Effectiveness of Web-Based Interventions on Clinical Outcomes and Lifestyle Modifications in Women Planning to Conceive: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Eligibility Criteria
2.3. Literature Search
2.4. Study Identification and Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis
2.7. Ethical Approval
3. Results
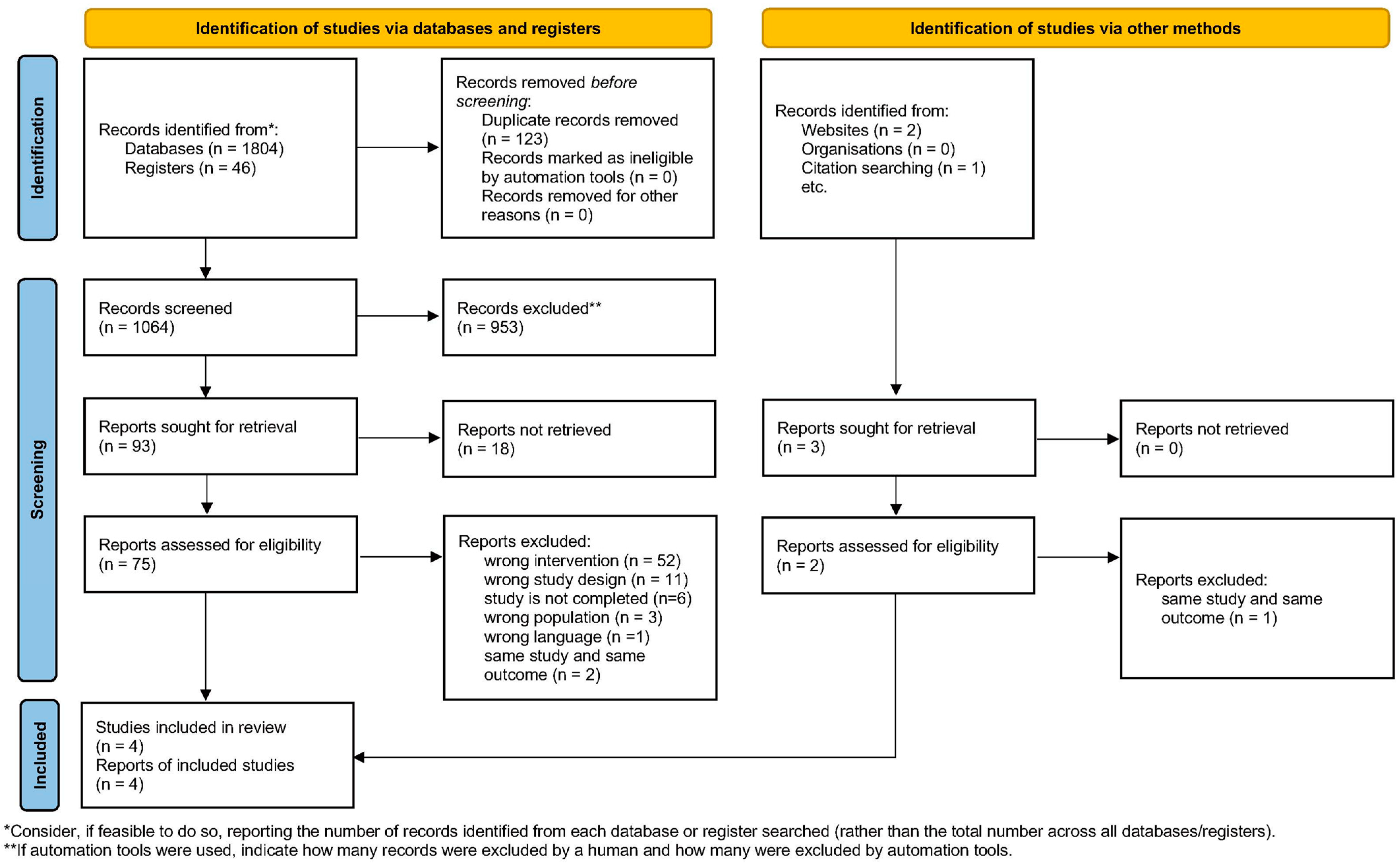
3.1. Study Selection
3.2. Study Characteristics
3.3. Intervention Characteristics
3.4. Quality Assessment of the Included Studies
3.5. Intervention Outcomes
3.5.1. Anthropometric Indicators
3.5.2. Physiological and Biochemical Indicators
3.5.3. Mental Health Status
3.5.4. Pregnancy-Related Outcomes
3.5.5. Food Intake (Vegetable and Fruit Intake)
3.5.6. Folic Acid Intake
3.5.7. Smoking and Alcohol Consumption
3.5.8. Physical Activity
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robbins, C.L.; D’Angelo, D.; Zapata, L.; Boulet, S.L.; Sharma, A.J.; Adamski, A.; Farfalla, J.; Stampfel, C.; Verbiest, S.; Kroelinger, C. Preconception Health Indicators for Public Health Surveillance. J. Women’s Health 2018, 27, 430–443. [Google Scholar] [CrossRef]
- World Health Organization. Meeting to Develop a Global Consensus on Preconception Care to Reduce Maternal and Childhood Mortality and Morbidity. 2012. Available online: https://iris.who.int/bitstream/handle/10665/78067/9789241505000_eng.pdf (accessed on 25 March 2024).
- Stephenson, J.; Heslehurst, N.; Hall, J.; Schoenaker, D.A.J.M.; Hutchinson, J.; Cade, J.E.; Poston, L.; Barrett, G.; Crozier, S.R.; Barker, M.; et al. Before the beginning: Nutrition and lifestyle in the preconception period and its importance for future health. Lancet 2018, 391, 1830–1841. [Google Scholar] [CrossRef] [PubMed]
- Shawe, J.; Steegers, A.P.E.; Verbiest, S. Preconception Health and Care: A Life Course Approach; Springer: New York, NY, USA, 2020. [Google Scholar]
- Hassan, M.A.; Killick, S.R. Negative lifestyle is associated with a significant reduction in fecundity. Fertil. Steril. 2004, 81, 384–392. [Google Scholar] [CrossRef]
- Barker, D.J. The developmental origins of chronic adult disease. Acta Paediatr. 2004, 93, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Schoenaker, D.A.; Hinton, W.; Poston, L.; Barker, M.; Alwan, N.A.; Godfrey, K.; Hanson, M.; de Lusignan, S.; UK Preconception Partnership. A wake-up call for preconception health: A clinical review. Br. J. Gen. Pract. 2021, 71, 233–236. [Google Scholar] [CrossRef]
- Hall, J.; Chawla, M.; Watson, D.; Jacob, C.M.; Schoenaker, D.; Connolly, A.; Barrett, G.; Stephenson, J. Addressing reproductive health needs across the life course: An integrated, community-based model combining contraception and preconception care. Lancet Public Health 2023, 8, e76–e84. [Google Scholar] [CrossRef] [PubMed]
- Schoenaker, D.; Connolly, A.; Stephenson, J. Preconception care in primary care: Supporting patients to have healthier pregnancies and babies. Br. J. Gen. Pract. 2022, 72, 152. [Google Scholar] [CrossRef]
- Cecconi, C.; Adams, R.; Cardone, A.; Declaye, J.; Silva, M.; Vanlerberghe, T.; Guldemond, N.; Devisch, I.; van Vugt, J. Generational differences in healthcare: The role of technology in the path forward. Front. Public Health 2025, 13, 1546317. [Google Scholar] [CrossRef]
- Whitaker, S.D. Population, Migration, and Generations in Urban Neighborhoods. 2019. Available online: https://www.clevelandfed.org/publications/economic-commentary/2019/ec-201908-population-migration-and-generations-in-urban-neighborhoods (accessed on 25 March 2024).
- Musgrave, L.; Cheney, K.; Dorney, E.; Homer, C.S.E.; Gordon, A. Addressing Preconception Behavior Change Through Mobile Phone Apps: Systematic Review and Meta-analysis. J. Med. Internet Res. 2023, 25, e41900. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.4. 2023. Available online: https://training.cochrane.org/handbook/current (accessed on 25 March 2024).
- Flemyng, E.; Moore, T.H.; Boutron, I.; Higgins, J.P.T.; Hróbjartsson, A.; Nejstgaard, C.H.; Dwan, K. Using Risk of Bias 2 to assess results from randomised controlled trials: Guidance from Cochrane. BMJ Evid.-Based Med. 2023, 28, 260–266. [Google Scholar] [CrossRef]
- Hanafiah, A.N.; Aagaard-Hansen, J.; Ch Cheah, J.; Norris, S.A.; Karim, Z.B.; Skau, J.K.; Ali, Z.M.; Biesma, R.; Matzen, P.; Sulaiman, L.H.; et al. Effectiveness of a complex, pre-conception intervention to reduce the risk of diabetes by reducing adiposity in young adults in Malaysia: The Jom Mama project—A randomised controlled trial. J. Glob. Health 2022, 12, 04053. [Google Scholar] [CrossRef] [PubMed]
- Jack, B.W.; Bickmore, T.; Yinusa-Nyahkoon, L.; Reichert, M.; Julce, C.; Sidduri, N.; Martin-Howard, J.; Zhang, Z.; Woodhams, E.; Fernandez, J.; et al. Improving the health of young African American women in the preconception period using health information technology: A randomised controlled trial. Lancet Digit. Health 2020, 2, e475–e485. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.Y.B.; Steegers-Theunissen, R.; Willemsen, S.; Wellstead, S.; Cheong, Y.; Macklon, N. Smartphone-based lifestyle coaching modifies behaviours in women with subfertility or recurrent miscarriage: A randomized controlled trial. Reprod. Biomed. Online 2021, 43, 111–119. [Google Scholar] [CrossRef]
- Oostingh, E.C.; Koster, M.P.H.; van Dijk, M.R.; Willemsen, S.P.; Broekmans, F.J.M.; Hoek, A.; Goddijn, M.; Klijn, N.F.; van Santbrink, E.J.P.; Steegers, E.A.P.; et al. First effective mHealth nutrition and lifestyle coaching program for subfertile couples undergoing in vitro fertilization treatment: A single-blinded multicenter randomized controlled trial. Fertil. Steril. 2020, 114, 945–954. [Google Scholar] [CrossRef]
- Oei, T.P.S.; Sawang, S.; Goh, Y.W.; Mukhtar, F. Using the Depression Anxiety Stress Scale 21 (DASS-21) across cultures. Int. J. Psychol. 2013, 48, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Prochaska, J.O.; Velicer, W.F. The transtheoretical model of health behavior change. Am. J. Health Promot. 1997, 12, 38–48. [Google Scholar] [CrossRef]
- Huijgen, N.A.; van de Kamp, M.E.; Twigt, J.M.; de Vries, J.H.M.; Eilers, P.H.C.; Steegers, E.A.P.; Laven, J.S.E.; Steegers-Theunissen, R.P.M. The Preconception Dietary Risk score; a simple tool to assess an inadequate habitual diet for clinical practice. e-SPEN J. 2014, 9, e13–e19. [Google Scholar] [CrossRef]
- Boutron, I.; Tubach, F.; Giraudeau, B.; Ravaud, P. Blinding was judged more difficult to achieve and maintain in nonpharmacologic than pharmacologic trials. J. Clin. Epidemiol. 2004, 57, 543–550. [Google Scholar] [CrossRef]
- Friedberg, J.P.; Lipsitz, S.R.; Natarajan, S. Challenges and recommendations for blinding in behavioral interventions illustrated using a case study of a behavioral intervention to lower blood pressure. Patient Educ. Couns. 2010, 78, 5–11. [Google Scholar] [CrossRef]
- Rains, J.C.; Penzien, D.B. Behavioral research and the double-blind placebo-controlled methodology: Challenges in applying the biomedical standard to behavioral headache research. Headache 2005, 45, 479–486. [Google Scholar] [CrossRef]
- Boutron, I.; Moher, D.; Tugwell, P.; Giraudeau, B.; Poiraudeau, S.; Nizard, R.; Ravaud, P. A checklist to evaluate a report of a nonpharmacological trial (CLEAR NPT) was developed using consensus. J. Clin. Epidemiol. 2005, 58, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Davidson, K.W.; Goldstein, M.; Kaplan, R.M.; Kaufmann, P.G.; Knatterud, G.L.; Orleans, C.T.; Spring, B.; Trudeau, K.J.; Whitlock, E.P. Evidence-based behavioral medicine: What is it and how do we achieve it? Ann. Behav. Med. 2003, 26, 161–171. [Google Scholar] [CrossRef]
- Fleming, T.P.; Watkins, A.J.; Velazquez, M.A.; Mathers, J.C.; Prentice, A.M.; Stephenson, J.; Barker, M.; Saffery, R.; Yajnik, C.S.; Eckert, J.J.; et al. Origins of lifetime health around the time of conception: Causes and consequences. Lancet 2018, 391, 1842–1852. [Google Scholar] [CrossRef]
- Barker, M.; Dombrowski, S.U.; Colbourn, T.; Fall, C.H.D.; Kriznik, N.M.; Lawrence, W.T.; Norris, S.A.; Ngaiza, G.; Patel, D.; Skordis-Worrall, J.; et al. Intervention strategies to improve nutrition and health behaviours before conception. Lancet 2018, 391, 1853–1864. [Google Scholar] [CrossRef]
- Moholdt, T.; Hawley, J.A. Maternal Lifestyle Interventions: Targeting Preconception Health. Trends Endocrinol. Metab. 2020, 31, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Tobias, D.K.; Zhang, C.; Chavarro, J.; Bowers, K.; Rich-Edwards, J.; Rosner, B.; Mozaffarian, D.; Hu, F.B. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am. J. Clin. Nutr. 2012, 96, 289–295. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guidelines on Physical Activity and Sedentary Behaviour. 2020. Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 25 March 2024).
- Garnæs, K.K.; Mørkved, S.; Salvesen, Ø.; Moholdt, T. Exercise Training and Weight Gain in Obese Pregnant Women: A Randomized Controlled Trial (ETIP Trial). PLoS Med. 2016, 13, e1002079. [Google Scholar] [CrossRef]
- Seneviratne, S.N.; Jiang, Y.; Derraik, J.; McCowan, L.; Parry, G.K.; Biggs, J.B.; Craigie, S.; Gusso, S.; Peres, G.; Rodrigues, R.O.; et al. Effects of antenatal exercise in overweight and obese pregnant women on maternal and perinatal outcomes: A randomised controlled trial. BJOG 2016, 123, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Gaston, A.; Cramp, A. Exercise during pregnancy: A review of patterns and determinants. J. Sci. Med. Sport 2011, 14, 299–305. [Google Scholar] [CrossRef]
- Floyd, R.L.; Jack, B.W.; Cefalo, R.; Atrash, H.; Mahoney, J.; Herron, A.; Husten, C.; Sokol, R.J. The clinical content of preconception care: Alcohol, tobacco, and illicit drug exposures. Am. J. Obstet. Gynecol. 2008, 199, S333–S339. [Google Scholar] [CrossRef]
- Jack, B.W.; Atrash, H.; Coonrod, D.V.; Moos, M.K.; O’Donnell, J.; Johnson, K. The clinical content of preconception care: An overview and preparation of this supplement. Am. J. Obstet. Gynecol. 2008, 199, S266–S279. [Google Scholar] [CrossRef] [PubMed]
- Evans-Hoeker, E.A.; Eisenberg, E.; Diamond, M.P.; Legro, R.S.; Alvero, R.; Coutifaris, C.; Casson, P.R.; Christman, G.M.; Hansen, K.R.; Zhang, H.; et al. Reproductive Medicine Network. Major depression, antidepressant use, and male and female fertility. Fertil. Steril. 2018, 109, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Wesselink, A.K.; Hatch, E.E.; Rothman, K.J.; Weuve, J.L.; Aschengrau, A.; Song, R.J.; Wise, L.A. Perceived Stress and Fecundability: A Preconception Cohort Study of North American Couples. Am. J. Epidemiol. 2018, 187, 2662–2671. [Google Scholar] [CrossRef] [PubMed]
- Jácome, C.; Oliveira, D.; Marques, A.; Sá-Couto, P. Prevalence and impact of urinary incontinence among female athletes. Int. J. Gynaecol. Obstet. 2011, 114, 60–63. [Google Scholar] [CrossRef]
- Rubin, N.; Cerentini, T.M.; Schlöttgen, J.; do Nascimento Petter, G.; Bertotto, A.; La Verde, M.; Gullo, G.; Telles da Rosa, L.H.; Viana da Rosa, P.; Della Méa Plentz, R. Urinary incontinence and quality of life in high-performance swimmers: An observational study. Health Care Women Int. 2024, 45, 1446–1455. [Google Scholar] [CrossRef]
- Howard, G.S. Response-Shift Bias: A Problem in Evaluating Interventions with Pre/Post Self-Reports. Eval. Rev. 1980, 4, 93–106. [Google Scholar] [CrossRef]
- Saris, W.E.; Gallhofer, I.N. Estimation of Reliability, Validity, and Method Effects. In Design, Evaluation, and Analysis of Questionnaires for Survey Research; John Wiley & Sons, Inc.: New Jersey, NB, Canada, 2014; pp. 190–207. [Google Scholar]
- Biemer, P.P.; Groves, R.M.; Lyberg, L.E.; Mathiowetz, N.A.; Sudman, S. (Eds.) Measurement Errors in Surveys; John Wiley & Sons, Inc.: New Jersey, NB, Canada, 2013. [Google Scholar]
- Koller, K.; Pankowska, P.K.; Brick, C. Identifying bias in self-reported pro-environmental behavior. Curr. Res. Ecol. Soc. Psychol. 2023, 4, 100087. [Google Scholar] [CrossRef]
| PICO Component | Description |
|---|---|
| Population | Reproductive-aged women or couples wishing to conceive, including those experiencing infertility or undergoing ART. |
| Intervention | Web-based or technology-assisted lifestyle interventions (e.g., eHealth, mHealth, mobile apps, telemedicine, digital education). |
| Comparison | Standard care or no intervention. |
| Outcomes | Food intake, folic acid intake, physical activity, smoking, alcohol consumption, stress management, and clinical and biochemical indicators. |
| Author/Year | Country/ Setting | Study Design | Participants | Sample Size | Intervention | Control | Evaluation Period | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Hanafiah et al., 2022 [15] | Malaysia/district based | Individual RCT | Women aged 20–39 years; nulliparous; not pregnant; | 549 women | Provided lifestyle challenges that the young couples could select, information on healthy lifestyles, and included a total of six contact points with community health promotors | Standard care provided by public health clinics | At 33 weeks from baseline | Clinical outcomes: waist circumstance; weight; BMI; blood pressure; HbA1c; total cholesterol; high-density lipoprotein (HDL); triglycerides; depression; anxiety; stress Lifestyle modification outcomes: Vegetable and Fruit intake; Proportion size of rice and bread; Frequency of fried Foods, fast food, carbonated drinks, pastries, and sweet local delicacies; Vigorous/Moderate job-related physical activity; Vigorous/Moderate leisure physical activity; |
| Jack et al., 2020 [16] | USA/ web based | Individual RCT | African American or Black or both; women aged 18–34 years; currently not pregnant | 528 women | Visiting the Gabby character online and receiving an introductory engagement dialogue (log on at least once every 2 weeks) | Letter listing the risks identified and encouraging them to discuss these risks with a clinician | At 6 (24 w) and 12 (48 w) months after intervention | Clinical outcomes: anxiety; depression; stress; underweight; overweight Lifestyle modification outcomes: stages of change (SoC) [19] scores in the following: emotional and mental health (7 items); environmental issues (8 items); family planning (2 items); genetic health history (4 items); health care and programs (2 items); health conditions and medicine (17 items); immunizations and vaccines (8 items); infection diseases (12 items); men and health care (3 items); nutrition and activity (15 items); relationships (3 items); reproductive health (17 items); substance use (3 items) |
| Ng et al., 2021 [17] | UK/gynecological outpatient department | Individual RCT | Subfertility or recurrent miscarriage; women aged 18–45 years; actively trying to conceive | 262 women | Personalized smartphone lifestyle coaching program and emails (maximum of three per week) with feedback on progress, recommendations, tips, facts, and recipes | Standard preconception advice | At 12 and 24 weeks after randomization | Clinical outcomes: pregnancy at 24 weeks after randomization Lifestyle modification outcomes: fruit intake; vegetable intake; taking folic acid supplements; smoking; alcohol |
| Oostingh et al., 2020 [18] | Netherlands/IVF centers | Individual RCT | Women aged 18–45 years; starting their IVF/ICSI treatment within the next 3 months | 626 women | Coaching via smart phone based on sex, pregnancy status, and behaviors and monitoring changes in their identified risk behaviors and to assess pregnancy status | “Light” version of Smarter Pregnancy | At 24 weeks after completion of program | Clinical outcomes: serum folate levels of women; pregnancy rates at 52 weeks Lifestyle modification outcomes: vegetable intake; fruit intake; folic acid supplement use; dietary risk score; smoking; alcohol consumption; lifestyle risk score |
| Outcomes | Hanafiah et al., 2022 [15] | Jack et al., 2020 [16] | Ng et al., 2021 [17] | Oostingh et al., 2020 [18] | |||||
|---|---|---|---|---|---|---|---|---|---|
| Measurement | Results | Measurement | Results | Measurement | Results | Measurement | Results | ||
| Clinical outcomes | Anthropometric Indicators | Waist circumstance; weight; BMI; blood pressure (systolic); blood pressure (diastolic) | 36 w: Waist circumstance: IG mean (SD); 82.6 (13.9) vs. CG mean (SD); 81.7 (13.9), p = 0.547. Weight: IG mean (SD); 66.7 (18.8) vs. CG mean (SD); 65.4 (17.5), p = 0.528. BMI: IG mean (SD) 27.3 (7.4) vs. CG mean (SD); 27.0 (7.0), p = 0.701. Blood pressure (systolic); IG mean (SD); 107.6 (14.0) vs. CG mean (SD); 104.7 (10.6), p = 0.031. Blood pressure (diastolic); IG mean (SD); 72.3 (10.5) vs. CG mean (SD); 70.9 (9.1), p = 0.230. | Underweight; overweight | Underweight: the data are unavailable due to missing data for the underweight group in the IG. Overweight: 24 w: IG mean; 3.85 vs. CG mean; 3.64, p = 0.30; 48 w: IG mean; 4.02 vs. CG mean; 4.05 p = 0.88. | Not reported | Not reported | ||
| Physiological and Biochemical Indicators | HbA1c; total cholesterol; high-density lipoprotein (HDL); triglycerides | 36 w: HbA1c IG mean (SD); 5.3 (0.6) vs. CG mean (SD); 5.2 (0.5), p = 0.093. Total cholesterol; IG mean (SD); 4.86 (0.9) vs. CG mean (SD); 4.79 (0.9), p = 0.527. HDL: IG mean (SD); 1.45 (0.4) vs. CG mean (SD); 1.43 (0.4), p = 0.632. | Not reported | Not reported | Serum folate levels of women | 12 w: IG median 48.6 (IQR 28.8–64.1) nmol/L vs. CG median 30.1 (IQR 17.9–51.9) nmol/L | |||
| Mental Health Status | Stress, moderate or severe (DASS-21) [19] | 36 w: moderate; IG; 15.9% vs. CG; 16.3%, severe; IG; 2.1% vs. CG; 6.3%, p = 0.19. | Stress (PSS) | 24 w: IG mean; 4.04 vs. CG mean; 3.60, p = 0.21. 48 w: IG mean; 4.09 vs. CG mean; 3.89, p = 0.52. | Not reported | Not reported | |||
| Depression, moderate or severe (DASS-21) [19] | 36 w: moderate; IG; 34.5% vs. CG; 33.1%, severe; IG; 2% vs. CG; 6%, p = 0.64 | Potential Depression (PHQ-2) | 24 w: IG mean; 3 vs. CG mean; 2.77, p = 0.66. 48 w: IG mean; 3.27 vs. CG mean; 3.58, p = 0.41. | Not reported | Not reported | ||||
| Anxiety moderate or severe (DASS-21) [19] | 36 w: moderate; IG; 20.0% vs. CG; 19.4%, severe; IG; 1.4% vs. CG; 3.1%, p = 0.60. | Not reported | Not reported | Not reported | |||||
| Pregnancy-related outcomes | Not reported | Not reported | Pregnancy | 24 w: difference between IG and CG; 2.83 (95% CI 0.35 to 57.76) | Pregnancy | 52w: difference between IG and CG; 0.807 (95% CI 0.574 to 1.134) | |||
| Lifestyle modification outcomes | Vegetable intake | Vegetable intake/week | 36 w: IG mean ± SD; 9.4 ± 7.8 vs. CG mean ± SD; 8.7 ± 6.1, p = 0.458. | SoC score of bad diet or food choices (<5 daily servings of fruits and vegetables and/or regular intake of junk food) | 24 w: IG mean; 3.53 vs. CG mean; 3.27, p = 0.27. 48 w: IG mean; 3.55 vs. CG mean; 3.39, p = 0.57. | Vegetable intake risk score (“0” adequate to “3” inadequate) | 12 w: difference between IG and CG; −0.21 (95% CI −0.48 to 0.03) 24 w: difference between IG and CG; 0.00 (95% CI −0.30 to 0.27) | number of vegetables > 200 g/day | 24 w: IG; 40% vs. CG; 28% 36 w: IG; 33% vs. CG; 21% |
| Fruit intake | Fruit intake/week | 36 w: IG mean ± SD; 5.8 ± 4.8 vs. CG mean ± SD; 5.1 ± 4.2, p = 0.199. | SoC score of bad diet or food choices (<5 daily servings of fruits and vegetables and/or regular intake of junk food) | 24 w: IG mean; 3.53 vs. CG mean; 3.27, p = 0.27. 48 w: IG mean; 3.55 vs. CG mean; 3.39, p = 0.57. | Fruit intake risk score (“0” adequate to “3” inadequate) | 12 w: difference between IG and CG; −0.14 (95% CI −0.60 to 0.07) 24 w: difference between IG and CG; −0.21 (95% CI −0.50 to 0.66) | Fruits, >2 pieces per day | 24 w: IG; 67% vs. CG; 44% 36 w: IG; 68% vs. CG; 57% | |
| Folic acid intake | Not reported | SoC score of not using multivitamin with folic acid or folic acid supplement | 24 w: IG mean; 3.29 vs. CG mean; 3.13, p = 0.47. 48 w: IG mean; 3.45 vs. CG mean; 3.32, p = 0.61. | Taking folic acid supplements (“0” adequate to “3” inadequate) | 12 w: difference between IG and CG; −0.04 (95% CI −0.29 to 0.21) 24 w: difference between IG and CG; −0.16 (95% CI −0.42 to 0.09) | Taking adequate folic acid supplement | 24 w: IG; 97% vs. CG; 99% 36 w: IG; 96% vs. CG; 96% | ||
| Smoking | Not reported | SoC score of any current tobacco use | 24 w: IG mean; 2.46 vs. CG mean; 2.38, p = 0.90. 48 w: IG mean; 3.54 vs. CG mean; 3.4, p = 0.89. | Smoking risk score (“0” no smoking to “6” 15 or more cigarettes/day) | 12 w: difference between IG and CG; 0.02 (95% CI −0.01 to 0.10) 24 w: difference between IG and CG; 0.08 (95% CI −0.02 to 0.28) | No smoking | 24 w: IG; 93% vs. CG; 92% 36 w: IG; 80% vs. CG; 91% | ||
| Alcohol consumption | Not reported | SoC score of excessive alcohol (≥4 drinks in a day over the past year) | 24 w: IG mean; 3.45 vs. CG mean; 3.67, p = 0.50. 48 w: IG mean; 4.03 vs. CG mean; 3.54, p = 0.13. | Alcohol risk score (“0” no alcohol intake to “3” 3 or more alcohol beverages/day) | 12 w: difference between IG and CG; 0.0 (95% CI −0.14 to 0.09) 24 w: difference between IG and CG; −0.02 (95% CI −0.15 to 0.10) | No alcohol consumption | 24 w: IG; 78% vs. CG; 73% 36 w: IG; 69% vs. CG; 75% | ||
| SoC score of excessive alcohol (drinking more than twice a week) | 24 w: IG mean; 3.69 vs. CG mean; 3.74, p = 0.88. 48 w: IG mean; 4.09 vs. CG mean; 3.66, p = 0.19. | ||||||||
| Physical Activity | Vigorous job-related physical activity, mins/week | 36 w: IG mean ± SD; 259.9 ± 389.7 vs. CG mean ± SD; 153.8 ± 280.2, p = 0.032 | SoC score of not enough exercise | 24 w: IG mean; 3.25 vs. CG mean; 3.35, p = 0.60. 48 w: IG mean; 3.50 vs. CG mean; 3.51, p = 0.97. | Not reported | Not reported | |||
| Moderate job-related physical activity, mins/week | 36 w: IG mean ± SD; 749.0 ± 822.0 vs. CG mean ± SD; 550.0 ± 725.4, p = 0.058. | ||||||||
| Vigorous leisure physical activity, mins/week | 36 w: IG mean ± SD; 120.5 ± 143.6 vs. CG mean ± SD; 138.5 ± 164.7, p = 0.417. | ||||||||
| Moderate leisure physical activity, mins/week | 36 w: IG mean ± SD; 271.8 ± 463.2 vs. CG mean ± SD; 328.6 ± 607.0, p = 0.434. | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, H.; Tun, P.P.; Liu, S.; Ota, E.; Arata, N. Effectiveness of Web-Based Interventions on Clinical Outcomes and Lifestyle Modifications in Women Planning to Conceive: A Systematic Review. Healthcare 2025, 13, 1037. https://doi.org/10.3390/healthcare13091037
Suzuki H, Tun PP, Liu S, Ota E, Arata N. Effectiveness of Web-Based Interventions on Clinical Outcomes and Lifestyle Modifications in Women Planning to Conceive: A Systematic Review. Healthcare. 2025; 13(9):1037. https://doi.org/10.3390/healthcare13091037
Chicago/Turabian StyleSuzuki, Hitomi, Phyu Phyu Tun, Shuxian Liu, Erika Ota, and Naoko Arata. 2025. "Effectiveness of Web-Based Interventions on Clinical Outcomes and Lifestyle Modifications in Women Planning to Conceive: A Systematic Review" Healthcare 13, no. 9: 1037. https://doi.org/10.3390/healthcare13091037
APA StyleSuzuki, H., Tun, P. P., Liu, S., Ota, E., & Arata, N. (2025). Effectiveness of Web-Based Interventions on Clinical Outcomes and Lifestyle Modifications in Women Planning to Conceive: A Systematic Review. Healthcare, 13(9), 1037. https://doi.org/10.3390/healthcare13091037






