance study based on FDA adverse event reporting system. Front. Public Health 2022, 10, 996179. [Google Scholar] [CrossRef] [PubMed]
- Butuca, A.; Dobrea, C.M.; Arseniu, A.M.; Frum, A.; Chis, A.A.; Rus, L.L.; Ghibu, S.; Juncan, A.M.; Muntean, A.C.; Lazăr, A.E.; et al. An Assessment of Semaglutide Safety Based on Real World Data: From Popularity to Spontaneous Reporting in EudraVigilance Database. Biomedicines 2024, 12, 1124. [Google Scholar] [CrossRef] [PubMed]
- Amirthalingam, P. A Descriptive Analysis from VigiAccess on Drug-related Problems Associated with the Glucagon-like Peptide-1 Receptor Agonists. Curr. Drug Saf. 2025, 20, e15748863367086. [Google Scholar] [CrossRef] [PubMed]
- Waldrop, S.W.; Johnson, V.R.; Stanford, F.C. Inequalities in the provision of GLP-1 receptor agonists for the treatment of obesity. Nat. Med. 2024, 30, 22–25. [Google Scholar] [CrossRef] [PubMed]
- NHS Black Country Integrated Care Board. Our Five Year Joint Forward Plan. Available online: https://blackcountry.icb.nhs.uk/about-us/our-priorities/our-5-year-joint-forward-plan (accessed on 28 December 2024).
- OpenPrescribing. Semaglutide (0601023AW). Available online: https://openprescribing.net/chemical/0601023AW/ (accessed on 24 October 2024).
- Medicines & Healthcare Products Regulatory Agency. Yellow Card: Interactive Drug Analysis Profile. Available online: https://info.mhra.gov.uk/drug-analysis-profiles/dap.html?drug=./UK_EXTERNAL/NONCOMBINED/UK_NON_000329053901.zip&agency=MHRA (accessed on 24 October 2024).
- Higher Education Statistics Agency. Socioeconomic Index for Small Areas (SEISA)–England Map. Available online: https://www.hesa.ac.uk/data-and-analysis (accessed on 24 October 2024).
- Gov.UK. Yellow Card: Please Help to Reverse the Decline in Reporting of Suspected Adverse Drug Reactions. Available online: https://www.gov.uk/drug-safety-update/yellow-card-please-help-to-reverse-the-decline-in-reporting-of-suspected-adverse-drug-reactions (accessed on 21 November 2024).
- Kunutsor, S.K.; Khunti, K.; Seidu, S. Racial, ethnic and regional differences in the effect of sodium-glucose co-transporter 2 inhibitors and glucagon-like peptide 1 receptor agonists on cardiovascular and renal outcomes: A systematic review and meta-analysis of cardiovascular outcome trials. J. R. Soc. Med. 2024, 117, 267–283. [Google Scholar] [CrossRef] [PubMed]
- DeSouza, C.; Cariou, B.; Garg, S.; Lausvig, N.; Navarria, A.; Fonseca, V. Efficacy and Safety of Semaglutide for Type 2 Diabetes by Race and Ethnicity: A Post Hoc Analysis of the SUSTAIN Trials. J. Clin. Endocrinol. Metab. 2020, 105, 543–556. [Google Scholar] [CrossRef] [PubMed]
- The Pharmaceutical Journal. Pharmacy Regulator to Require ‘Two-Way Communication’ in New Draft Guidance. Available online: https://pharmaceutical-journal.com/article/news/pharmacy-regulator-to-require-two-way-communication-in-new-draft-guidance (accessed on 1 January 2025).
- Frias, J.P.; Davies, M.J.; Rosenstock, J.; Manghi, F.C.P.; Lando, L.F.; Bergman, B.K.; Liu, B.; Cui, X.; Brown, K. Tirzepatide versus Semaglutide Once Weekly in Patients with Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Agardh, E.; Allebeck, P.; Hallqvist, J.; Moradi, T.; Sidorchuk, A. Type 2 diabetes incidence and socio-economic position: A systematic review and meta-analysis. Int. J. Epidemiol. 2011, 40, 804–818. [Google Scholar] [CrossRef] [PubMed]
- Kilvert, A.; Fox, C. Health inequalities and diabetes. Pract. Diabetes 2023, 40, 19–24a. [Google Scholar] [CrossRef]
| Ethnic Category | Number of Semaglutide Prescriptions | % of Total Semaglutide Prescriptions | % Ethnicity Make-Up of West Midlands Region Studied (Census, 2021) |
|---|---|---|---|
| White (incl. British and Other) | 1157 | 82.5 | 84.8 |
| White British | 580 | 41.3 | 82.4 |
| British or Mixed British | 412 | 29.4 | n/a |
| Other White and European | 165 | 11.8 | 2.4 |
| South Asian | 101 | 7.2 | 7.2 |
| Black (African, Caribbean, or Other) | 38 | 2.7 | 2.5 |
| Mixed Ethnicity | 18 | 1.3 | 2.8 |
| Other (Asian, South/Central American) | 32 | 2.3 | 2.5 |
| Undisclosed | 53 | 3.8 | n/a |
| Total patients prescribed semaglutide | 1403 | n/a | n/a |
| Total Number of Adverse Drug Reactions Reported from 2020 to 2024 | Total Prescription Number from 2020 to 2024 | Incidence of Adverse Drug Reactions from 2020 to 2024 (%) | |
|---|---|---|---|
| National (A) | 9429 | 4,500,312 | 0.21 |
| The WM area studied | 23 | 1244 | 1.85 |
| Total Number of Reactions | % of All Reported Adverse Drug Reactions | |
|---|---|---|
| Gastrointestinal disorders | 5116 | 54.1% |
| General and administration site reactions | 1259 | 13.3% |
| Nervous system disorders | 1009 | 10.7% |
| Psychiatric, skin, and metabolism disorders | 754 | 8.0% |
| Psychiatric | 305 | |
| Skin and subcutaneous tissue | 216 | |
| Metabolism and nutrition | 233 | |
| Injury, investigations, and procedural issues | 540 | 5.7% |
| Injury/poisoning | 275 | |
| Investigations | 250 | |
| Surgical/medical procedures | 10 | |
| Other organ/system disorders | 728 | 7.7% |
| Includes cardiac, hepatic, respiratory, infections, renal, endocrine, and vascular disorders. | ||
| Fatalities | 23 | 0.2% |
| Total number of ADRs | 9429 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Whorton, A.; Osman, S.; Johal, J.; Baig, S.; Jones, A.M.; Jalal, Z. Prescribing Patterns and Adverse Effects of Semaglutide: A Real-World Comparative Evaluation. Healthcare 2026, 14, 35. https://doi.org/10.3390/healthcare14010035
Whorton A, Osman S, Johal J, Baig S, Jones AM, Jalal Z. Prescribing Patterns and Adverse Effects of Semaglutide: A Real-World Comparative Evaluation. Healthcare. 2026; 14(1):35. https://doi.org/10.3390/healthcare14010035
Chicago/Turabian StyleWhorton, Abigail, Samira Osman, Jaspal Johal, Sarah Baig, Alan M. Jones, and Zahraa Jalal. 2026. "Prescribing Patterns and Adverse Effects of Semaglutide: A Real-World Comparative Evaluation" Healthcare 14, no. 1: 35. https://doi.org/10.3390/healthcare14010035
APA StyleWhorton, A., Osman, S., Johal, J., Baig, S., Jones, A. M., & Jalal, Z. (2026). Prescribing Patterns and Adverse Effects of Semaglutide: A Real-World Comparative Evaluation. Healthcare, 14(1), 35. https://doi.org/10.3390/healthcare14010035







