COVID-19 Vaccine Uptake Inequality Among Children: A Multidimensional Demographic Analysis
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Inequalities in Uptake by Children Age-Race Groups
3.1.1. 16–17-Year-Olds
3.1.2. 12–15-Year-Olds
3.1.3. 5–11-Year-Olds
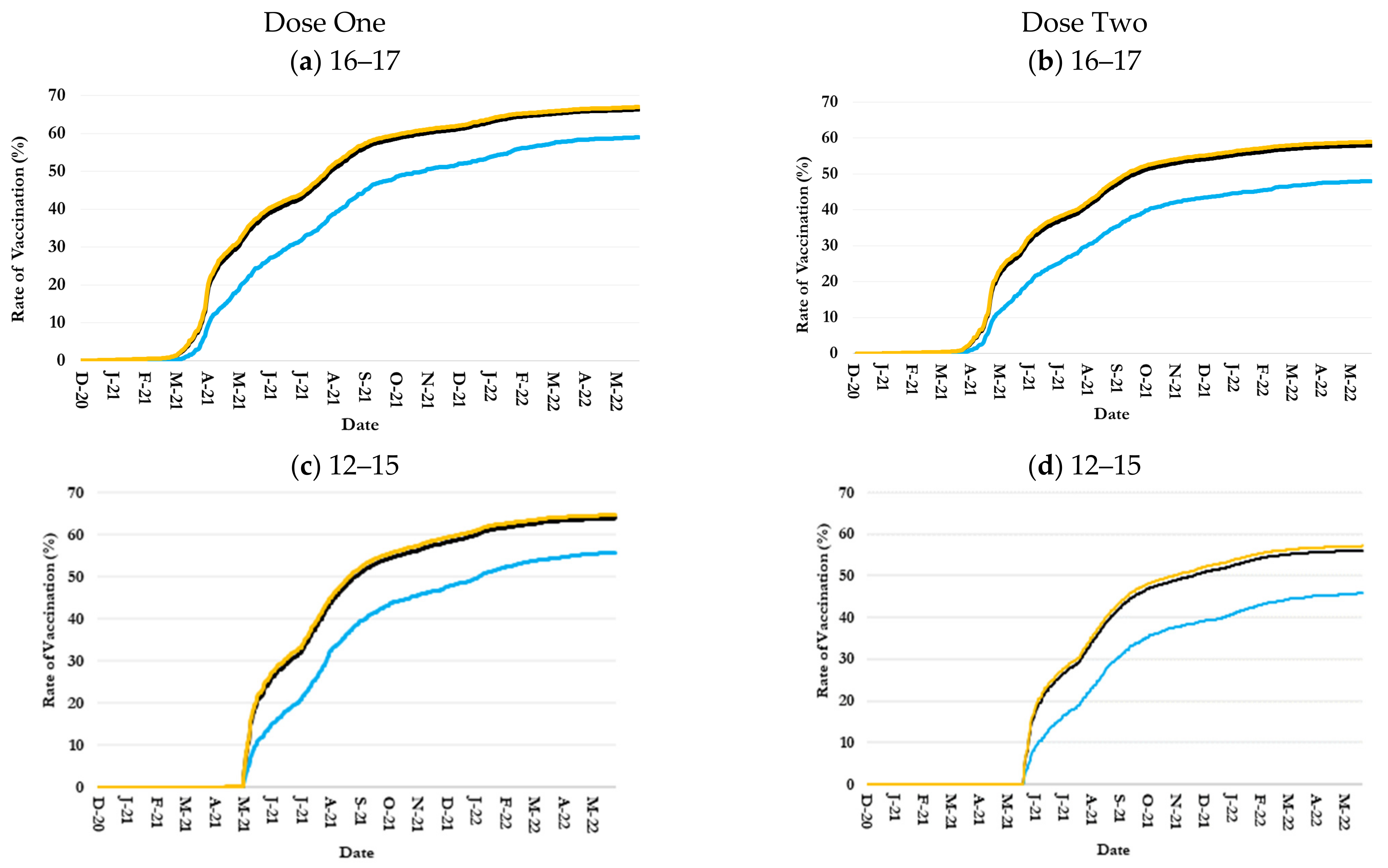
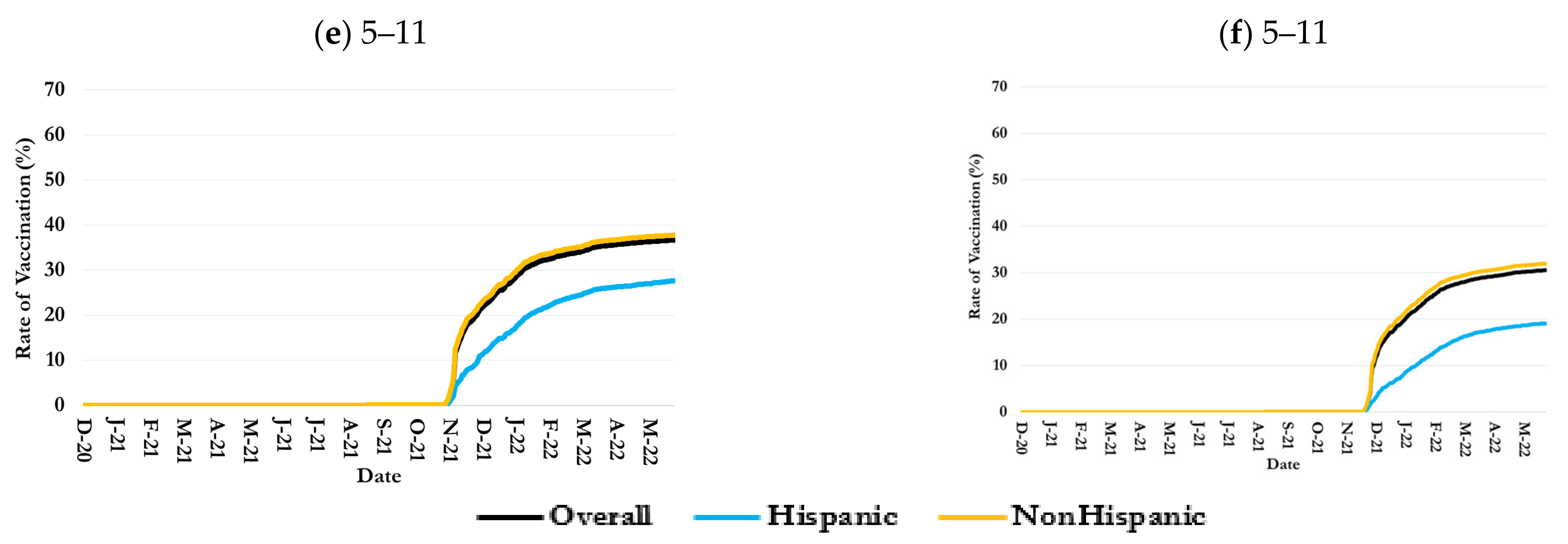
3.2. Inequalities in Uptake by Children Age-Ethnicity Groups
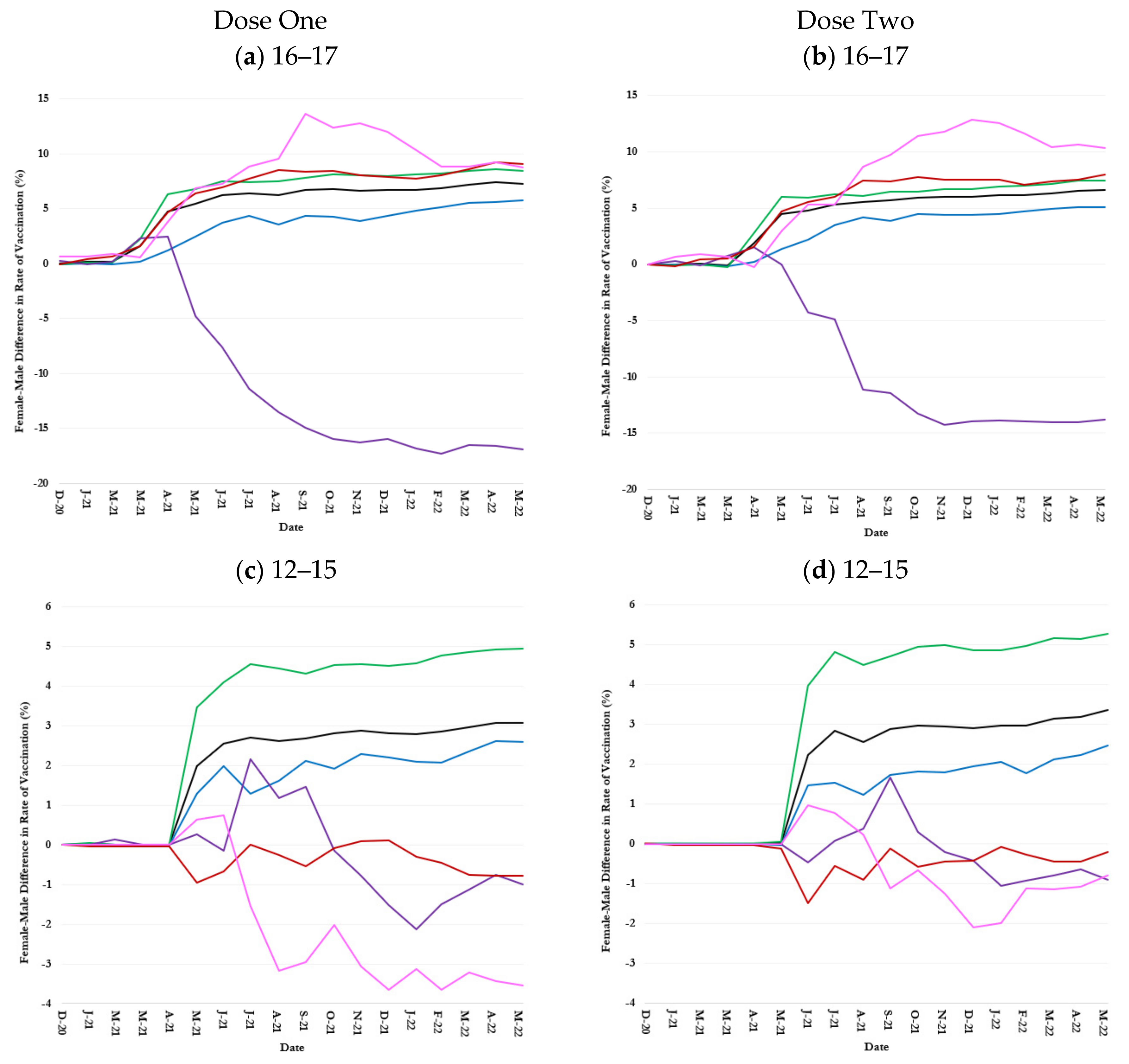
3.3. Inequalities in Uptake by Children Age-Race-Sex Groups
3.3.1. 16–17-Year-Olds
3.3.2. 12–15-Year-Olds
3.3.3. 5–11-Year-Olds
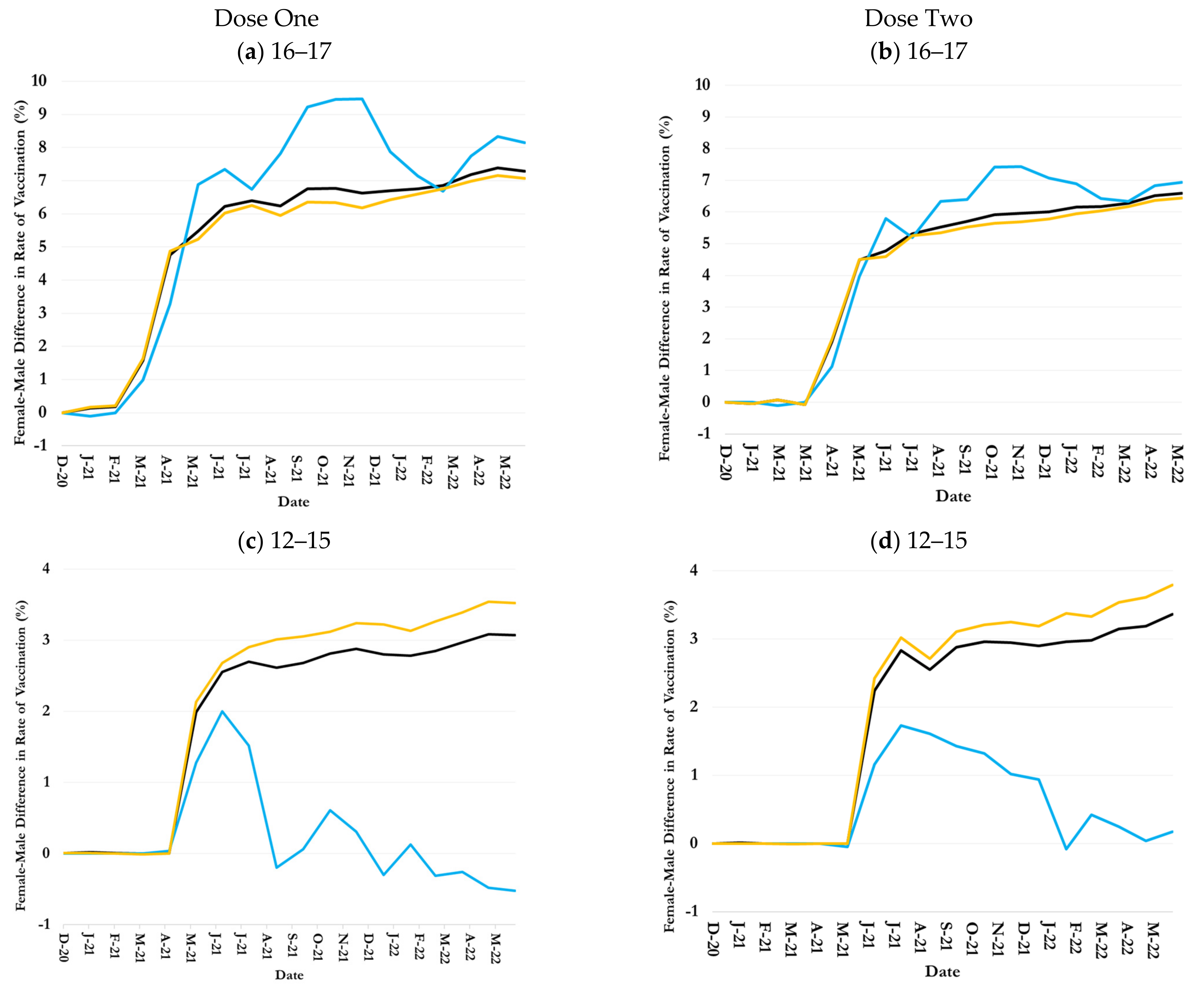
3.4. Inequalities in Uptake by Children Age-Ethnicity-Sex Groups
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mortaz, E.; Tabarsi, P.; Varahram, M.; Folkerts, G.; Adcock, I.M. The immune response and immunopathology of COVID-19. Front. Immunol. 2020, 11, 2037. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan, H.; Guo, W. Clinical characteristics of children with COVID-19: A meta-analysis. Front. Pediatr. 2020, 8, 431. [Google Scholar] [CrossRef] [PubMed]
- Kelvin, A.A.; Halperin, S. COVID-19 in children: The link in the transmission chain. Lancet Infect. Dis. 2020, 20, 633–634. [Google Scholar] [CrossRef]
- CDC. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Available online: https://archive.cdc.gov/#/details?url=https://emergency.cdc.gov/han/2020/han00432.asp (accessed on 5 March 2025).
- Feldstein, L.R.; Tenforde, M.W.; Friedman, K.G.; Newhams, M.; Rose, E.B.; Dapul, H.; Soma, V.L.; Maddux, A.B.; Mourani, P.M.; Bowens, C.; et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021, 325, 1074–1087. [Google Scholar] [CrossRef]
- Cull, B.; Harris, M.; Black, L.; Ray, G. Children and COVID-19: State Data Report. Available online: https://downloads.aap.org/AAP/PDF/AAP%20and%20CHA%20-%20Children%20and%20COVID-19%20State%20Data%20Report%204.29.21%20FINAL.pdf (accessed on 17 January 2025).
- McKune, S.L.; Acosta, D.; Diaz, N.; Brittain, K.; Beaulieu, D.J.; Maurelli, A.T.; Nelson, E.J. Psychosocial health of school-aged children during the initial COVID-19 safer-at-home school mandates in Florida: A cross-sectional study. BMC Public Health 2021, 21, 603. [Google Scholar] [CrossRef]
- Pandey, S.C.; Pande, V.; Sati, D.; Upreti, S.; Samant, M. Vaccination strategies to combat novel corona virus SARS-CoV-2. Life Sci. 2020, 256, 117956. [Google Scholar] [CrossRef]
- Hinton, D. Pfizer-BioNTech COVID-19 Vaccine EUA Letter of Authorization Reissued 05/10/2021. US Food & Drug Administration Website. 2021. Available online: https://www.fda.gov/media/144412/download (accessed on 17 January 2025).
- Food and Drug Administration. FDA Takes Additional Action in Fight Against COVID-19 By Issuing Emergency Use Authorization for Second COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid?utm_source=chatgpt.com (accessed on 17 January 2025).
- Food and Drug Administration. FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-issues-emergency-use-authorization-third-covid-19-vaccine?utm_source=chatgpt.com (accessed on 17 January 2025).
- American Journal of Managed Care. A Timeline of COVID-19 Vaccine Developments in 2021. Available online: https://www.ajmc.com/view/a-timeline-of-covid-19-vaccine-developments-in-2021 (accessed on 17 January 2025).
- Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Adolescents in Another Important Action in Fight Against Pandemic. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use (accessed on 17 January 2025).
- Food and Drug Administration. FDA Authorizes Pfizer-BioNTech COVID-19 Vaccine for Emergency Use in Children 5 Through 11 Years of Age. Available online: https://www.fda.gov/news-events/press-announcements/fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use-children-5-through-11-years-age (accessed on 17 January 2025).
- HHS. COVID-19 Vaccines. Available online: https://www.hhs.gov/coronavirus/covid-19-vaccines/index.html (accessed on 17 January 2025).
- Immunize.org. Vaccine History Timeline. Available online: https://www.immunize.org/vaccines/vaccine-timeline/ (accessed on 25 January 2025).
- Tai, D.B.G.; Shah, A.; Doubeni, C.A.; Sia, I.G.; Wieland, M.L. The disproportionate impact of COVID-19 on racial and ethnic minorities in the United States. Clin. Infect. Dis. 2021, 72, 703–706. [Google Scholar] [CrossRef]
- Ndugga, N.; Hill, L.; Artiga, S.; Haldar, S. Latest Data on COVID-19 Vaccinations by Race/Ethnicity. Kais Family Found. 2021. Available online: https://www.kff.org/coronavirus-covid-19/issue-brief/latest-data-on-covid-19-vaccinations-race-ethnicity/ (accessed on 25 January 2025).
- Valier, M.R. Racial and ethnic differences in COVID-19 vaccination coverage among children and adolescents aged 5–17 years and parental intent to vaccinate their children—National Immunization Survey–Child COVID Module, United States, December 2020–September 2022. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1–8. [Google Scholar] [CrossRef]
- Karimi, S.M.; Parh, Y.A.; Shakib, S.H.; Zarei, H.; Aranha, V.; Graham, A.; Allen, T.; Khan, S.M.; Moghadami, M.; Antimisiaris, D.; et al. COVID-19 vaccine uptake inequality among older adults: A multidimensional demographic analysis. Am. J. Infect. Control 2025, 53, 115–125. [Google Scholar] [CrossRef]
- Karimi, S.M.; Khan, S.M.; Moghadami, M.; Parh, Y.A.; Shakib, S.H.; Zarei, H.; Poursafargholi, S.; Little, B.B. Multidimensional Demographic Analyses of COVID-19 Vaccine Inequality in the United States: A Systematic Review. Healthcare 2025, 13, 139. [Google Scholar] [CrossRef]
- United States Census Bureau. Jefferson County, Kentucky. Available online: https://www.census.gov/quickfacts/fact/table/jeffersoncountykentucky/PST045224 (accessed on 10 April 2025).
- U.S. Food & Drug. FDA Approves First COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 17 January 2025).
- Murthy, N.C.; Zell, E.; Fast, H.E.; Murthy, B.P.; Meng, L.; Saelee, R.; Vogt, T.; Chatham-Stephens, K.; Ottis, C.; Shaw, L.; et al. Disparities in first dose COVID-19 vaccination coverage among children 5–11 years of age, United States. Emerg. Infect. Dis. 2022, 28, 986. [Google Scholar] [CrossRef] [PubMed]
- Taddio, A.; Ilersich, A.; McMurtry, C.M.; Bucci, L.M.; MacDonald, N.E. Managing pain and fear: Playing your CARDs to improve the vaccination experience. Can. Commun. Dis. Rep. 2021, 47, 87. [Google Scholar] [CrossRef] [PubMed]
- Rane, M.S.; Robertson, M.M.; Westmoreland, D.A.; Teasdale, C.A.; Grov, C.; Nash, D. Intention to vaccinate children against COVID-19 among vaccinated and unvaccinated US parents. JAMA Pediatr. 2022, 176, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Elbel, B.; Heng, L.; Konty, K.J.; Day, S.E.; Rothbart, M.W.; Abrams, C.; Lee, D.C.; Thorpe, L.E.; Schwartz, A.E. COVID-19 vaccines for children: Racial and ethnic disparities in New York City. Prev. Med. Rep. 2023, 35, 102357. [Google Scholar] [CrossRef]
- Brown, C.; Morlock, A.; Blakolmer, K.; Heidari, E.; Morlock, R. COVID-19 vaccination and race—A nationwide survey of vaccination status, intentions, and trust in the US general population. J. Manag. Care Spec. Pharm. 2022, 28, 1429–1438. [Google Scholar] [CrossRef]
- Siegel, M.; Critchfield-Jain, I.; Boykin, M.; Owens, A.; Muratore, R.; Nunn, T.; Oh, J. Racial/ethnic disparities in state-level COVID-19 vaccination rates and their association with structural racism. J. Racial Ethn. Health Disparities 2022, 9, 2361–2374. [Google Scholar] [CrossRef]
- Teasdale, C.A.; Ratzan, S.; Rauh, L.; Lathan, H.S.; Kimball, S.; El-Mohandes, A. COVID-19 vaccine coverage and hesitancy among New York City parents of children aged 5–11 years. Am. J. Public Health 2022, 112, 931–936. [Google Scholar] [CrossRef]
- Park, C.; Zabala, P.V.; Trisnadi, A.I. COVID-19 vaccine or booster hesitancy among children aged 6 month-5 years, 5–11 years, and 12–17 years in the United States: An analytic cross-sectional study. Prev. Med. Rep. 2023, 36, 102436. [Google Scholar] [CrossRef]
- U. C. Bureau. Week 44 Household Pulse Survey. Available online: https://www.census.gov/data/tables/2022/demo/hhp/hhp44.html (accessed on 8 February 2025).
- Opel, D.J.; Mangione-Smith, R.; Taylor, J.A.; Korfiatis, C.; Wiese, C.; Catz, S.; Martin, D.P. Development of a survey to identify vaccine-hesitant parents: The parent attitudes about childhood vaccines survey. Hum. Vaccines 2011, 7, 419–425. [Google Scholar] [CrossRef]
- Budiman, A.; Ruiz, N.G. Key Facts About Asian Americans, a Diverse and Growing Population. 2021. Available online: https://www.pewresearch.org/short-reads/2021/04/29/key-facts-about-asian-americans/ (accessed on 8 February 2025).
- Wang, Z.; Jamal, A.; Wang, R.; Dan, S.; Kappagoda, S.; Kim, G.; Palaniappan, L.; Long, J.; Singh, J.; Srinivasan, M. Disparities and trends in routine adult vaccination rates among disaggregated Asian American subgroups, National Health Interview Survey 2006–2018. AJPM Focus 2023, 2, 100044. [Google Scholar] [CrossRef]
- Catholic Charities Refugee Resettlement in Kentucky. Refugee Resettlement in Kentucky. Available online: https://www.kentuckyrefugees.org/refugees-in-kentucky/ (accessed on 10 April 2025).
- Singh, G.K.; Lee, H.; Azuine, R.E. Marked disparities in COVID-19 vaccination among US children and adolescents by racial/ethnic, socioeconomic, geographic, and health characteristics, United States, December 2021–April 2022. Int. J. Matern. Child Health AIDS 2022, 11, e598. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. COVID Data Tracker. Available online: https://covid.cdc.gov/covid-data-tracker (accessed on 27 January 2025).
- Schiff, J.; Schmidt, A.R.; Pham, P.K.; Pérez, J.B.; Pannaraj, P.S.; Chaudhari, P.P.; Liberman, D.B. Parental attitudes in the pediatric emergency department about the COVID-19 vaccine. Vaccine 2022, 40, 7328–7334. [Google Scholar] [CrossRef] [PubMed]
- Flores, G. Culture and the patient-physician relationship: Achieving cultural competency in health care. J. Pediatr. 2000, 136, 14–23. [Google Scholar] [CrossRef]
- Williams, J.T.; Rice, J.D.; Lou, Y.; Bayliss, E.A.; Federico, S.G.; Hambidge, S.J.; O’leary, S.T. Parental vaccine hesitancy and vaccination disparities in a safety-net system. Pediatrics 2021, 147, e2020010710. [Google Scholar] [CrossRef]
- Kaiser Family Foundation. Poverty Rate by Race/Ethnicity. Available online: https://www.kff.org/other/state-indicator/poverty-rate-by-raceethnicity/?currentTimeframe=1&selectedRows=%7B%22states%22:%7B%22kentucky%22:%7B%7D%7D%7D&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D (accessed on 27 January 2025).
- Hamel, L.; Lopes, L.; Sparks, G.; Kirzinger, A.; Kearney, A.; Stokes, M.; Brodie, M. KFF COVID-19 Vaccine Monitor: October 2021. Available online: https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-october-2021/ (accessed on 20 February 2025).
- Seixas, B.V.; Macinko, J. Unavailability of paid sick leave among parents is a barrier for children’s utilization of nonemergency health services: Evidence from the National Health Interview Survey. Int. J. Health Plan. Manag. 2020, 35, 1083–1097. [Google Scholar] [CrossRef]
- Alfieri, N.L.; Kusma, J.D.; Heard-Garris, N.; Davis, M.M.; Golbeck, E.; Barrera, L.; Macy, M.L. Parental COVID-19 vaccine hesitancy for children: Vulnerability in an urban hotspot. BMC Public Health 2021, 21, 1662. [Google Scholar] [CrossRef]
- Weller, B.E.; Faubert, S.J.; Ault, A.K. Youth access to medical homes and medical home components by race and ethnicity. Matern. Child Health J. 2020, 24, 241–249. [Google Scholar] [CrossRef]
- Lu, P.-J.; O’Halloran, A.; Bryan, L.; Kennedy, E.D.; Ding, H.; Graitcer, S.B.; Santibanez, T.A.; Meghani, A.; Singleton, J.A. Trends in racial/ethnic disparities in influenza vaccination coverage among adults during the 2007–08 through 2011–12 seasons. Am. J. Infect. Control 2014, 42, 763–769. [Google Scholar] [CrossRef]
- Barker, L.E.; Chu, S.Y.; Li, Q.; Shaw, K.M.; Santoli, J.M. Disparities between white and African-American children in immunization coverage. J. Natl. Med. Assoc. 2006, 98, 130. [Google Scholar]
- Nelson, A. Unequal treatment: Confronting racial and ethnic disparities in health care. J. Natl. Med. Assoc. 2002, 94, 666. [Google Scholar]
- King, W.D. Examining African Americans’ mistrust of the health care system: Expanding the research question. Commentary on “Race and trust in the health care system”. Public Health Rep. 2003, 118, 366. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Arbelaez, J.J.; Cooper, L.A. Patient–physician relationships and racial disparities in the quality of health care. Am. J. Public Health 2003, 93, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Berenson, A.B.; Rahman, M. Gender differences among low income women in their intent to vaccinate their sons and daughters against human papillomavirus infection. J. Pediatr. Adolesc. Gynecol. 2012, 25, 218–220. [Google Scholar] [CrossRef][Green Version]
- Cunningham-Erves, J.; Mayer, C.S.; Han, X.; Fike, L.; Yu, C.; Tousey, P.M.; Schlundt, D.G.; Gupta, D.K.; Mumma, M.T.; Walkley, D.; et al. Factors influencing intent to receive COVID-19 vaccination among Black and White adults in the southeastern United States, October–December 2020. Hum. Vaccines Immunother. 2021, 17, 4761–4798. [Google Scholar] [CrossRef]
- Lu, P.J.; O’Halloran, A.; Williams, W.W.; Lindley, M.C.; Farrall, S.; Bridges, C.B. Racial and ethnic disparities in vaccination coverage among adult populations in the US. Vaccine 2015, 33, D83–D91. [Google Scholar] [CrossRef]




| Dose Number | Race | Ethnicity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| White | Black | Asian | Some Other Races | Multi-Racial | Hispanic | Non- Hispanic | |||
| (a) 16–17 | |||||||||
| Vaccination Rate (%) | |||||||||
| 1 | 66.4 | 73.7 | 48.7 | 67.2 | 71.7 | 64.8 | 59.1 | 67.0 | |
| 2 | 58.0 | 64.8 | 41.2 | 58.6 | 60.0 | 58.8 | 48.0 | 58.9 | |
| Difference from the Overall Vaccination Rate (%) | |||||||||
| 1 | 0.0 | +7.3 | –17.7 | +0.8 | +5.3 | –1.6 | –7.3 | +0.6 | |
| 2 | 0.0 | +6.8 | –16.8 | +0.6 | +2.0 | +0.8 | –10.0 | +0.9 | |
| Female Vaccination Rate (%) | |||||||||
| 1 | 70.0 | 77.9 | 51.7 | 62.0 | 77.5 | 69.4 | 63.0 | 70.5 | |
| 2 | 61.3 | 68.5 | 43.8 | 54.5 | 66.3 | 62.8 | 51.4 | 62.2 | |
| Female Minus Male Difference in Vaccination Rate (%) | |||||||||
| 1 | +7.3 | +8.5 | +5.8 | –16.9 | +8.8 | +9.1 | +8.2 | +7.1 | |
| 2 | +6.6 | +7.4 | +5.1 | –13.8 | +10.4 | +8.0 | +6.9 | +6.4 | |
| (b) 12–15 | |||||||||
| Vaccination Rate (%) | |||||||||
| 1 | 63.9 | 70.5 | 45.9 | 60.1 | 71.5 | 70.1 | 55.8 | 64.7 | |
| 2 | 56.1 | 62.9 | 38.8 | 54.4 | 55.2 | 63.3 | 45.8 | 57.2 | |
| Difference from the Overall Vaccination Rate (%) | |||||||||
| 1 | 0.0 | +6.6 | –18.0 | –3.8 | +7.6 | +6.2 | –8.1 | +0.8 | |
| 2 | 0.0 | +6.8 | –17.3 | –1.7 | –0.9 | +7.2 | –10.3 | +1.1 | |
| Female Vaccination Rate (%) | |||||||||
| 1 | 65.5 | 73.1 | 47.2 | 59.9 | 69.7 | 69.7 | 55.4 | 66.5 | |
| 2 | 57.8 | 65.6 | 40.0 | 54.3 | 54.9 | 63.2 | 45.8 | 59.1 | |
| Female Minus Male Difference in Vaccination Rate (%) | |||||||||
| 1 | +3.1 | +5.0 | +2.6 | –0.1 | –3.7 | –0.8 | –0.6 | +3.5 | |
| 2 | +3.4 | +5.3 | +2.5 | –0.9 | –0.7 | –0.3 | +0.1 | +3.8 | |
| (c) 5–11 | |||||||||
| Vaccination Rate (%) | |||||||||
| 1 | 36.7 | 43.4 | 22.4 | 43.4 | 50.9 | 38.4 | 27.6 | 37.8 | |
| 2 | 30.6 | 35.5 | 17.2 | 36.7 | 37.0 | 32.9 | 19.1 | 32.0 | |
| Difference from the Overall Vaccination Rate (%) | |||||||||
| 1 | 0.0 | +6.7 | –14.3 | +6.7 | +14.2 | +1.7 | –9.1 | +1.2 | |
| 2 | 0.0 | +5.6 | –13.4 | +6.1 | +6.0 | +2.9 | –11.5 | +1.4 | |
| Female (%) | |||||||||
| 1 | 36.9 | 41.4 | 22.5 | 43.3 | 53.2 | 38.9 | 28.5 | 37.9 | |
| 2 | 30.8 | 35.6 | 17.4 | 35.8 | 39.4 | 33.5 | 19.6 | 32.1 | |
| Female Minus Male Difference in Vaccination Rate (%) | |||||||||
| 1 | +0.4 | +0.2 | +0.2 | –0.3 | +4.7 | +1.1 | +1.8 | +0.2 | |
| 2 | +0.3 | +0.0 | +0.5 | –2.8 | +4.9 | +1.1 | +0.8 | +0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi, S.M.; Moghadami, M.; Parh, M.Y.A.; Shakib, S.H.; Zarei, H.; Aranha, V.; Poursafargholi, S.; Allen, T.; Little, B.B.; Antimisiaris, D.; et al. COVID-19 Vaccine Uptake Inequality Among Children: A Multidimensional Demographic Analysis. Healthcare 2025, 13, 1019. https://doi.org/10.3390/healthcare13091019
Karimi SM, Moghadami M, Parh MYA, Shakib SH, Zarei H, Aranha V, Poursafargholi S, Allen T, Little BB, Antimisiaris D, et al. COVID-19 Vaccine Uptake Inequality Among Children: A Multidimensional Demographic Analysis. Healthcare. 2025; 13(9):1019. https://doi.org/10.3390/healthcare13091019
Chicago/Turabian StyleKarimi, Seyed M., Mana Moghadami, Md Yasin Ali Parh, Shaminul H. Shakib, Hamid Zarei, Venetia Aranha, Sepideh Poursafargholi, Trey Allen, Bert B. Little, Demetra Antimisiaris, and et al. 2025. "COVID-19 Vaccine Uptake Inequality Among Children: A Multidimensional Demographic Analysis" Healthcare 13, no. 9: 1019. https://doi.org/10.3390/healthcare13091019
APA StyleKarimi, S. M., Moghadami, M., Parh, M. Y. A., Shakib, S. H., Zarei, H., Aranha, V., Poursafargholi, S., Allen, T., Little, B. B., Antimisiaris, D., McKinney, W. P., Chen, Y.-T., Ingram, T., & Graham, A. (2025). COVID-19 Vaccine Uptake Inequality Among Children: A Multidimensional Demographic Analysis. Healthcare, 13(9), 1019. https://doi.org/10.3390/healthcare13091019







