The Effects of Motor Imagery on Trapeziometacarpal Osteoarthritis in Women During the Post-Surgical Immobilization Period: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Participants
2.1.2. Inclusion Criteria
2.1.3. Exclusion Criteria
2.2. Study Variables and Measurement Instruments
2.2.1. Sociodemographic Variables
2.2.2. Clinical Variables
2.3. Interventions
Assessment
- -
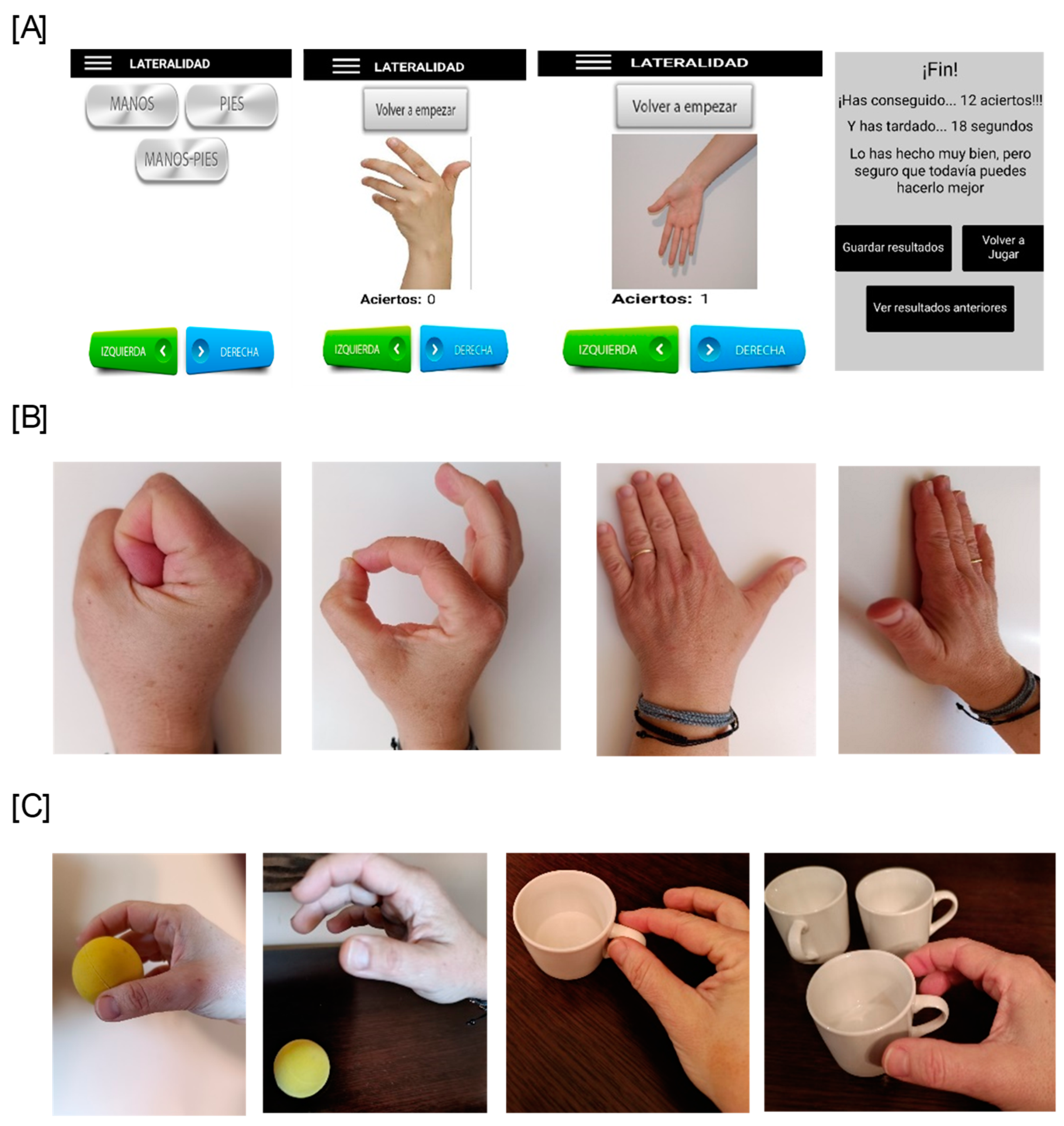
- During the 3 weeks of postoperative immobilization, laterality training was performed twice daily (morning and evening) to assess the patients’ left–right discrimination (implicit MI).
- -
- During the first two weeks of MI training, patients performed internal hand visualization, mentally repeating manual gestures (thumb abduction and extension, fist opening and closing, and finger opposition). These exercises were completed four times daily in 10-min training sessions with 4-h intervals (explicit MI). At the end of the second week, each participant was contacted by telephone to reinforce therapeutic adherence, to check that the participants performed the exercises, and to remind them of the exercises they were to add during the third week (explicit first-person motor imagery using two functional activities: picking up a tennis ball from a table and dropping it, and picking up a coffee cup and placing it on a shelf). During this phase, the total intervention time was increased to 15 min.
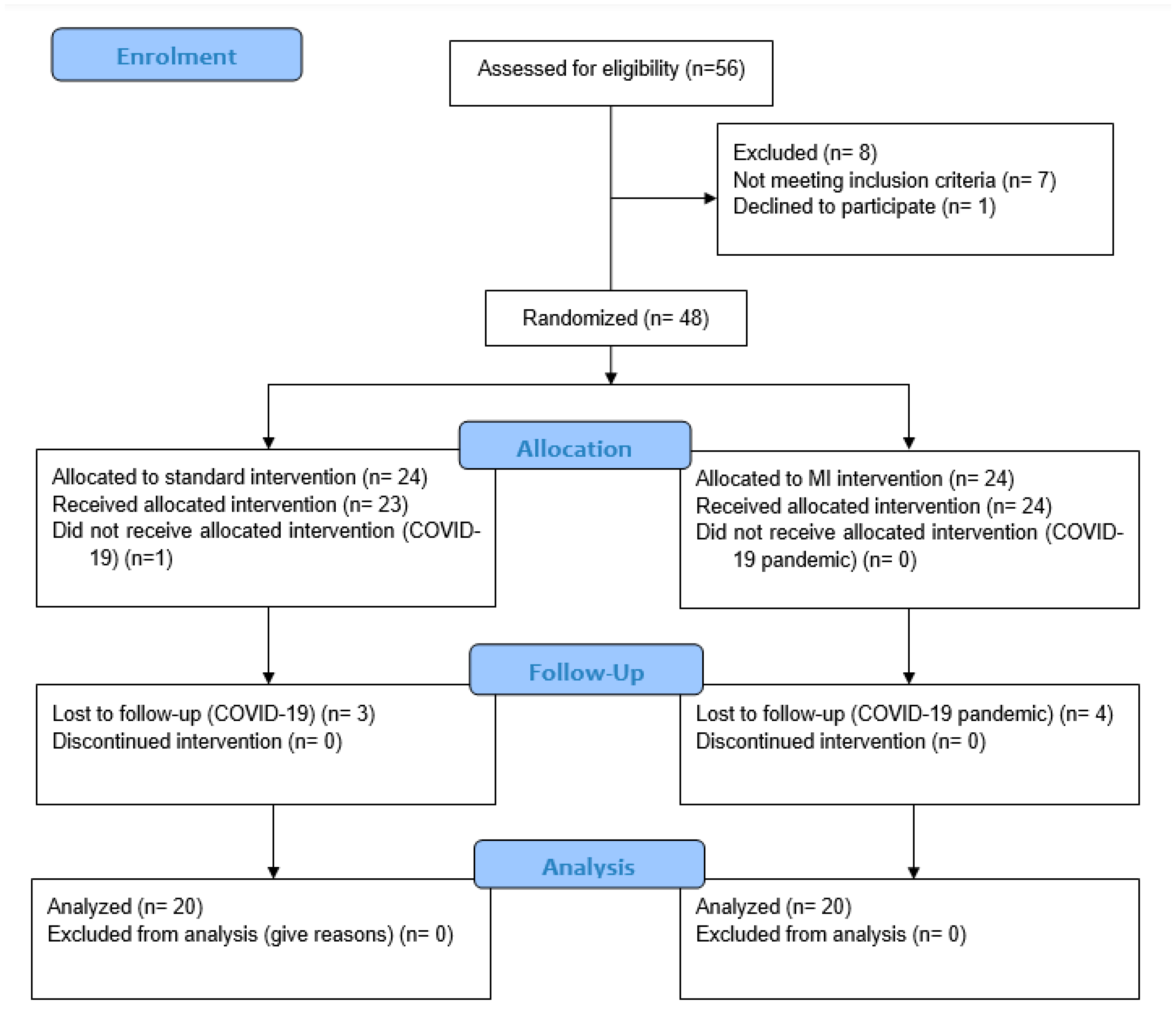
2.4. Sample Size
2.5. Randomization
2.6. Blinding
2.7. Statistical Analysis
2.8. Data Collection Methods
2.9. Ethical Considerations
2.10. Funding
3. Results
4. Discussion
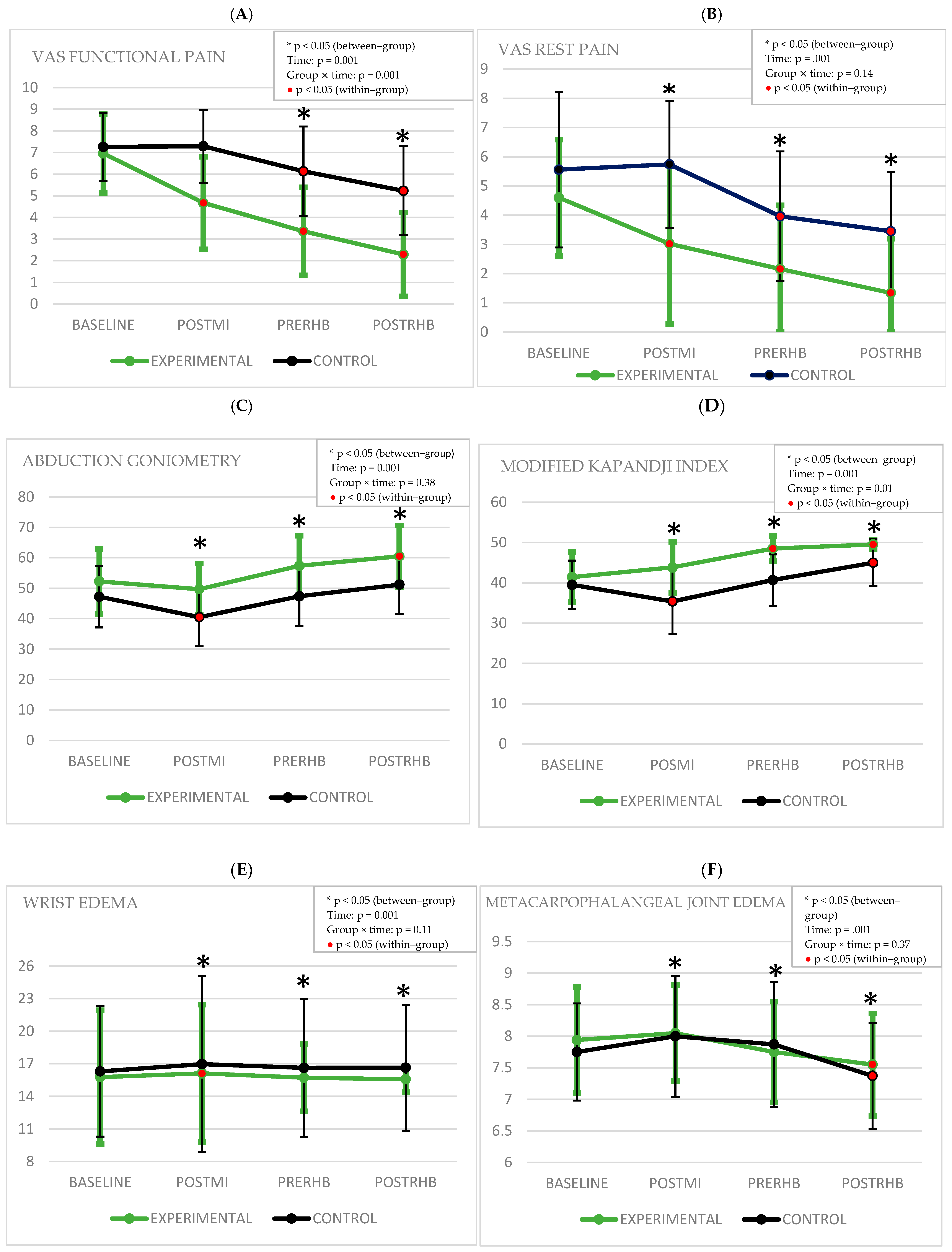
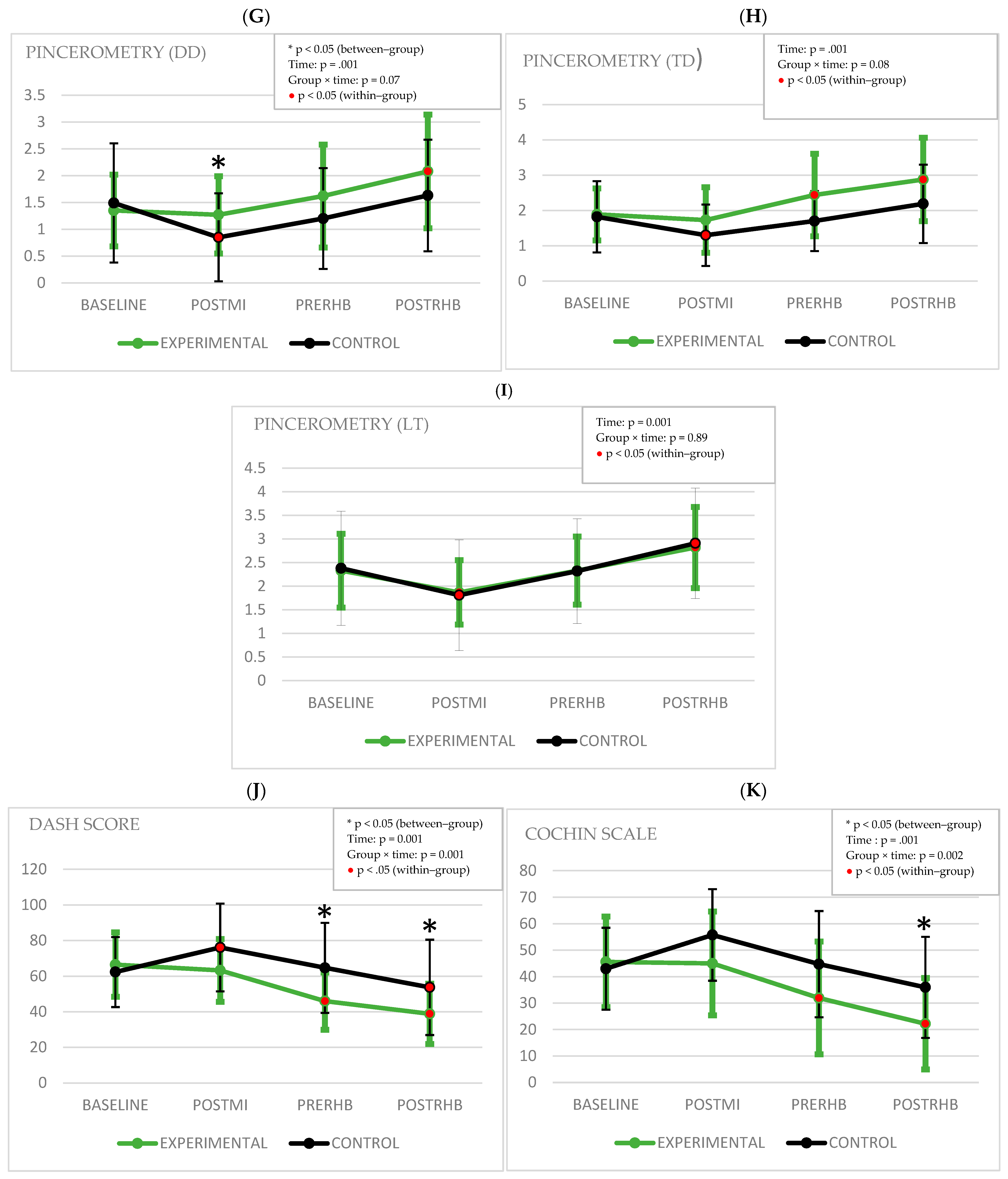
4.1. Pain
4.2. Range of Motion and Mobility
4.3. Edema
4.4. Strength
4.5. Quality of Life in Relation to Upper Limb Problems Dependent on Hand Function and Occupational Performance and Functionality
4.6. Minimum Clinically Important Difference
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ladd, A.L.; Weiss, A.P.C.; Crisco, J.J.; Hagert, E.; Wolf, J.M.; Glickel, S.Z.; Yao, J. The thumb carpometacarpal joint: Anatomy, hormones, and biomechanics. Instr. Course Lect. 2013, 62, 165–179. [Google Scholar] [PubMed]
- Wilkens, S.C.; Meghpara, M.M.; Ring, D.; Coert, J.H.; Jupiter, J.B.; Chen, N.C. Trapeziometacarpal Arthrosis. JBJS Rev. 2019, 7, E8. [Google Scholar] [CrossRef]
- Brandt, K.D.; Radin, E.L.; Dieppe, P.A.; Van de Putte, L. Yet more evidence that osteoarthritis is not a cartilage disease. Ann. Rheum. Dis. 2006, 65, 1261. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, L.; Nevitt, M.C.; Niu, J.; Goggins, J.P.; Aliabadi, P.; Yu, W.; Lui, L.Y.; Felson, D.T. Lower prevalence of hand osteoarthritis among Chinese subjects in Beijing compared with white subjects in the United States: The Beijing osteoarthritis study. Arthritis Rheum. 2003, 48, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.; Peat, G.; Nicholls, E.; van der Windt, D.; Myers, H.; Dziedzic, K. Subsets of symptomatic hand osteoarthritis in community-dwelling older adults in the United Kingdom: Prevalence, inter-relationships, risk factor profiles and clinical characteristics at baseline and 3-years. Osteoarthr. Cartil. 2013, 21, 1674. [Google Scholar] [CrossRef]
- Armstrong, A.L.; Hunter, J.B.; Davis, T.R.C. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J. Hand Surg. 1994, 19, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Sodha, S.; Ring, D.; Zurakowski, D.; Jupiter, J.B. Prevalence of osteoarthrosis of the trapeziometacarpal joint. J. Bone Jt. Surg. 2005, 87, 2614–2618. [Google Scholar] [CrossRef]
- Gandola, M.; Bruno, M.; Zapparoli, L.; Saetta, G.; Rolandi, E.; De Santis, A.; Banfi, G.; Zerbi, A.; Sansone, V.; Paulesu, E. Functional brain effects of hand disuse in patients with trapeziometacarpal joint osteoarthritis: Executed and imagined movements. Exp. Brain Res. 2017, 235, 3227–3241. [Google Scholar] [CrossRef]
- Flor, H.; Braun, C.; Elbert, T.; Birbaumer, N. Extensive Reorganization of Primary Somatosensory Cortex in Chronic Back Pain Patients. 1997. Available online: http://www.ub.uni-konstanz.de/kops/volltexte/2007/4138/ (accessed on 15 November 2023).
- Tsao, H.; Danneels, L.A.; Hodges, P.W. ISSLS prize winner: Smudging the motor brain in young adults with recurrent low back pain. Spine 2011, 36, 1721–1727. [Google Scholar] [CrossRef]
- Wolfe, T.; Chu, J.Y.; Woods, T.; Lubahn, J.D. A systematic review of postoperative hand therapy management of basal joint arthritis. Clin. Orthop. Relat. Res. 2014, 472, 1190–1197. [Google Scholar] [CrossRef]
- Stenhamre, H.; Nannmark, U.; Lindahl, A. Surgery for thumb (trapeziometacarpal joint) osteoarthritis (Review) summary of findings for the main comparison. Br. Med. Bull. 2009, 126, 79–84. [Google Scholar]
- Knightly, N.; Sullivan, P. Surgery for Trapeziometacarpal Joint Osteoarthritis: A Meta-Analysis on Efficacy and Safety. J. Hand Surg. 2021, 26, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Furlan, L.; Conforto, A.B.; Cohen, L.G.; Sterr, A. Upper Limb Immobilisation: A Neural Plasticity Model with Relevance to Poststroke Motor Rehabilitation. Neural Plast. 2016, 2016, 8176217. [Google Scholar] [CrossRef] [PubMed]
- Bassolino, M.; Campanella, M.; Bove, M.; Fadiga, L. Training the Motor Cortex by Observing the Actions of Others During Immobilization. Cereb. Cortex 2014, 24, 3268. [Google Scholar] [CrossRef]
- Debarnot, U.; Huber, C.; Guillot, A.; Schwartz, S. Sensorimotor representation and functional motor changes following short-term arm immobilization. Behav. Neurosci. 2018, 132, 595–603. [Google Scholar] [CrossRef]
- Burianova, H.; Sowman, P.F.; Marstaller, L.; Rich, A.N.; Williams, M.A.; Savage, G.; Al-Janabi, S.; de Lissa, P.; Johnson, B.W. Adaptive Motor Imagery: A Multimodal Study of Immobilization-Induced Brain Plasticity. Cereb. Cortex 2016, 26, 1072–1080. [Google Scholar] [CrossRef]
- Pelletier, R.; Higgins, J.; Bourbonnais, D. Addressing Neuroplastic Changes in Distributed Areas of the Nervous System Associated With Chronic Musculoskeletal Disorders. Phys. Ther. 2015, 95, 1582–1591. [Google Scholar] [CrossRef]
- Debarnot, U.; Di Rienzo, F.; Daligault, S.; Schwartz, S. Motor Imagery Training During Arm Immobilization Prevents Corticomotor Idling: An EEG Resting-State Analysis. Brain Topogr. 2020, 33, 327–335. [Google Scholar] [CrossRef]
- Newsom, J.; Knight, P.; Balnave, R. Use of Mental Imagery to Limit Strength Loss After Immobilization. Instrumentation 2003, 12, 249–259. [Google Scholar] [CrossRef]
- Avanzino, L.; Giannini, A.; Tacchino, A.; Pelosin, E.; Ruggeri, P.; Bove, M. Motor imagery influences the execution of repetitive finger opposition movements. Neurosci. Lett. 2009, 466, 11–15. [Google Scholar] [CrossRef]
- Gentili, R.; Han, C.E.; Schweighofer, N.; Papaxanthis, C. Motor learning without doing: Trial-by-trial improvement in motor performance during mental training. J. Neurophysiol. 2010, 104, 774–783. [Google Scholar] [CrossRef] [PubMed]
- Foerster, Á.; Rocha, S.; Wiesiolek, C.; Chagas, A.P.; Machado, G.; Silva, E.; Fregni, F.; Monte-Silva, K. Site-specific effects of mental practice combined with transcranial direct current stimulation on motor learning. Eur. J. Neurosci. 2013, 37, 786–794. [Google Scholar] [CrossRef]
- Feltz, D.L.; Landers, D.M. The Effects of Mental Practice on Motor Skill Learning and Performance: A Meta-analysis. J. Sport Psychol. 2016, 5, 25–57. [Google Scholar] [CrossRef]
- Moseley, G.L.; Flor, H. Targeting cortical representations in the treatment of chronic pain: A review. Neurorehabilit. Neural Repair 2012, 26, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Persichetti, A.S.; Avery, J.A.; Huber, L.; Merriam, E.P.; Martin, A. Layer-specific contributions to imagined and executed hand movements in human primary motor cortex. Curr. Biol. 2020, 30, 1721. [Google Scholar] [CrossRef]
- Rienzo FDi Debarnot, U.; Daligault, S.; Saruco, E.; Delpuech, C.; Doyon, J.; Collet, C.; Guillot, A. Online and Offline Performance Gains Following Motor Imagery Practice: A Comprehensive Review of Behavioral and Neuroimaging Studies. Front. Hum. Neurosci. 2016, 10, 315. [Google Scholar] [CrossRef]
- Guillot, A. Neurophysiological Foundations and Practical Applications of Motor Imagery. In The Cambridge Handbook of the Imagination; Cambridge University Press: Cambridge, UK, 2020; pp. 207–226. [Google Scholar]
- Hétu, S.; Grégoire, M.; Saimpont, A.; Coll, M.P.; Eugène, F.; Michon, P.E.; Jackson, P.L. The neural network of motor imagery: An ALE meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 930–949. [Google Scholar] [CrossRef]
- Ji, E.K.; Wang, H.H.; Jung, S.J.; Lee, K.B.; Kim, J.S.; Jo, L.; Hong, B.Y.; Lim, S.H. Graded motor imagery training as a home exercise program for upper limb motor function in patients with chronic stroke: A randomized controlled trial. Medicine 2021, 100, E24351. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, X.; Zhang, K.; Wang, X.; Sun, C.; Zhu, B.; Xu, Y.; Fan, M.; Tong, S.; Guo, X.; et al. Motor network reorganization after motor imagery training in stroke patients with moderate to severe upper limb impairment. CNS Neurosci. Ther. 2022, 29, 619–632. [Google Scholar] [CrossRef]
- Limakatso, K.; Madden, V.J.; Manie, S.; Parker, R. The effectiveness of graded motor imagery for reducing phantom limb pain in amputees: A randomised controlled trial. Physiotherapy 2020, 109, 65–74. [Google Scholar] [CrossRef]
- Birinci, T.; Kaya Mutlu, E.; Altun, S. The efficacy of graded motor imagery in post-traumatic stiffness of elbow: A randomized controlled trial. J. Shoulder Elb. Surg. 2022, 31, 2147–2156. [Google Scholar] [CrossRef]
- Prado Robles, E.; Delgado Gil, J.A. Efectos de la imaginería motora aplicada durante el periodo de inmovilización o posquirúrgico en miembro superior: Una revisión sistemática. Fisioterapia 2019, 41, 219–226. [Google Scholar] [CrossRef]
- Gandola, M.; Zapparoli, L.; Saetta, G.; De Santis, A.; Zerbi, A.; Banfi, G.; Sansone, V.; Bruno, M.; Paulesu, E. Thumbs up: Imagined hand movements counteract the adverse effects of post-surgical hand immobilization. Clinical, behavioral, and fMRI longitudinal observations. Neuroimage Clin. 2019, 23, 101838. [Google Scholar] [CrossRef]
- Prado-Robles, E.; Delgado-Gil, J.Á.; Navarro-Prada, S.R.; Rodríguez-Martín, B.; Gómez-Martínez, M.; Seco-Calvo, J. The effects of motor imagery on trapeziometacarpal osteoarthritis in women during the post-surgical immobilisation period: A protocol for a randomised clinical trial. Br. J. Occup. Ther. 2022, 86, 531–539. [Google Scholar] [CrossRef]
- Díez Burón, F.; Marcos Vidal, J.M.; Baticón Escudero, P.M.; Montes Armenteros, A.; Bermejo López, J.C.; Merino Garcia, M. Concordancia entre la escala verbal numérica y la escala visual analógica en el seguimiento del dolor agudo postoperatorio. Rev. Esp. Anestesiol. Reanim. 2011, 58, 279–282. [Google Scholar] [CrossRef]
- Nikolic, Z.; Boland, P.J.; Athanasian, E.A. The Critical Difference in the DASH Outcome Measure After Essential Upper Extremity Tumor Surgery. J. Shoulder Elb. Surg. 2022, 30, e602–e609. [Google Scholar]
- Hervás, M.T.; Navarro Collado, M.J.; Peiró, S.; Rodrigo Pérez, J.L.; López Matéu, P.; Martínez Tello, I. Versión Española del cuestionario DASH. Adaptación transcultural, fiabilidad, validez y sensibilidad a los cambios. Med. Clin. 2006, 127, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Arreguín Reyes, R.; López López, C.O.; Álvarez Hernández, E.; Medrano Ramírez, G.; Montes Castillo, M.L.; Vázquez-Mellado, J. Evaluación de la función de la mano en las enfermedades reumáticas. Validación y utilidad de los cuestionarios AUSCAN, m-SACRAH, DASH y Cochin en Español. Reumatol. Clin. 2012, 8, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Lefevre-Colau, M.M.; Poiraudeau, S.; Oberlin, C.; Demaille, S.; Fermanian, J.; Rannou, F.; Revel, M. Reliability, validity, and responsiveness of the modified kapandji index for assessment of functional mobility of the rheumatoid hand1. Arch. Phys. Med. Rehabil. 2003, 84, 1032–1038. [Google Scholar] [CrossRef]
- Delprat, J.; Ehrler, S.; Meyer, J.-C. Muñeca y mano: Examen articular. EMC-Kinesiterapia-Med. Física 2005, 26, 1–19. [Google Scholar] [CrossRef]
- Holzbauer, M.; Hopfner, M.; Haslhofer, D.; Kwasny, O.; Duscher, D.; Froschauer, S.M. Radial and palmar active range of motion measurement: Reliability of six methods in healthy adults. J. Plast. Surg. Hand Surg. 2021, 55, 41–47. [Google Scholar] [CrossRef]
- Mathiowetz, V.; Weber, K.; Volland, G.; Kashman, N. Reliability and validity of grip and pinch strength evaluations. J. Hand Surg. 1984, 9, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Dilek, B.; Ayhan, C.; Yagci, G.; Yakut, Y. Effectiveness of the graded motor imagery to improve hand function in patients with distal radius fracture: A randomized controlled trial. J. Hand Ther. 2018, 31, 2–9.e1. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 2013. [Google Scholar]
- Apaza, J.A.S.; Franco, J.V.A.; Meza, N.; Madrid, E.; Loézar, C.; Garegnani, L. Minimal clinically important difference: The basics. Medwave 2021, 21, e8149. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal clinically important difference of the disabilities of the arm, shoulder and hand outcome measure (DASH) and its shortened version (quickDASH). J. Orthop. Sports Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef]
- Daste, C.; Abdoul, H.; Foissac, F.; Lefèvre-Colau, M.M.; Poiraudeau, S.; Rannou, F.; Nguyen, C. Patient acceptable symptom state for patient-reported outcomes in people with non-specific chronic low back pain. Ann. Phys. Rehabil. Med. 2022, 65, 101451. [Google Scholar] [CrossRef]
- Dahaghin, S.; Bierma-Zeinstra, S.M.A.; Ginai, A.Z.; Pols, H.A.; Hazes, J.M.; Koes, B.W. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study). Ann. Rheum. Dis. 2005, 64, 682–687. [Google Scholar] [CrossRef]
- Haara, M.M.; Arokoski, J.P.A.; Kröger, H.; Kärkkäinen, A.; Manninen, P.; Knekt, P.; Impivaara, O.; Heliövaara, M. Association of radiological hand osteoarthritis with bone mineral mass: A population study. Rheumatology 2005, 44, 1549–1554. [Google Scholar] [CrossRef]
- Bijsterbosch, J.; Visser, W.; Kroon, H.M.; Stamm, T.; Meulenbelt, I.; Huizinga, T.W.; Kloppenburg, M. Thumb base involvement in symptomatic hand osteoarthritis is associated with more pain and functional disability. Ann. Rheum. Dis. 2010, 69, 585–587. [Google Scholar] [CrossRef]
- Gehrmann, S.V.; Tang, J.; Li, Z.M.; Goitz, R.J.; Windolf, J.; Kaufmann, R.A. Motion deficit of the thumb in CMC joint arthritis. J. Hand Surg. Am. 2010, 35, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Kjeken, I.; Dagfinrud, H.; Slatkowsky-Christensen, B.; Mowinckel, P.; Uhlig, T.; Kvien, T.K.; Finset, A. Activity limitations and participation restrictions in women with hand osteoarthritis: Patients’ descriptions and associations between dimensions of functioning. Ann. Rheum. Dis. 2005, 64, 1633. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.R.; Lin, C.-W.C.; Smeets, R.J.E.M.; Taylor, D.; Law, R.; Lorimer Moseley, G. Spatially defined disruption of motor imagery performance in people with osteoarthritis. Rheumatology 2012, 51, 1455–1464. [Google Scholar] [CrossRef]
- Zapparoli, L.; Seghezzi, S.; Sacheli, L.M.; Verga, C.; Banfi, G.; Paulesu, E. Eyes wide shut: How visual cues affect brain patterns of simulated gait. Hum. Brain Mapp. 2020, 41, 4248. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rosa, J.J.; Natali, F.; Tettamanti, A.; Cursi, M.; Velikova, S.; Comi, G.; Gatti, R.; Leocani, L. Action observation and motor imagery in performance of complex movements: Evidence from EEG and kinematics analysis. Behav. Brain Res. 2015, 281, 290–300. [Google Scholar] [CrossRef]
- Limakatso, K.; Corten, L.; Parker, R. The effects of graded motor imagery and its components on phantom limb pain and disability in upper and lower limb amputees: A systematic review protocol. Syst. Rev. 2016, 5, 145. [Google Scholar] [CrossRef]
- Stenekes, M.W.; Geertzen, J.H.; Nicolai, J.-P.A.; De Jong, B.M.; Mulder, T. Effects of motor imagery on hand function during immobilization after flexor tendon repair. Arch. Phys. Med. Rehabil. 2009, 90, 553–559. [Google Scholar] [CrossRef]
- Guillot, A.; Lebon, F.; Vernay, M.; Girbon, J.P.; Doyon, J.; Collet, C. Effect of motor imagery in the rehabilitation of burn patients. J. Burn Care Res. 2009, 30, 686–693. [Google Scholar] [CrossRef]
- Frenkel, M.O.; Herzig, D.S.; Gebhard, F.; Mayer, J.; Becker, C.; Einsiedel, T. Mental practice maintains range of motion despite forearm immobilization: A pilot study in healthy persons. J. Rehabil. Med. 2014, 46, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.; Pongrac, J.; Buckholz, E. The measurement of imagery ability. Hum. Mov. Sci. 1985, 4, 107–118. [Google Scholar] [CrossRef]
- Atienza, F.; Balaguer, I.; Garcia-Merita, M.L. Factor analysis and reliability of the Movement Imagery Questionnaire. Percept. Mot. Skills 1994, 78, 1323–1328. [Google Scholar] [CrossRef]
- Breivik, H.; Collett, B.; Ventafridda, V.; Cohen, R.; Gallacher, D. Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. Eur. J. Pain 2006, 10, 287. [Google Scholar] [CrossRef] [PubMed]
- Robles-Molina, M.J.; López-Caba, F.; Gómez-Sánchez, R.C.; Cárdenas-Grande, E.; Pajares-López, M.; Hernández-Cortés, P. Trapeziectomy with ligament reconstruction and tendon interposition versus a trapeziometacarpal prosthesis for the treatment of thumb basal joint osteoarthritis. Orthopedics 2017, 40, e681–e686. [Google Scholar] [CrossRef] [PubMed]
- Cebrian-Gomez, R.; Lizaur-Utrilla, A.; Sebastia-Forcada, E.; Lopez-Prats, F.A. Outcomes of cementless joint prosthesis versus tendon interposition for trapeziometacarpal osteoarthritis: A prospective study. J. Hand Surg. (Eur. Vol.) 2018, 44, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Weibull, A.; Flondell, M.; Rosén, B.; Björkman, A. Cerebral and clinical effects of short-term hand immobilisation. Eur. J. Neurosci. 2011, 33, 699–704. [Google Scholar] [CrossRef]
- Moseley, G.L. Is successful rehabilitation of complex regional pain syndrome due to sustained attention to the affected limb? A randomised clinical trial. Pain 2005, 114, 54–61. [Google Scholar] [CrossRef]
- de Souza, N.S.; Martins, A.C.G.; Bastos VHdo, V.; Orsini, M.; Leite, M.A.; Teixeira, S.; Velasques, B.; Ribeiro, P.; Bittencourt, J.; Matta, A.P.; et al. Motor Imagery and Its Effect on Complex Regional Pain Syndrome: An Integrative Review. Neurol. Int. 2015, 7, 58–61. [Google Scholar] [CrossRef]
- Moukarzel, M.; Di Rienzo, F.; Lahoud, J.-C.; Hoyek, F.; Collet, C.; Guillot, A.; Hoyek, N. The therapeutic role of motor imagery during the acute phase after total knee arthroplasty: A pilot study. Disabil. Rehabil. 2017, 41, 926–933. [Google Scholar] [CrossRef]
- Zapparoli, L.; Sacheli, L.M.; Seghezzi, S.; Preti, M.; Stucovitz, E.; Negrini, F.; Pelosi, C.; Ursino, N.; Banfi, G.; Paulesu, E. Motor imagery training speeds up gait recovery and decreases the risk of falls in patients submitted to total knee arthroplasty. Sci. Rep. 2020, 10, 8917. [Google Scholar] [CrossRef]
- Moseley, G.L. Graded motor imagery is effective for long-standing complex regional pain syndrome: A randomised controlled trial. Pain 2004, 108, 192–198. [Google Scholar] [CrossRef]
- Hoyek, N.; Di Rienzo, F.; Collet, C.; Hoyek, F.; Guillot, A. The therapeutic role of motor imagery on the functional rehabilitation of a stage II shoulder impingement syndrome. Disabil. Rehabil. 2014, 36, 1113–1119. [Google Scholar] [CrossRef]
- Yap BWDa Lim, E.C.W. The Effects of Motor Imagery on Pain and Range of Motion in Musculoskeletal Disorders. Clin. J. Pain 2019, 35, 87–99. [Google Scholar]
- Galonski, T.; Mansfield, C.; Moeller, J.; Miller, R.; Rethman, K.; Briggs, M.S. Does graded motor imagery benefit individuals with knee pain: A systematic review and meta-analysis. J. Bodyw. Mov. Ther. 2023, 35, 130–139. [Google Scholar] [CrossRef]
- Limakatso, K.; Cashin, A.G.; Williams, S.; Devonshire, J.; Parker, R.; McAuley, J.H. The Efficacy of Graded Motor Imagery and Its Components on Phantom Limb Pain and Disability: A Systematic Review and Meta-Analysis. Can. J. Pain 2023, 7, 2188899. [Google Scholar] [CrossRef]
- Ríos-León, M.; Cuñado-González, Á.; Domínguez-Fernández, S.; Martín-Casas, P. Effectiveness of motor imagery in complex regional pain syndrome: A systematic review with meta-analysis. Pain Pract. 2024, 24, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Zalucki, N.; Birklein, F.; Marinus, J.; van Hilten, J.J.; Luomajoki, H. Thinking about movement hurts: The effect of motor imagery on pain and swelling in people with chronic arm pain. Arthritis Rheum. 2008, 59, 623–631. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Linton, S.J. Fear-avoidance and its consequences in chronic musculoskeletal pain: A state of the art. Pain 2000, 85, 317–332. [Google Scholar] [CrossRef] [PubMed]
- Roberson, L.; Giurintano, D.J. Objective Measures of Joint Stiffness. J. Hand Ther. 1995, 8, 163–166. [Google Scholar] [CrossRef]
- DeCicco, P.V.; Fisher, M.M. The effects of proprioceptive neuromuscular facilitation stretching on shoulder range of motion in overhand athletes. J. Sports Med. Phys. Fit. 2005, 45, 183–187. [Google Scholar]
- Williamson, E.M.; Marshall, P.H. Motor Control of the Knee as a Function of Age and Range of Motion. Exp. Aging Res. 2009, 35, 457–468. [Google Scholar] [CrossRef]
- Skirven, T.M.; Osterman, A.L. Rehabilitation of the Hand and Upper Extremity—NLM Catalog—NCBI; Elsevier Mosby: Philadelphia, PA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/nlmcatalog?term=101775113%5BNLM%20Unique%20ID%5D (accessed on 13 August 2024).
- Kanthack, T.F.D.; Guillot, A.; Papaxanthis, C.; Guizard, T.; Collet, C.; Di Rienzo, F. Neurophysiological insights on flexibility improvements through motor imagery. Behav. Brain Res. 2017, 331, 159–168. [Google Scholar] [CrossRef]
- Liu, X.J.; Ge, S.; Cordova, A.; Yaghi, Z.; Jiang, B.Y.; Yue, G.H.; Yao, W.X. Elderly may benefit more from motor imagery training in gaining muscle strength than young adults: A systematic review and meta-analysis. Front. Psychol. 2022, 13, 1052826. [Google Scholar] [CrossRef] [PubMed]
- Paravlic, A.H.; Slimani, M.; Tod, D.; Marusic, U.; Milanovic, Z.; Pisot, R. Effects and Dose–Response Relationships of Motor Imagery Practice on Strength Development in Healthy Adult Populations: A Systematic Review and Meta-analysis. Sports Med. 2018, 48, 1165–1187. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Mahato, N.K.; Nakazawa, M.; Law, T.D.; Thomas, J.S. The power of the mind: The cortex as a critical determinant of muscle strength/weakness. J. Neurophysiol. 2014, 112, 3219–3226. [Google Scholar] [CrossRef] [PubMed]
- Ruffino, C.; Papaxanthis, C.; Lebon, F. Neural plasticity during motor learning with motor imagery practice: Review and perspectives. Neuroscience 2017, 341, 61–78. [Google Scholar] [CrossRef]
- Jiang, C.; Ranganathan, V.K.; Zhang, J.; Siemionow, V.; Yue, G.H. Motor effort training with low exercise intensity improves muscle strength and descending command in aging. Medicine 2016, 95, e3291. [Google Scholar] [CrossRef]
- Yue, G.; Cole, K.J. Strength increases from the motor program: Comparison of training with maximal voluntary and imagined muscle contractions. J. Neurophysiol. 1992, 67, 1114–1123. [Google Scholar] [CrossRef]
- Park, J.H. Influence of mental practice on upper limb muscle activity and activities of daily living in chronic stroke patients. J. Phys. Ther. Sci. 2016, 28, 1061. [Google Scholar] [CrossRef]
- Méndez-Rebolledo, G.; Gatica-Rojas, V.; Torres-Cueco, R.; Albornoz-Verdugo, M.; Guzmán-Muñoz, E. Update on the effects of graded motor imagery and mirror therapy on complex regional pain syndrome type 1: A systematic review. J. Back. Musculoskelet. Rehabil. 2017, 30, 441–449. [Google Scholar] [CrossRef]
- Shauver, M.J.; Chang, K.W.C.; Chung, K.C. Contribution of functional parameters to patient-rated outcomes after surgical treatment of distal radius fractures. J. Hand Surg. Am. 2014, 39, 436–442. [Google Scholar] [CrossRef]
- Cella, D.; Riley, W.; Stone, A.; Reeve, B.; Yount, S.; Amtmann, D.; Bode, R.; Buysse, D.; Choi, S.; Cook, K.; et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010, 63, 1179–1194. [Google Scholar] [CrossRef]
| Variables | EG | CG | X2/t |
|---|---|---|---|
| Age (Mean ± SD) | 62.65 (6.01) | 61.80 (5.52) | 0.64 |
| Employment Situation (A/R) | 10/10 | 10/10 | 1.00 |
| Surgery (AR/TP) | 5/15 | 6/14 | 0.72 |
| Dominant hand (R/L) | 19 /1 | 18 /2 | 0.54 |
| Operated hand (R/L) | 10 /10 | 14 /6 | 0.33 |
| Variable | Difference Base−postMI (95% CI) | Difference Base−preRHB (95% CI) | Difference Base−postRHB (95% CI) | Time F (p) | Time × Group | ||
|---|---|---|---|---|---|---|---|
| F (p) | η2p | ||||||
| VAS FP | EG | 2.28 * (1.34\3.22) | 3.59 * (2.6\4.58) | 4.67 * (3.47\5.68) | 44.07 (0.001) | 7.97 (0.001) | 0.17 |
| CG | −0.03 (−0.97\0.91) | 1.13 * (0.14\2.12) | 2.03 * (0.83\3.23) | ||||
| VAS RP | EG | 1.58 * (0.25\2.92) | 2.44 * (1.15\3.73) | 3.26 * (0.79\2.56) | 21.52 (0.001) | 1.94 (0.14) | 0.04 |
| CG | −0.18 (−1.51\1.15) | 1.60 * (0.31\2.88) | 2.10 * (0.90\3.31) | ||||
| GONIOMETRY | EG | 2.55 (−2.56\7.66) | −5.1 (−10.63\0.43) | −8.25 * (−14.1\−2.42) | 15.7 (0.001) | 0.92 (0.38) | 0.25 |
| CG | 6.7 * (1.58\11.81) | −0.15 (−5.68\5.38) | −4 (−9.82\1.82) | ||||
| KAPANDJI | EG | −2.4 (−6.27\1.47) | −7.05 * (−10.2\−3.88) | −8.1 * (−10.66\−5.33) | 28.24 (0.001) | 5 (0.01) | 0.11 |
| CG | 4.1 * 80.22\7.97) | −1.2 (−4.36\1.96) | −5.5 * (−8.06\−2.93) | ||||
| EDEMA (WRIST) | EG | −0.34 * (−6.55\−0.35) | 0.05 (−0.31\0.41) | 0.2 (−0.2\0.6) | 9.06 (0.001) | 2.26 (0.11) | 0.08 |
| CG | −0.67 * (−0.98\−0.36) | −0.32 (−0.68\0.35) | −0.34 (−0.74\0.06) | ||||
| EDEMA (MCF) | EG | −0.1 (−0.4\0.18) | 0.19 (−0.14\0.52) | 0.39 * (0.1\0.68) | 10.87 (0.001) | 1.03 (0.37) | 0.02 |
| CG | −0.25 (−0.54\0.04) | −0.12 (−0.460.21) | 0.38 * (0.08\0.67) | ||||
| PINCEROMETRY (DD) | EG | 0.11 (−0.26\0.49) | −0.27 (−0.7\0.16) | −0.73 * (−1.18\−0.28) | 15.4 (0.001) | 2.8 (0.07) | 0.06 |
| CG | 0.63 * (0.26\1.01) | 0.29 (−0.14\0.72) | −0.13 (−0.58\0.31) | ||||
| PINCEROMETRY (TD) | EG | 0.16 (−0.24\0.57) | −0.54 * (−1.01\−0.07) | −0.98 * (−1.48\−0.48) | 18.76 (0.001) | 2.44 (0.08) | 0.06 |
| CG | 0.52 * (0.11\0.92) | 0.12 (−0.34\0.59 | −0.36 (−0.86\0.13) | ||||
| PINCEROMETRY (LT) | EG | 0.46 * (0.04\0.88) | 0.002 (−0.35\0.47) | −0.48 * (−0.93\−0.03) | 21.48 (0.001) | 0.128 (0.89) | 0.003 |
| CG | 0.57 * (0.15\0.99) | 0.006 −0.35\0.47) | −0.52 * (−0.97\−0.07) | ||||
| DASH | EG | 3.26 (−4.28\10.81) | 20.52 * (12.42\28.61) | 27.66 * (19.42\35.9) | 36.32 (0.001) | 8.74 (0.001) | 0.18 |
| CG | −13.82 * (−21.37\−6.26) | −2.33 (−10.43\5.75) | 8.61 * (0.36\16.85) | ||||
| COCHIN | EG | 0.6 (−5.45\6.65) | 13.65 * (6.56\20.73) | 23.4 * (15.41\31.39) | 35.74 (0.001) | 6.31 (0.002) | 0.14 |
| CG | −12.8 * (−18.85\−6.75) | −1.75 −8.83\5.32) | 7.02 (−0.97\15.01) | ||||
| Variable | GROUP (Mean ± SD) | p Value | Effect Size | |
|---|---|---|---|---|
| EG | CG | |||
| VASFP | ||||
| Baseline | 6.96 (1.83) | 7.26 (1.56) | 0.41 | |
| PostMI | 4.67 (2.14) | 7.29 (1.68) | 0.001 | 1.36 |
| PreRHB | 3.36 (2.03) | 6.13 (2.07) | 0.0001 | 1.34 |
| PostRHB | 2.29 (1.94) | 5.23 (2.06) | 0.001 | 1.46 |
| VASRP | ||||
| Baseline | 4.60 (1.99) | 5.56 (2.66) | 0.2 | |
| PostMI | 3.02 (2.74) | 5.74 (2.18) | 0.002 | 1.01 |
| PreRHB | 2.16 (2.18) | 3.96 (2.22) | 0.013 | 0.81 |
| PostRHB | 1.34 (1.86) | 3.45 (2.03) | 0.002 | 0.92 |
| GONIOMETRY | ||||
| Baseline | 52.25 (10.69) | 47.20 (10.05) | 0.132 | |
| PostMI | 49.70 (8.47) | 40.50 (9.58) | 0.003 | 1.01 |
| PreRHB | 57.35 (9.89) | 47.35 (9.70) | 0.003 | 1.02 |
| PostRHB | 60.50 (10.11) | 51.20 (9.60) | 0.005 | 0.23 |
| KAPANDJI | ||||
| Baseline | 41.45 (6.17) | 39.5 (6.02) | 0.31 | |
| PostMI | 43.85 (6.34) | 35.4 (8.11) | 0.001 | 1.16 |
| PreRHB | 48.5 (3.10) | 40.7 (6.39) | 0.001 | 1.55 |
| PostRHB | 49.55 (1.19) | 45 (5.81) | 0.001 | 1.18 |
| EDEMA (WRIST) | ||||
| Baseline | 15.77 (6.17) | 16.3 (6.02) | 0.17 | |
| PostMI | 16.12 (6.34) | 16.97 (8.11) | 0.04 | 0.88 |
| PreRHB | 15.72 (3.10) | 16.62 (6.39) | 0.001 | 0.92 |
| PostRHB | 15.57 (1.19) | 16.64 (5.81) | 0.001 | 0.98 |
| EDEMA (MCP) | ||||
| Baseline | 7.94 (0.85) | 7.75 (0.77) | 0.45 | |
| PostMI | 8.05 (0.76) | 8 (0.96) | 0.08 | 0.06 |
| PreRHB | 7.75 (0.80) | 7.87 (0.99) | 0.67 | 0.13 |
| PostRHB | 7.55 (0.81) | 7.37 (0.84) | 0.49 | 0.21 |
| PINCEROMETRY (DD) | ||||
| Baseline | 1.35 (0.67) | 1.49 (1.11) | 0.82 | |
| PostMI | 1.27 (0.72) | 0.85 (0.82) | 0.030 | 0.48 |
| PreRHB | 1.62 (0.96) | 1.20 (0.94) | 0.11 | 0.44 |
| PostRHB | 2.08 (1.06) | 1.63 (1.04) | 0.18 | 0.43 |
| PINCEROMETRY (TD) | ||||
| Baseline | 1.89 (0.74) | 1.82 (1.01) | 0.81 | |
| PostMI | 1.73 (0.93) | 1.30 (0.87) | 0.14 | 0.47 |
| PreRHB | 2.44 (1.17) | 1.70 (0.85) | 0.08 | 0.71 |
| PostRHB | 2.88 (1.18) | 2.19 (1.11) | 0.06 | 0.60 |
| PINCEROMETRY (LT) | ||||
| Baseline | 2.33 (0.78) | 2.38 (1.21) | 0.87 | |
| PostMI | 1.87 (0.68) | 1.81 (1.17) | 0.85 | 0.06 |
| PreRHB | 2.33 (0.72) | 2.32 (1.11) | 0.98 | 0.01 |
| PostRHB | 2.82 (0.86) | 2.91 (1.17) | 0.78 | 0.08 |
| DASH | ||||
| Baseline | 66.53 (18.12) | 62.37 (19.69) | 0.57 | |
| PostMI | 63.26 (17.61) | 76.19 (24.66) | 0.06 | 0.60 |
| PreRHB | 46.01 (16.11) | 64.71 (25.30) | 0.008 | 0.88 |
| PostRHB | 38.87 (16.91) | 53.76 (26.81) | 0.04 | 0.66 |
| COCHIN | ||||
| Baseline | 45.60 (17.11) | 43.00 (15.49) | 0.61 | |
| PostMI | 45.00 (19.61) | 55.80 (17.32) | 0.07 | 0.58 |
| PreRHB | 31.95 (21.31) | 44.75 (20.09) | 0.58 | 0.61 |
| PostRHB | 22.20 (17.27) | 35.98 (19.09) | 0.02 | 0.75 |
| Variable | Exceed MCID Baseline−v | Exceed MCID Baseline−postRHB | |||||
|---|---|---|---|---|---|---|---|
| YES | NO | OR (95% CI) | YES | NO | OR (95% CI) | ||
| VAS FP | EG | 13 (65%) | 7 (35%) | 16.7 * (2.97–93.88) | 16 (80%) | 4 (20%) | 4 * (0.98–16.27) |
| CG | 2 (10%) | 18 (90%) | 10 (50%) | 10 (50%) | |||
| VAS RP | EG | 8 (40%) | 12 (60%) | 1.55 (0.42–5.76) | 13 (65%) | 7 (35%) | 2.27 (0.63–8.1) |
| CG | 6 (30%) | 14 (70%) | 9 (45%) | 11 (55%) | |||
| DASH | EG | 4 (20%) | 16 (80%) | 2.2 (0.36–13.97) | 16 (80%) | 4 (20%) | 7.4 * (1.77–31.04) |
| CG | 2 (10%) | 18 (90%) | 7 (35%) | 13 (65%) | |||
| COCHIN | EG | 7 (35%) | 13 (65%) | 2.1 (0.51–9) | 18 (90%) | 2 (10%) | 9 * (1.63–49.44) |
| CG | 4 (20%) | 16 (80%) | 10 (50%) | 10 (50%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prado-Robles, E.; Delgado-Gil, J.Á.; Seco-Calvo, J. The Effects of Motor Imagery on Trapeziometacarpal Osteoarthritis in Women During the Post-Surgical Immobilization Period: A Randomized Clinical Trial. Healthcare 2025, 13, 1011. https://doi.org/10.3390/healthcare13091011
Prado-Robles E, Delgado-Gil JÁ, Seco-Calvo J. The Effects of Motor Imagery on Trapeziometacarpal Osteoarthritis in Women During the Post-Surgical Immobilization Period: A Randomized Clinical Trial. Healthcare. 2025; 13(9):1011. https://doi.org/10.3390/healthcare13091011
Chicago/Turabian StylePrado-Robles, Eva, Jose Ángel Delgado-Gil, and Jesús Seco-Calvo. 2025. "The Effects of Motor Imagery on Trapeziometacarpal Osteoarthritis in Women During the Post-Surgical Immobilization Period: A Randomized Clinical Trial" Healthcare 13, no. 9: 1011. https://doi.org/10.3390/healthcare13091011
APA StylePrado-Robles, E., Delgado-Gil, J. Á., & Seco-Calvo, J. (2025). The Effects of Motor Imagery on Trapeziometacarpal Osteoarthritis in Women During the Post-Surgical Immobilization Period: A Randomized Clinical Trial. Healthcare, 13(9), 1011. https://doi.org/10.3390/healthcare13091011







