Menopause and Diabetes Risk Along with Trajectory of β-Cell Function and Insulin Sensitivity: A Community-Based Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Procedures and Variables
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BMI | Body Mass Index |
| CIs | Confidence Intervals |
| DI | Disposition Index |
| DIHOMA-β, HOMA-IR | Disposition Index based on HOMA-β and HOMA-IR |
| DI IGI60, ISI | Disposition Index based on IGI60 and Composite ISI |
| FPG | Fasting Plasma Glucose |
| GEE | Generalized Estimating Equation |
| HbA1c | Glycated Hemoglobin A1c |
| HDL | High-Density Lipoprotein |
| HOMA-β | Homeostasis Model Assessment of β-cell function |
| HOMA-IR | Homeostasis Model Assessment of Insulin Resistance |
| HRT | Hormone Replacement Therapy |
| IGI60 | 60 min Insulinogenic Index |
| ISI | Insulin Sensitivity Index |
| KoGES | Korean Genome and Epidemiology Study |
| LMM | Linear Mixed Model |
| NIH | National Institute of Health |
| OGTT | Oral Glucose Tolerance Test |
| OR | Odds Ratio |
| PG | Plasma Glucose |
| SD | Standard Deviation |
| TC | Total Cholesterol |
| TG | Triglycerides |
| β-cell | Beta-cell |
References
- El Khoudary, S.R.; Greendale, G.; Crawford, S.L.; Avis, N.E.; Brooks, M.M.; Thurston, R.C.; Karvonen-Gutierrez, C.; Waetjen, L.E.; Matthews, K. The menopause transition and women’s health at midlife: A progress report from the Study of Women’s Health across the Nation (SWAN). Menopause 2019, 26, 1213–1227. [Google Scholar] [CrossRef]
- Sowers, M.R.; Zheng, H.; McConnell, D.; Nan, B.; Harlow, S.D.; Randolph, J.F., Jr. Estradiol rates of change in relation to the final menstrual period in a population-based cohort of women. J. Clin. Endocrinol. Metab. 2008, 93, 3847–3852. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef]
- Lambrinoudaki, I.; Paschou, S.A.; Armeni, E.; Goulis, D.G. The interplay between diabetes mellitus and menopause: Clinical implications. Nat. Rev. Endocrinol. 2022, 18, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Szmuilowicz, E.D.; Stuenkel, C.A.; Seely, E.W. Influence of menopause on diabetes and diabetes risk. Nat. Rev. Endocrinol. 2009, 5, 553–558. [Google Scholar] [CrossRef]
- Kim, C. Does menopause increase diabetes risk? Strategies for diabetes prevention in midlife women. Women’s Health 2012, 8, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Slopien, R.; Wender-Ozegowska, E.; Rogowicz-Frontczak, A.; Meczekalski, B.; Zozulinska-Ziolkiewicz, D.; Jaremek, J.D.; Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; et al. Menopause and diabetes: EMAS clinical guide. Maturitas 2018, 117, 6–10. [Google Scholar] [CrossRef]
- Karvonen-Gutierrez, C.A.; Park, S.K.; Kim, C. Diabetes and Menopause. Curr. Diab. Rep. 2016, 16, 20. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, M.; Liu, Y.; Sun, X.; Wang, B.; Zhao, Y.; Liu, D.; Liu, X.; Zhang, D.; Liu, F.; et al. Association of menopause and type 2 diabetes mellitus. Menopause 2019, 26, 325–330. [Google Scholar] [CrossRef]
- Soriguer, F.; Morcillo, S.; Hernando, V.; Valdés, S.; Ruiz de Adana, M.S.R.; Olveira, G.; Fuentes, E.G.; González, I.; Tapia, M.J.; Esteva, I.; et al. Type 2 diabetes mellitus and other cardiovascular risk factors are no more common during menopause: Longitudinal study. Menopause 2009, 16, 817–821. [Google Scholar] [CrossRef]
- Ko, B.J.; Jung, J.H.; Han, K.; Nam, G.E. Age at Menopause and Development of Type 2 Diabetes in Korea. JAMA Netw. Open 2025, 8, e2455388. [Google Scholar] [CrossRef] [PubMed]
- Brand, J.S.; Van Der Schouw, Y.T.; Onland-Moret, N.C.; Sharp, S.J.; Ong, K.K.; Khaw, K.T.; Ardanaz, E.; Amiano, P.; Boeing, H.; Chirlaque, M.D.; et al. Age at menopause, reproductive life span, and type 2 diabetes risk: Results from the EPIC-InterAct study. Diabetes Care 2013, 36, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Song, L.; Li, H.; Liu, B.; Zheng, X.; Zhang, L.; Yuan, J.; Liang, Y.; Wang, Y. Association between earlier age at natural menopause and risk of diabetes in middle-aged and older Chinese women: The Dongfeng–Tongji cohort study. Diabetes Metab. 2017, 43, 345–350. [Google Scholar] [CrossRef]
- Park, S.K.; Harlow, S.D.; Zheng, H.; Karvonen-Gutierrez, C.; Thurston, R.C.; Ruppert, K.; Janssen, I.; Randolph, J.F. Association between changes in oestradiol and follicle-stimulating hormone levels during the menopausal transition and risk of diabetes. Diabet. Med. 2017, 34, 531–538. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Manson, J.E.; Stevenson, J.C.; Fonseca, V.A. Menopausal hormone therapy and type 2 diabetes prevention: Evidence, mechanisms, and clinical implications. Endocr. Rev. 2017, 38, 173–188. [Google Scholar] [CrossRef]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and diagnosis of diabetes: Standards of care in diabetes-2023. Diabetes Care 2023, 46, S19–S40. [Google Scholar] [CrossRef]
- Ohn, J.H.; Kwak, S.H.; Cho, Y.M.; Lim, S.; Jang, H.C.; Park, K.S.; Cho, N.H. 10-year trajectory of β-cell function and insulin sensitivity in the development of type 2 diabetes: A community-based prospective cohort study. Lancet Diabetes Endocrinol. 2016, 4, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Han, B.G.; KoGES Group. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef]
- National Institute of Health. The Korean Genome and Epidemiology Study. 2025. Available online: https://www.nih.go.kr/ko/main/contents.do?menuNo=300564 (accessed on 24 April 2025).
- Cho, S.Y.; Hong, E.J.; Nam, J.M.; Han, B.; Chu, C.; Park, O. Opening of the national biobank of Korea as the infrastructure of future biomedical science in Korea. Osong Public Health Res. Perspect. 2012, 3, 177–184. [Google Scholar] [CrossRef]
- Tura, A.; Kautzky-Willer, A.; Pacini, G. Insulinogenic indices from insulin and C-peptide: Comparison of beta-cell function from OGTT and IVGTT. Diabetes Res. Clin. Pr. 2006, 72, 298–301. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Matsuda, M. Reduced time points to calculate the composite index. Diabetes Care 2010, 33, e93. [Google Scholar] [CrossRef]
- Park, S.Y.; Gautier, J.F.; Chon, S. Assessment of insulin secretion and insulin resistance in human. Diabetes Metab. J. 2021, 45, 641–654. [Google Scholar] [CrossRef]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef]
- Hubbard, A.E.; Ahern, J.; Fleischer, N.L.; Van der Laan, M.; Lippman, S.A.; Jewell, N.; Bruckner, T.; Satariano, W.A. To GEE or not to GEE: Comparing population average and mixed models for estimating the associations between neighborhood risk factors and health. Epidemiology 2010, 21, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.I.; Weaver, N.E.; Hendricks, A.E. Accessible analysis of longitudinal data with linear mixed effects models. Dis. Model. Mech. 2022, 15, dmm048025. [Google Scholar] [CrossRef]
- LeBlanc, E.S.; Kapphahn, K.; Hedlin, H.; Desai, M.; Parikh, N.I.; Liu, S.; Parker, D.R.; Anderson, M.; Aroda, V.; Sullivan, S.; et al. Reproductive history and risk of type 2 diabetes mellitus in postmenopausal women: Findings from the Women’s Health Initiative. Menopause 2017, 24, 64–72. [Google Scholar] [CrossRef]
- Mishra, S.R.; Waller, M.; Chung, H.F.; Mishra, G.D. Epidemiological studies of the association between reproductive lifespan characteristics and risk of type 2 diabetes and hypertension: A systematic review. Maturitas 2022, 155, 14–23. [Google Scholar] [CrossRef]
- Kim, C.; Edelstein, S.L.; Crandall, J.P.; Dabelea, D.; Kitabchi, A.E.; Hamman, R.F.; Montez, M.G.; Perreault, L.; Foulkes, M.A.; Barrett-Connor, E.; et al. Menopause and risk of diabetes in the Diabetes Prevention Program. Menopause 2011, 18, 857–868. [Google Scholar] [CrossRef]
- Greendale, G.A.; Sternfeld, B.; Huang, M.; Han, W.; Karvonen-Gutierrez, C.; Ruppert, K.; Cauley, J.A.; Finkelstein, J.S.; Jiang, S.F.; Karlamangla, A.S. Changes in body composition and weight during the menopause transition. JCI Insight 2019, 4, e124865. [Google Scholar] [CrossRef] [PubMed]
- Fenton, A. Weight, Shape, and Body Composition Changes at Menopause. J. Midlife Health 2021, 12, 187–192. [Google Scholar] [CrossRef]
- Cho, N.H.; Jang, H.C.; Park, H.K.; Cho, Y.W. Waist circumference is the key risk factor for diabetes in Korean women with history of gestational diabetes. Diabetes Res. Clin. Pr. 2006, 71, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.H.; Lee, J.H.; Kim, J.W.; Cho, J.H.; Choi, Y.H.; Ko, S.H.; Zimmet, P.; Son, H.Y. Epidemic obesity and type 2 diabetes in Asia. Lancet 2006, 11, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, S.; Tabák, A.G.; Akbaraly, T.N.; Hulmán, A.; Kivimäki, M.; Forouhi, N.G.; Iso, H.; Brunner, E.J. Age trajectories of glycaemic traits in non-diabetic South Asian and white individuals: The Whitehall II cohort study. Diabetologia 2015, 58, 534–542. [Google Scholar] [CrossRef]
- Muscelli, E.; Kozàkovà, M.; Flyvbjerg, A.; Kyriakopoulou, K.; Astiarraga, B.D.; Glintborg, D.; Konrad, T.; Favuzzi, A.; Petrie, J.; RISC investigators. The effect of menopause on carotid artery remodeling, insulin sensitivity, and plasma adiponectin in healthy women. Am. J. Hypertens. 2009, 22, 364–370. [Google Scholar] [CrossRef]
- Toth, M.J.; Sites, C.K.; Eltabbakh, G.H.; Poehlman, E.T. Effect of menopausal status on insulin-stimulated glucose disposal: Comparison of middle-aged premenopausal and early postmenopausal women. Diabetes Care 2000, 23, 801–806. [Google Scholar] [CrossRef]
- Walton, C.; Godsland, I.F.; Proudler, A.J.; Wynn, V.; Stevenson, J.C. The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. Eur. J. Clin. Investig. 1993, 23, 466–473. [Google Scholar] [CrossRef]
- Bergman, R.N.; Ader, M.; Huecking, K.; Van Citters, G. Accurate assessment of β-cell function: The hyperbolic correction. Diabetes 2002, 51, S212–S220. [Google Scholar] [CrossRef]
- Schnedl, W.J.; Stepan, V.M.; Roller, R.E.; Lipp, R.W. HbA1c determination with high-performance liquid chromatography. Diabetes Care 2000, 23, 874–875. [Google Scholar] [CrossRef]
- Riant, E.; Waget, A.; Cogo, H.; Arnal, J.F.; Burcelin, R.; Gourdy, P. Estrogens protect against high-fat diet-induced insulin resistance and glucose intolerance in mice. Endocrinology 2009, 150, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Bryzgalova, G.; Gao, H.; Ahrén, B.; Zierath, J.R.; Galuska, D.; Steiler, T.L.; Dahlman-Wright, K.; Nilsson, S.; Gustafsson, J.A.; Efendic, S.; et al. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: Insulin sensitivity in the liver. Diabetologia 2006, 49, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Le May, C.; Wong, W.P.S.; Ward, R.D.; Clegg, D.J.; Marcelli, M.; Korach, K.S.; Mauvais-Jarvis, F. Importance of extranuclear estrogen receptor-α and membrane G protein–coupled estrogen receptor in pancreatic islet survival. Diabetes 2009, 58, 2292–2302. [Google Scholar] [CrossRef]
- Tiano, J.P.; Mauvais-Jarvis, F. Importance of oestrogen receptors to preserve functional β-cell mass in diabetes. Nat. Rev. Endocrinol. 2012, 8, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Andrikopoulos, S.; Verchere, C.B.; Wang, F.; Hull, R.L.; Vidal, J. Oophorectomy promotes islet amyloid formation in a transgenic mouse model of type II diabetes. Diabetologia 2000, 43, 1309–1312. [Google Scholar] [CrossRef]
- Le May, C.; Chu, K.; Hu, M.; Ortega, C.S.; Simpson, E.R.; Korach, K.S.; Tsai, M.J.; Mauvais-Jarvis, F. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc. Natl. Acad. Sci. USA 2006, 103, 9232–9237. [Google Scholar] [CrossRef]

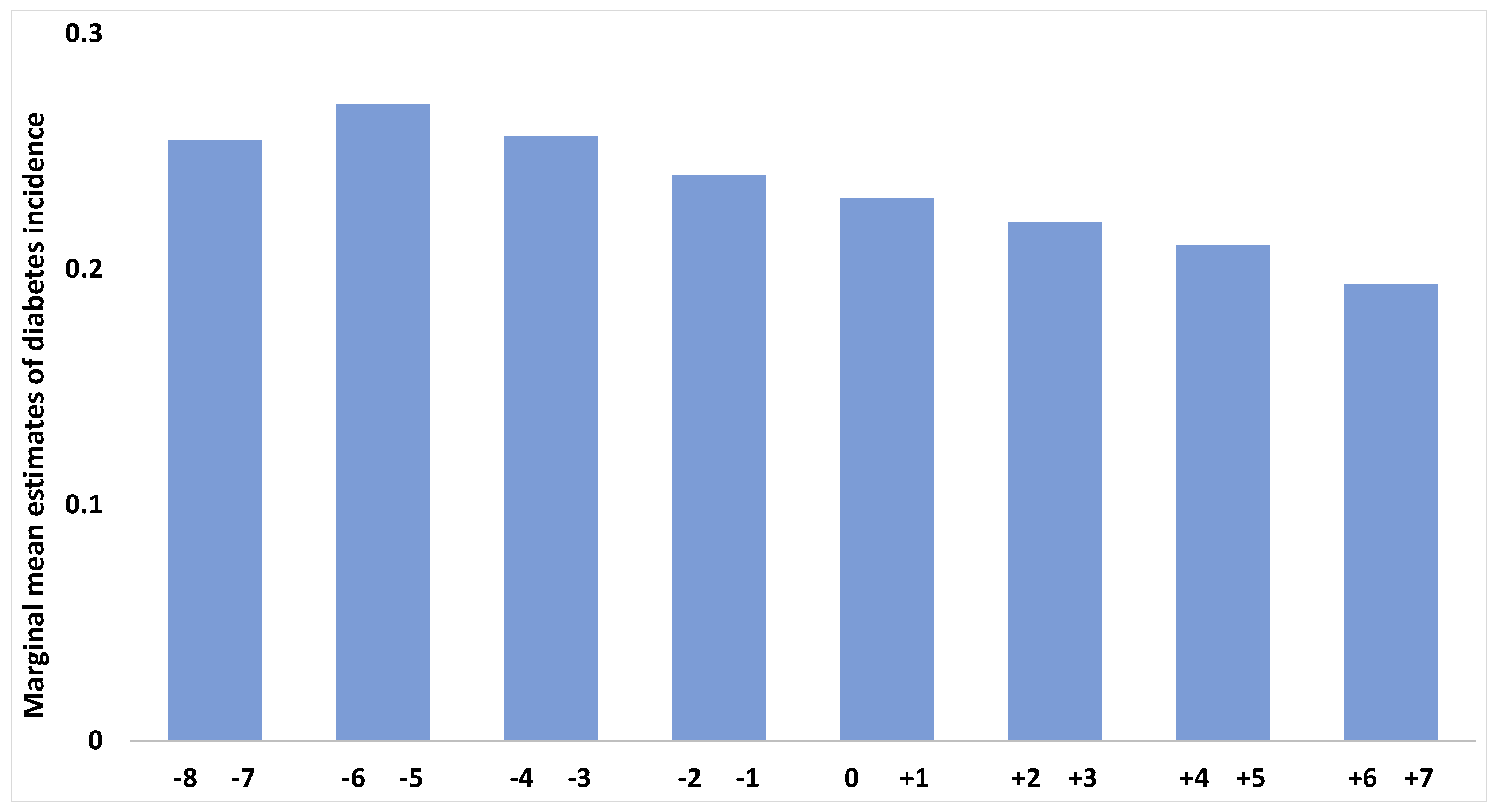

| Non- Progressor to Diabetes (N = 996) | Women with Premenopausal Incident Diabetes (N = 36) | Women with Perimenopausal Incident Diabetes (N = 77) | Women with Postmenopausal Incident Diabetes (N = 115) | p-Value | Statistical Significance of Two-Groups Comparison (p < 0.05) * | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean n | SD % | Mean n | SD % | Mean n | SD % | Mean n | SD % | ||||
| Age (years) | 44.1 | 3.0 | 42.7 | 2.3 | 44.5 | 3.1 | 44.9 | 2.8 | 0.001 | a, d, e | |
| Age at menopause (years) | 50.8 | 2.7 | 51.7 | 2.5 | 51.3 | 2.6 | 50.2 | 2.8 | 0.007 | e | |
| Reproductive lifetime (years) | 35.5 | 3.1 | 36.8 | 3.0 | 36.2 | 3.1 | 35.0 | 3.0 | 0.006 | e | |
| BMI (kg/m2) † | 23.9 | 1.1 | 25.3 | 1.1 | 25.6 | 1.1 | 25.4 | 1.1 | <0.001 | b, c | |
| Waist circumference (cm) † | 76.5 | 1.1 | 78.7 | 1.1 | 80.3 | 1.1 | 80.4 | 1.1 | <0.001 | b, c | |
| FPG (mg/dL) | 79.8 | 6.6 | 85.9 | 11.7 | 86.6 | 8.9 | 83.3 | 7.8 | <0.001 | a, b, c, f | |
| HbA1c | 5.4 | 0.3 | 5.6 | 0.4 | 5.7 | 0.4 | 5.6 | 0.3 | <0.001 | a, b, c | |
| Fasting insulin (μIU/mL) † | 6.5 | 1.8 | 7.4 | 1.8 | 7.2 | 1.9 | 8.2 | 1.5 | 0.001 | c | |
| IGI60 † | 7.3 | 3.9 | 5.3 | 2.3 | 4.8 | 4.3 | 7.4 | 3.0 | 0.065 | ||
| HOMA-β † | 153.1 | 2.0 | 136.3 | 2.0 | 119.4 | 2.0 | 160.3 | 1.8 | 0.011 | b, f | |
| Composite ISI † | 10.2 | 1.7 | 6.7 | 1.6 | 7.4 | 1.9 | 7.4 | 1.7 | <0.001 | a, b, c | |
| HOMA-IR † | 1.34 | 1.8 | 1.56 | 1.9 | 1.53 | 2.0 | 1.68 | 1.6 | <0.001 | c | |
| Disposition index IGI, ISI † | 70.6 | 3.6 | 35.8 | 2.4 | 33.3 | 3.6 | 49.8 | 2.6 | <0.001 | a, b, c | |
| Disposition index HOMA-β,HOMA-IR † | 119.4 | 1.7 | 87.4 | 2.1 | 78.3 | 1.7 | 95.2 | 1.7 | <0.001 | a, b, c | |
| Total cholesterol (mg/dL) † | 175.9 | 1.2 | 178.1 | 1.2 | 181.7 | 1.2 | 180.6 | 1.2 | 0.173 | ||
| HDL cholesterol (mg/dL) † | 46.5 | 1.2 | 43.2 | 1.2 | 43.4 | 1.2 | 43.0 | 1.3 | <0.001 | b, c | |
| Triglycerides (mg/dL) † | 106.3 | 1.4 | 128.7 | 1.8 | 132.4 | 1.5 | 134.1 | 1.5 | <0.001 | b, c, d | |
| Study Site (region) | 0.278 | ||||||||||
| Ansan | 618 | 62.0 | 28 | 77.8 | 47.0 | 61.0 | 70.0 | 60.9 | |||
| Ansung | 378 | 38.0 | 8 | 22.2 | 30.0 | 39.0 | 45.0 | 39.1 | |||
| Education | 0.256 | ||||||||||
| less than 7 years | 166 | 16.7 | 4 | 11.1 | 14 | 18.4 | 28 | 24.6 | |||
| 7~12 years | 726 | 73.1 | 26 | 72.2 | 55 | 72.4 | 72 | 63.2 | |||
| more than 12 years | 101 | 10.2 | 6 | 16.7 | 7 | 9.2 | 14 | 12.3 | |||
| Marital status | 0.331 | ||||||||||
| single | 8 | 0.8 | 1 | 2.8 | 1 | 1.3 | 1 | 0.9 | |||
| married | 944 | 95.1 | 33 | 91.7 | 70 | 90.9 | 109 | 95.6 | |||
| divorced/separated/bereaved | 41 | 4.1 | 2 | 5.6 | 6 | 7.8 | 4 | 3.5 | |||
| Income (Korean won) | 0.364 | ||||||||||
| less than one million | 163 | 16.8 | 7 | 19.4 | 17 | 22.7 | 22 | 19.5 | |||
| one to three million | 581 | 60.0 | 16 | 44.4 | 40 | 53.3 | 68 | 60.2 | |||
| more than three million | 225 | 23.2 | 13 | 36.1 | 18 | 24.0 | 23 | 20.4 | |||
| Family history of diabetes (yes) | 232 | 23.3 | 15 | 41.7 | 27 | 35.1 | 36 | 31.3 | 0.004 | a, b | |
| History of GDM (yes) | 10 | 1.0 | 1 | 2.8 | 1 | 1.3 | 0 | 0.0 | 0.332 | ||
| Hypertension (yes) | 195 | 19.6 | 12 | 33.3 | 35 | 45.5 | 53 | 46.1 | <0.001 | b, c | |
| Smoking | |||||||||||
| never/former | 953 | 98.0 | 30 | 88.2 | 73 | 96.1 | 108 | 97.3 | 0.003 | a | |
| current | 19 | 2.0 | 4 | 11.8 | 3 | 3.9 | 3 | 2.7 | |||
| Exercise | |||||||||||
| none | 561 | 61.0 | 23 | 63.9 | 40 | 55.6 | 66 | 61.1 | 0.185 | ||
| one or two times per week | 86 | 9.4 | 2 | 5.6 | 2 | 2.8 | 13 | 12.0 | |||
| three times or more per week | 272 | 29.6 | 11 | 30.6 | 30 | 41.7 | 29 | 26.9 | |||
| Alcohol consumption | |||||||||||
| none | 657 | 67.9 | 18 | 51.4 | 52 | 68.4 | 73 | 64.6 | 0.003 | a, b | |
| less than 20 g per day | 296 | 30.6 | 14 | 40.0 | 19 | 25.0 | 37 | 32.7 | |||
| 20 g or more per day | 14 | 1.4 | 3 | 8.6 | 5 | 6.6 | 3 | 2.7 | |||
| OR | (95% CI) | p-Value | |
|---|---|---|---|
| Age at menopause | 0.96 | (0.90, 1.02) | 0.210 |
| Premenopausal aging | 1.03 | (1.02, 1.04) | <0.001 |
| Perimenopausal aging | 1.00 | (0.99, 1.01) | 0.862 |
| Postmenopausal aging | 0.96 | (0.95, 0.97) | <.001 |
| BMI * | 6.89 | (3.01, 15.82) | <.001 |
| Waist circumference * | 2.25 | (1.41, 3.61) | 0.001 |
| HDL cholesterol * | 1.00 | (0.82, 1.23) | 0.982 |
| Triglycerides * | 1.14 | (1.08, 1.22) | <.001 |
| Study site (region) | |||
| Ansan | 1.14 | (0.77, 1.70) | 0.515 |
| Ansung | 1 [Reference] | ||
| Education | |||
| more than 12 years | 0.78 | (0.38, 1.58) | 0.484 |
| 7~12 years | 0.89 | (0.56, 1.39) | 0.598 |
| less than 7 years | 1 [Reference] | ||
| Marital status | |||
| married | 0.81 | (0.17, 3.82) | 0.791 |
| divorced/separated/bereaved | 1.52 | (0.27, 8.66) | 0.639 |
| single | 1 [Reference] | ||
| Income (Korean won) | |||
| more than three million | 1.21 | (0.68, 2.17) | 0.515 |
| one to three million | 0.97 | (0.60, 1.57) | 0.897 |
| less than one million | 1 [Reference] | ||
| Family history of diabetes (yes) | 1.78 | (1.23, 2.57) | 0.002 |
| Hypertension (yes) | 2.99 | (2.12, 4.21) | <.001 |
| Smoking | |||
| current | 0.99 | (0.63, 1.57) | 0.978 |
| never/former | 1 [Reference] | ||
| Exercise | |||
| three times or more per week | 0.99 | (0.94, 1.04) | 0.589 |
| one or two times per week | 0.95 | (0.87, 1.04) | 0.273 |
| none | 1 [Reference] | ||
| Alcohol consumption | |||
| 20 g or more per day | 1.02 | (0.85, 1.21) | 0.863 |
| less than 20 g per day | 0.99 | (0.93, 1.04) | 0.627 |
| none | 1 [Reference] |
| Perimenopausal Incident Diabetes | Postmenopausal Incident Diabetes | |||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | p-Value | OR | (95% CI) | p-Value | |
| Model 1 * | ||||||
| IGI60 | ||||||
| Baseline | 0.86 | (0.72, 1.02) | 0.084 | 0.99 | (0.83, 1.17) | 0.893 |
| Δ premenopausal period | 1.16 | (1.01, 1.32) | 0.030 | 1.00 | (0.82, 1.20) | 0.962 |
| Δ perimenopausal period | 1.10 | (1.01, 1.19) | 0.031 | 1.02 | (0.88, 1.17) | 0.813 |
| Δ postmenopausal period | - | 1.12 | (0.99, 1.26) | 0.080 | ||
| Composite ISI | ||||||
| Baseline | 0.65 | (0.53, 0.80) | <0.001 | 0.77 | (0.60, 0.98) | 0.034 |
| Δ premenopausal period | 1.24 | (1.02, 1.49) | 0.027 | 1.33 | (0.97, 1.81) | 0.074 |
| Δ perimenopausal period | 1.01 | (0.96, 1.06) | 0.742 | 1.31 | (1.03, 1.65) | 0.027 |
| Δ postmenopausal period | - | 1.14 | (0.89, 1.45) | 0.295 | ||
| DI IGI60, ISI | ||||||
| Baseline | 0.93 | (0.89, 0.96) | <0.001 | 0.90 | (0.86, 0.94) | <0.001 |
| Δ premenopausal period | 1.07 | (1.03, 1.12) | 0.002 | 1.10 | (1.04, 1.16) | <0.001 |
| Δ perimenopausal period | 1.04 | (1.01, 1.06) | 0.007 | 1.09 | (1.04, 1.14) | 0.001 |
| Δ postmenopausal period | - | 1.08 | (1.04, 1.13) | <0.001 | ||
| Model 2 * | ||||||
| IGI60 | ||||||
| Baseline | 0.85 | (0.70, 1.03) | 0.099 | 0.99 | (0.84, 1.16) | 0.870 |
| Δ premenopausal period | 1.17 | (1.02, 1.33) | 0.023 | 1.00 | (0.83, 1.20) | 0.987 |
| Δ perimenopausal period | 1.10 | (1.01, 1.19) | 0.030 | 1.02 | (0.89, 1.17) | 0.789 |
| Δ postmenopausal period | - | 1.12 | (0.99, 1.26) | 0.063 | ||
| Composite ISI | ||||||
| Baseline | 0.60 | (0.49, 0.74) | <0.001 | 0.77 | (0.61, 0.98) | 0.032 |
| Δ premenopausal period | 1.62 | (1.28, 2.06) | <0.001 | 1.33 | (0.98, 1.81) | 0.072 |
| Δ perimenopausal period | 1.39 | (1.13, 1.71) | 0.002 | 1.30 | (1.03, 1.65) | 0.028 |
| Δ postmenopausal period | - | 1.14 | (0.90, 1.45) | 0.292 | ||
| DI IGI60, ISI | ||||||
| Baseline | 0.92 | (0.89, 0.95) | <0.001 | 0.90 | (0.86, 0.94) | <0.001 |
| Δ premenopausal period | 1.08 | (1.03, 1.12) | 0.001 | 1.10 | (1.04, 1.16) | <0.001 |
| Δ perimenopausal period | 1.04 | (1.01, 1.07) | 0.008 | 1.09 | (1.04, 1.14) | 0.001 |
| Δ postmenopausal period | - | 1.08 | (1.04, 1.13) | <0.001 | ||
| Model 3 * | ||||||
| IGI60 | ||||||
| Baseline | 0.76 | (0.59, 0.98) | 0.031 | 1.04 | (0.86, 1.25) | 0.690 |
| Δ premenopausal period | 1.31 | (1.03, 1.68) | 0.029 | 1.14 | (0.91, 1.43) | 0.245 |
| Δ perimenopausal period | 1.17 | (1.02, 1.33) | 0.023 | 1.07 | (0.89, 1.30) | 0.467 |
| Δ postmenopausal period | - | 1.28 | (1.07, 1.52) | 0.006 | ||
| Composite ISI | ||||||
| Baseline | 0.54 | (0.42, 0.70) | <0.001 | 0.73 | (0.59, 0.92) | 0.007 |
| Δ premenopausal period | 1.88 | (1.33, 2.67) | <0.001 | 1.37 | (1.01, 1.86) | 0.041 |
| Δ perimenopausal period | 1.61 | (1.20, 2.16) | 0.002 | 1.33 | (1.04, 1.71) | 0.024 |
| Δ postmenopausal period | - | 1.11 | (0.84, 1.46) | 0.477 | ||
| DI IGI60, ISI | ||||||
| Baseline | 0.91 | (0.87, 0.95) | <0.001 | 0.82 | (0.72, 0.93) | 0.003 |
| Δ premenopausal period | 1.06 | (1.01, 1.12) | 0.020 | 1.17 | (1.06, 1.30) | 0.003 |
| Δ perimenopausal period | 1.04 | (1.01, 1.08) | 0.025 | 1.17 | (1.04, 1.32) | 0.011 |
| Δ postmenopausal period | - | 1.14 | (1.06, 1.24) | 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, M.J.; Yu, J. Menopause and Diabetes Risk Along with Trajectory of β-Cell Function and Insulin Sensitivity: A Community-Based Cohort Study. Healthcare 2025, 13, 1062. https://doi.org/10.3390/healthcare13091062
Choi MJ, Yu J. Menopause and Diabetes Risk Along with Trajectory of β-Cell Function and Insulin Sensitivity: A Community-Based Cohort Study. Healthcare. 2025; 13(9):1062. https://doi.org/10.3390/healthcare13091062
Chicago/Turabian StyleChoi, Mi Jin, and Juyoun Yu. 2025. "Menopause and Diabetes Risk Along with Trajectory of β-Cell Function and Insulin Sensitivity: A Community-Based Cohort Study" Healthcare 13, no. 9: 1062. https://doi.org/10.3390/healthcare13091062
APA StyleChoi, M. J., & Yu, J. (2025). Menopause and Diabetes Risk Along with Trajectory of β-Cell Function and Insulin Sensitivity: A Community-Based Cohort Study. Healthcare, 13(9), 1062. https://doi.org/10.3390/healthcare13091062






