Surveying the Digital Cytology Workflow in Italy: An Initial Report on AI Integration Across Key Professional Roles
Abstract
1. Introduction
1.1. Historical Foundations of the Digital Cytology Workflow
1.2. An Advancement in the Digital Cytology Workflow: The Role of Virtual Microscopy [1] as a Transformative Technology in Microscopy
1.3. The Integration of Artificial Intelligence into the Digital Cytology Workflow
1.4. Collaborative Approaches to AI Integration in Digital Cytology: Identifying Challenges, Enablers, and the Path to Effective Implementation—Rationale for the Study
2. Methods
2.1. Study Design, Setting, and Participants
2.1.1. The Integrated Approach: VFG Embedded Within CAWI
- Efficiency in Data Collection:
- 2.
- Enhanced Participant Convenience:
- 3.
- Asynchronous Data Collection:
- 4.
- Cost-Effectiveness and Time Efficiency:
- 5.
- Streamlined Data Analysis:
2.1.2. Setting
2.1.3. Participants
- Laboratory Technicians: Professionals involved in the hands-on processes of cytological analysis, including sample preparation, processing, and initial evaluations.
- Medical Doctors: Specialists in pathology and diagnostic medicine, responsible for interpreting cytological results and guiding clinical decisions.
- Biologists: Professionals working within cytology laboratories, often providing diagnostic support and conducting research related to cell biology.
- Specialists in Health Professions of Diagnostic Technical Sciences: Professionals with specialized training in diagnostic imaging, medical laboratory technologies, and other technical sciences relevant to cytology practices.
- Participants were recruited from a diverse range of sources, including the following:
- Scientific societies specializing in cytology and pathology.
- Former students of university courses at Sapienza.
- Peer-to-peer recruitment methods through social media platforms (e.g., WhatsApp, LinkedIn) and professional networks.
2.2. Data Collection Instruments
2.2.1. Data Collection Tools: CAWI Instrument
2.2.2. Question Formats
- Single-choice questions: Used to gather straightforward, categorical data from participants, such as yes/no responses or selecting one option from a list.
- Multiple-choice questions: Allowed respondents to select more than one answer, providing a broader range of responses and allowing for nuanced data collection.
- Graded evaluation questions: These questions employed a six-level psychometric scale (ranging from 1 to 6) to assess participants’ familiarity and attitudes towards specific technologies or practices. The six levels enabled a more granular understanding of participants’ perceptions, which was vital for analyzing their familiarity with digital cytology and AI tools.
- Open-ended questions: These questions were selectively included to capture qualitative insights. Participants were given the opportunity to provide detailed, narrative responses that offered valuable context, particularly for understanding the factors influencing the adoption and use of AI in diagnostic workflows.
- Net Promoter Score (NPS) rating: in the CAWI, this question aimed to assess the participants’ willingness to recommend the procedure, with responses categorized into Promoters (9–10), Passives (7–8), and Detractors (0–6) and the final score derived by subtracting the percentage of Detractors from the percentage of Promoters.
2.2.3. Ethical Integrity and Efficiency
2.2.4. CAWI Methodology and Its Portability
2.3. Data Analysis Methodology
2.3.1. Descriptive Statistics
2.3.2. Inferential Statistics
2.3.3. Thematic Analysis
2.3.4. Data Cleaning and Preprocessing
2.3.5. Statistical Software Tools
3. Results
3.1. Demographic Characteristics of Participants
3.1.1. Sex Distribution
3.1.2. Educational Background
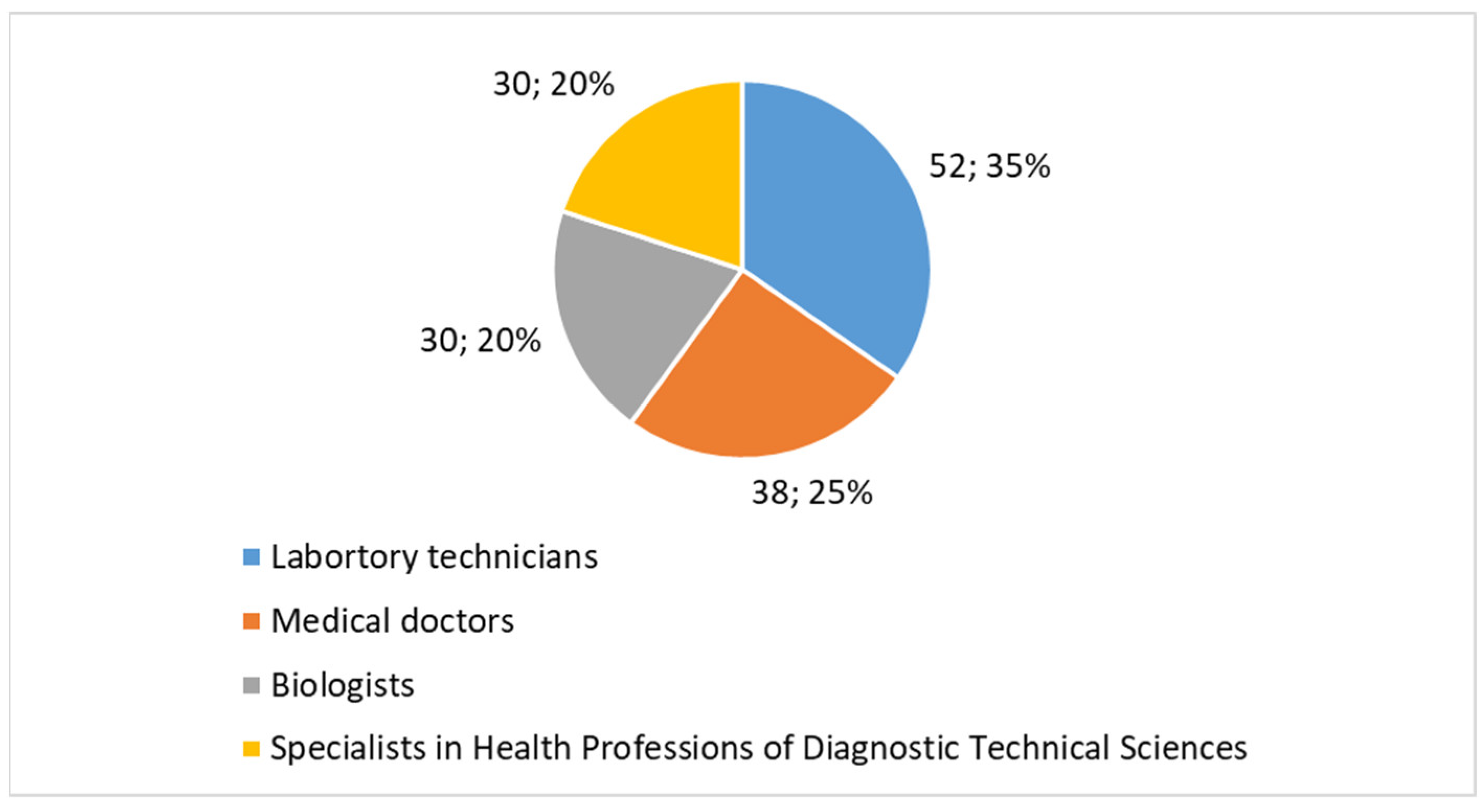
- Laboratory technicians comprised 35% (n = 52) of the participants. This group is a significant component of the cytology workforce in both public and private laboratories, where they play a key role in sample processing and analysis.
- Medical doctors represented 25% (n = 38) of the sample, reflecting the involvement of physicians, particularly specialists in pathology and diagnostic medicine, who oversee and interpret cytology results.
- Biologists made up 20% (n = 30), another essential group within cytology laboratories, often engaged in diagnostic support and research.
- Specialists in health professions of diagnostic technical sciences accounted for 20% (n = 30). This group includes professionals trained specifically in diagnostic imaging, technical sciences, and medical laboratory technologies, who contribute to advanced diagnostic procedures in cytology.
3.1.3. Postgraduate Training in Cytology
- A master’s in Cytopathology and Population Screening, which was the predominant specialization mentioned across the sample.
- Other notable programs included a specialization in Clinical Pathology and Cytology and master’s in Cervical Cytology and Population Screening. These qualifications are crucial for professionals working in both routine diagnostic and screening contexts, particularly in cervical cancer prevention and other related areas.
3.2. Familiarity and Use of Advanced Technologies in Digital Cytology
3.2.1. Familiarity with Digital Cytology
- Low familiarity (score 1−3): 50% (n = 75) of participants indicated low familiarity with digital cytology, with most scoring between 1 and 3, reflecting limited but existing exposure to digital platforms for cytological analysis.
- High familiarity (score 4−6): 50% (n = 75) reported higher familiarity with digital cytology, with most scores falling between 4 and 6, indicating a moderate to high level of comfort and experience with digital tools in cytology.
3.2.2. Familiarity with Artificial Intelligence (AI)
- Low familiarity (score 1–3): A significant 75% (n = 113) of participants had low familiarity with AI, with scores predominantly in the lower range (1–3), suggesting limited or no exposure to AI technologies in clinical practice.
- High familiarity (score 4–6): Only 25% (n = 37) reported higher familiarity (scores 4–6), indicating that most participants have little to no practical experience or understanding of AI’s role in diagnostic workflows.
3.2.3. Use of AI Tools in Digital Cytology Workflow
- Yes: Only 35% (n = 52) of participants had used AI tools in their cytology practice, highlighting a moderate level of the integration of AI in diagnostic work, though still relatively low compared to the total number of participants.
- No: A large 65% (n = 98) of respondents had not used AI tools, indicating significant barriers to the adoption of AI technologies in the field.
3.2.4. Types of AI Tools Used in Digital Cytology
- Automated Image Analysis: 50% (n = 26) used AI for automated image analysis to assist in identifying key cellular features and abnormalities.
- Support in Detecting Cellular Anomalies: 40% (n = 21) applied AI tools to help detect cellular anomalies, such as dysplasia or malignancy.
- Prediction of Diagnosis: 20% (n = 10) had used AI-based prediction tools for diagnosing conditions based on cytological findings.
- Other: 10% (n = 5) mentioned using additional AI applications not covered by the specific categories, suggesting some variability in the use of AI tools in cytology.
3.3. Perceptions on the Integration of AI in Digital Cytology
3.3.1. How Do You Think AI Can Be Useful in the Integration of Digital Cytology?
- Improving Diagnostic Precision: 70% (n = 105) of participants believe AI will significantly enhance the precision of cytological diagnoses. They highlighted AI’s potential in reducing human error and providing consistent, reliable results.
- Reducing Analysis and Reporting Time: 55% (n = 82) feel that AI could dramatically reduce the time required for analysis and reporting, which could lead to faster decision-making and more efficient clinical workflows.
- Supporting the Detection of Hard-to-Identify Cellular Anomalies: 50% (n = 75) see AI as a powerful tool for identifying subtle or hard-to-detect anomalies, such as early-stage malignancies or rare cytological features that could be missed by human analysts.
- Optimizing Workflow and Sample Management: 45% (n = 68) believe AI could optimize workflows, automate repetitive tasks, and help manage cytology samples more effectively, leading to a more streamlined laboratory operation.
- Enabling Remote Access and Faster Consultations: 30% (n = 45) see AI facilitating the possibility of remote access to cytological data, enabling quicker consultations between experts and potentially improving the overall quality of care.
- Other: 5% (n = 8) provided additional suggestions, such as enhancing collaboration across multidisciplinary teams or supporting research in cytology.
3.3.2. How Do You Think You Will Contribute to the Integration of AI in the Digital Cytology Workflow?
- Providing Training and Support for AI Tools: 50% (n = 75) indicated that they could contribute by offering training and guidance to colleagues, ensuring the proper usage and understanding of AI tools in everyday practice.
- Collaborating to Improve Diagnostic Accuracy: 30% (n = 45) believed their role would involve working closely with AI to refine its algorithms and improve its diagnostic capabilities, potentially offering feedback to developers.
- Adapting Workflows to Integrate AI Tools: 15% (n = 23) expressed a willingness to modify existing workflows to incorporate AI, adjusting processes to maximize the effectiveness of the new technologies.
- Other: 5% (n = 7) suggested other contributions, including being involved in the development of AI solutions specific to cytology needs or advocating for AI adoption in professional circles.
3.3.3. Do You Think AI Will Be
- Complementary to Human Work: 80% (n = 120) strongly believed that AI would complement human efforts rather than replace them, providing support and assistance in making more accurate and timely diagnoses.
- A Replacement for Human Work: 10% (n = 15) expressed concerns that AI might replace some human tasks, reflecting apprehension about job security and the future of clinical expertise.
- Unnecessary: 4% (n = 6) felt that AI would not be useful in digital cytology, potentially due to skepticism about or unfamiliarity with AI’s capabilities.
- Difficult to Integrate into the Workflow: 4% (n = 6) thought AI might be hard to incorporate into the existing workflow due to technical, procedural, or logistical challenges.
- Other: 2% (n = 3) indicated various views highlighting that AI could be a stepping stone to more automated systems or might lead to new forms of collaboration between clinicians and machines.
3.3.4. What Barriers Do You Think Could Slow Down the Adoption of AI in Digital Cytology?
- Resistance to Change from Professionals: 60% (n = 90) mentioned that resistance from cytology professionals could be a major challenge. This could stem from concerns about job displacement, reluctance to adopt new technologies, or discomfort with AI’s role in decision-making.
- High Costs for Implementation and Maintenance: 55% (n = 82) noted that the financial cost of implementing and maintaining AI tools could be a significant obstacle, particularly in settings with limited budgets or financial resources.
- Concerns Over Image Quality and Scanning: 40% (n = 60) expressed worries about the quality of images produced by AI systems, fearing that low-quality scans or insufficient data might lead to inaccurate diagnoses.
- Difficulty Integrating with Existing Systems: 35% (n = 52) pointed out the potential technical difficulties in integrating AI tools with existing laboratory and hospital systems, especially if these systems are outdated or incompatible with new technology.
- Need for Continuous Staff Training and Updates: 30% (n = 45) highlighted the need for ongoing staff training to keep up with the evolving AI tools, which might require a sustained investment in education and professional development.
- Data Management and Privacy Concerns: 25% (n = 38) raised concerns over the security and privacy of patient data when using AI tools, especially in light of stringent data protection regulations such as the GDPR.
- Lack of Sufficient Clinical Evidence Supporting AI Effectiveness: 20% (n = 30) felt that the limited amount of clinical evidence demonstrating AI’s effectiveness in improving cytological diagnostics might slow its adoption, as professionals often require robust data to support new technologies.
3.4. Evaluation of Training and Resources for AI Integration
3.4.1. How Do You Evaluate the Adequacy of Training and Resources Available to Use AI Tools in Your Practice?
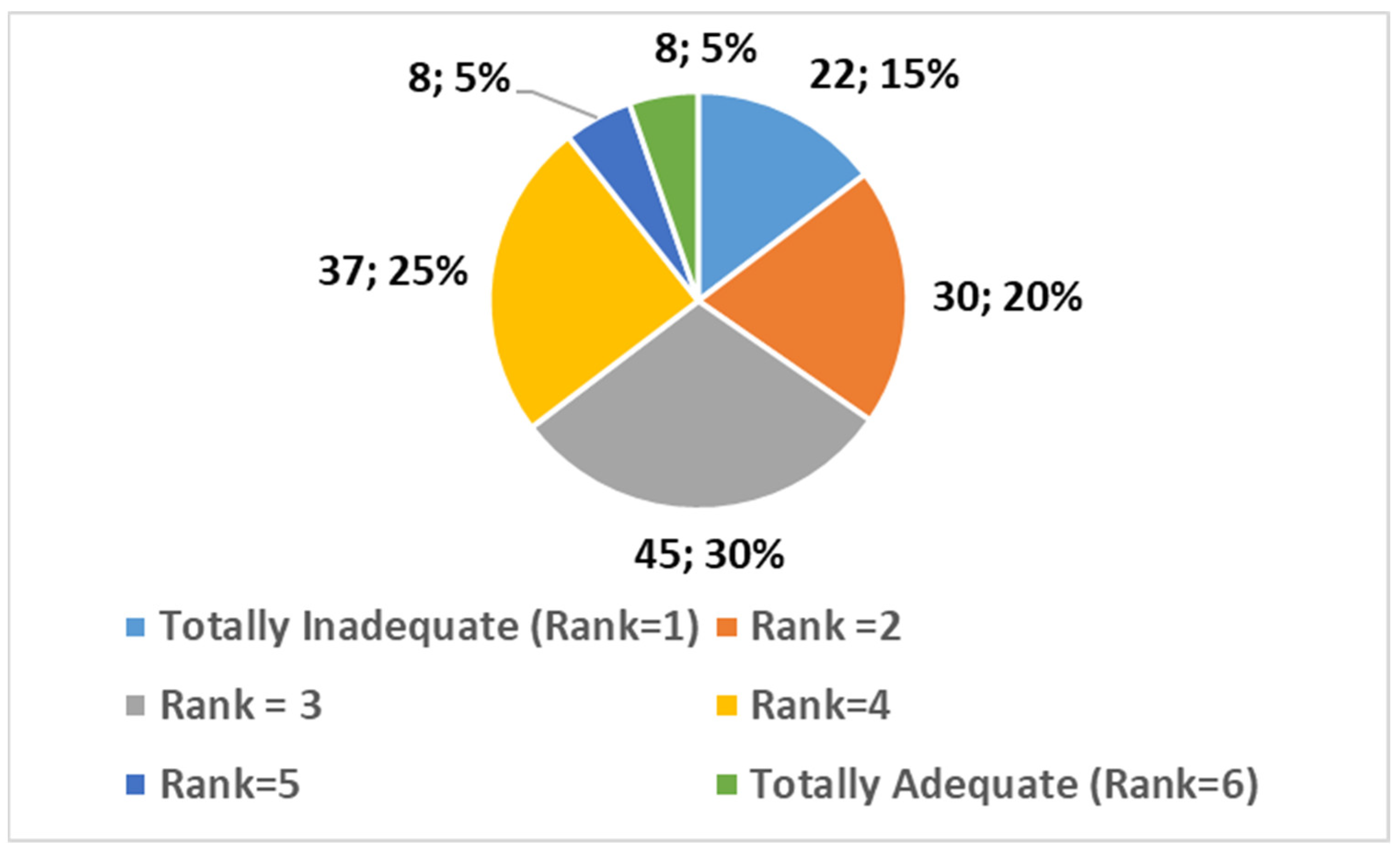
- Totally Inadequate (1): 15% (n = 22);
- 2: 20% (n = 30);
- 3: 30% (n = 45);
- 4: 25% (n = 37);
- 5: 5% (n = 8);
- Totally Adequate (6): 5% (n = 8).
3.4.2. Do You Have Any Comments or Observations?
3.4.3. How Likely Are You to Recommend This Survey to Others?
- Promoters (score 9–10): 60% (n = 90).
- Passives (score 7–8): 30% (n = 45).
- Detractors (score 0–6): 10% (n = 15).
4. Discussion
4.1. Summary and Highlights
4.2. Discussion of Added Value and Comparison to Existing Literature
Added Contribution of the Study
4.3. Comparing Consent and Acceptance in AI Integration in Digital Cytology: A Literature Context
4.4. Limitations of the Study
4.5. Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Pseudocode for the CAWI Survey
- Step 1: Participation Consent and Basic Demographics.
- (1)
- Participation Consent.
- ○
- Participation in this survey is voluntary, and data will be collected anonymously in compliance with applicable regulations. Consent is required to participate.
- ○
- Mandatory Response: Single choice.
- ■
- Yes.
- ■
- No (exit the survey).
- (2)
- Do you work in a cytology laboratory?
- ○
- Mandatory Response: Single choice.
- ■
- Yes.
- ■
- No (exit the survey).
- (3)
- What is your age?
- ○
- Mandatory Response: Single line text.
- ■
- Enter your age in years.
- ■
- The value must be a number.
- (4)
- What is your sex?
- ○
- Mandatory Response: Single choice.
- ■
- Male.
- ■
- Female.
- ■
- Prefer not to disclose.
- Step 2: Educational Background and Work Experience--.
- (5)
- What is your educational background?
- ○
- Mandatory Response: Single choice.
- ■
- Laboratory Technician.
- ■
- Doctor.
- ■
- Biologist.
- ■
- Specialist in diagnostic technical health sciences.
- ■
- Other.
- (6)
- Do you have a Master’s degree or specialization focused on cytology?
- ○
- Mandatory Response: Single choice.
- ■
- Yes.
- ■
- No (jump to question 8).
- (7)
- Please enter the title of your Master’s or specialization with a focus on cytology.
- ○
- Mandatory Response: Single line text.
- ■
- Enter your answer.
- (8)
- How familiar are you with digital cytology?
- ○
- Mandatory Response: Rating scale (1 = minimum; 6 = maximum).
- (9)
- How familiar are you with artificial intelligence (AI)?
- ○
- Mandatory Response: Rating scale (1 = minimum; 6 = maximum).
- (10)
- Have you ever used AI tools in the digital cytology workflow?
- ○
- Mandatory Response: Single choice.
- ■
- Yes.
- ■
- No (jump to question 12).
- (11)
- What type of AI tools have you used?
- ○
- Mandatory Response: Multiple choice (select all that apply).
- ■
- Automatic image analysis.
- ■
- Support in detecting cellular abnormalities.
- ■
- Diagnosis prediction.
- ■
- Other.
- Step 3: AI in Digital Cytology Integration--.
- (12)
- How do you think artificial intelligence can be useful in the integration of digital cytology?
- ○
- Mandatory Response: Multiple choice (select all that apply).
- ■
- Improving diagnostic accuracy.
- ■
- Reducing analysis and reporting times.
- ■
- Supporting the detection of hard-to-identify cellular abnormalities.
- ■
- Optimizing workflow and sample management.
- ■
- Enabling remote access and faster consultation.
- ■
- Other.
- (13)
- How do you think you can contribute to the integration of AI into digital cytology workflows?
- ○
- Mandatory Response: Single choice.
- ■
- Providing training and support in the use of AI tools.
- ■
- Collaborating to improve diagnostic accuracy.
- ■
- Adapting work processes to integrate AI tools.
- ■
- Other.
- (14)
- Do you think artificial intelligence will be:
- ○
- Mandatory Response: Single choice.
- ■
- Complementary to human work.
- ■
- A replacement for human work.
- ■
- Useless.
- ■
- Difficult to integrate into my workflow.
- ■
- Other.
- Step 4: Barriers to AI Adoption--.
- (15)
- What barriers do you think could slow down the adoption of artificial intelligence in digital cytology?
- ○
- Mandatory Response: Multiple choice (select all that apply).
- ■
- Resistance to change from professionals.
- ■
- High costs for implementation and maintenance.
- ■
- Concerns regarding image quality and scanning.
- ■
- Difficulty integrating with existing systems.
- ■
- Need for continuous staff training and updates.
- ■
- Data management and privacy issues.
- ■
- Lack of clinical evidence supporting AI effectiveness.
- ■
- Other.
- Step 5: Training and Resources
- (16)
- How would you rate the adequacy of the training and resources available to use AI tools in your practice?
- ○
- Mandatory Response: Rating scale (1 = totally inadequate; 6 = totally adequate).
- Step 6: Open Comments
- (17)
- Add any comments or observations.
- Text paragraph.
- Enter your answer.
- Step 7: Survey Recommendation
- (18)
- How likely are you to recommend our survey?
- ○
- Mandatory Response: Net Promoter Score (NPS) rating.
References
- Giansanti, D.; Grigioni, M.; D’Avenio, G.; Morelli, S.; Maccioni, G.; Bondi, A.; Giovagnoli, M.R. Virtual Microscopy and Digital Cytology: State of the Art. Ann. Ist. Super. Sanita 2010, 46, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Remmelinck, M.; Lopes, M.B.; Nagy, N.; Rorive, S.; Rombaut, K.; Decaestecker, C.; Kiss, R.; Salmon, I. How Could Static Telepathology Improve Diagnosis in Neuropathology? Anal. Cell Pathol. 2000, 21, 177–182. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Petrovichev, N.N.; Shabalova, I.P.; Sokolova, V.K.; Chistiakova, O.V.; Kareva, L.P.; Kovrigina, A.M.; Fedoseev, V.N.; Shchipalkin, V.I.; Solomatina, T.P. Feasibilities of Cytological Diagnosis in the Static Telepathology Mode. Klin. Lab. Diagn. 1999, 2, 21–24. [Google Scholar] [PubMed]
- Della Mea, V.; Cataldi, P.; Pertoldi, B.; Beltrami, C.A. Combining Dynamic and Static Robotic Telepathology: A Report on 184 Consecutive Cases of Frozen Sections, Histology and Cytology. Anal. Cell Pathol. 2000, 20, 33–39. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunn, B.E.; Choi, H.; Almagro, U.A.; Recla, D.L. Combined Robotic and Nonrobotic Telepathology as an Integral Service Component of a Geographically Dispersed Laboratory Network. Hum. Pathol. 2001, 32, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Demichelis, F.; Barbareschi, M.; Boi, S.; Clemente, C.; Dalla Palma, P.; Eccher, C.; Forti, S. Robotic Telepathology for Intraoperative Remote Diagnosis Using a Still-Imaging-Based System. Am. J. Clin. Pathol. 2001, 116, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.; Plaisant, C.; Hasegawa, H. Designing a Real-Time Telepathology Workstation To Mitigate Communication Delays. Interact. Comput. 1998, 11, 33–52. [Google Scholar] [CrossRef]
- Virtual Microscopy—Overview and Definition. Available online: https://www.virtual-microscopy.net/ (accessed on 28 February 2025).
- Digital Histology. Available online: https://digitalhistology.org/ (accessed on 28 February 2025).
- Welcome to www.Virtualmicroscopy.Co.Uk. Available online: https://www.virtualmicroscopy.co.uk/ (accessed on 28 February 2025).
- FDA Regulation of Whole Slide Imaging (WSI) Devices: Current Thoughts. Available online: https://www.cdc.gov/cliac/docs/addenda/cliac0212/Tab_15_Faison_CLIAC_2012Feb14_Whole_Slide_Imaging.pdf (accessed on 28 February 2025).
- Saini, T.; Bansal, B.; Dey, P. Digital Cytology: Current Status and Future Prospects. Diagn. Cytopathol. 2023, 51, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chiou, P.Z.; Jia, Y. Evaluating the Use of Virtual Microscopy in Cytology Education. J. Am. Soc. Cytopathol. 2023, 12, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, D.; Castrichella, L.; Giovagnoli, M.R. Telepathology Training in a Master of Cytology Degree Course. J. Telemed. Telecare. 2008, 14, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, A.D.; Mukherjee, M.S.; Lyden, E.R.; Radio, S.J. Virtual Microscopy in Cytotechnology Education: Application of Knowledge From Virtual to Glass. Cytojournal 2012, 9, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tizhoosh, H.R.; Pantanowitz, L. Artificial Intelligence and Digital Pathology: Challenges and Opportunities. J. Pathol. Inform. 2018, 9, 38. [Google Scholar] [CrossRef]
- McAlpine, E.D.; Pantanowitz, L.; Michelow, P.M. Challenges Developing Deep Learning Algorithms in Cytology. Acta Cytol. 2021, 65, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Ciaparrone, C.; Maffei, E.; L’Imperio, V.; Pisapia, P.; Eloy, C.; Fraggetta, F.; Zeppa, P.; Caputo, A. Computer-Assisted Urine Cytology: Faster, Cheaper, Better? Cytopathology 2024, 35, 634–641. [Google Scholar] [CrossRef]
- Zhang, L.; Wong, C.; Li, Y.; Huang, T.; Wang, J.; Lin, C. Artificial Intelligence Assisted Diagnosis of Early TC Markers and Its Application. Discov. Oncol. 2024, 15, 172. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Pisapia, P.; L’Imperio, V. Current Role of Cytopathology in the Molecular and Computational Era: The Perspective of Young Pathologists. Cancer Cytopathol. 2024, 132, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; Campennì, A.; Tuncel, M.; Petranović Ovčariček, P. Integrated Diagnostics of Thyroid Nodules. Cancers 2024, 16, 311. [Google Scholar] [CrossRef]
- Kim, D.; Sundling, K.E.; Virk, R.; Thrall, M.J.; Alperstein, S.; Bui, M.M.; Chen-Yost, H.; Donnelly, A.D.; Lin, O.; Liu, X.; et al. Digital Cytology Part 2: Artificial Intelligence in Cytology: A Concept Paper With Review and Recommendations From the American Society of Cytopathology Digital Cytology Task Force. J. Am. Soc. Cytopathol. 2024, 13, 97–110. [Google Scholar] [CrossRef]
- Kim, D.; Sundling, K.E.; Virk, R.; Thrall, M.J.; Alperstein, S.; Bui, M.M.; Chen-Yost, H.; Donnelly, A.D.; Lin, O.; Liu, X.; et al. Digital Cytology Part 1: Digital Cytology Implementation for Practice: A Concept Paper With Review and Recommendations From the American Society of Cytopathology Digital Cytology Task Force. J. Am. Soc. Cytopathol. 2024, 13, 86–96. [Google Scholar] [CrossRef]
- Malik, S.; Zaheer, S. ChatGPT as an Aid for Pathological Diagnosis of Cancer. Pathol. Res. Pract. 2024, 253, 154989. [Google Scholar] [CrossRef]
- Slabaugh, G.; Beltran, L.; Rizvi, H.; Deloukas, P.; Marouli, E. Applications of Machine and Deep Learning to Thyroid Cytology and Histopathology: A Review. Front. Oncol. 2023, 13, 958310. [Google Scholar] [CrossRef]
- Lebrun, L.; Salmon, I. Pathology and New Insights in Thyroid Neoplasms in the 2022 WHO Classification. Curr. Opin. Oncol. 2024, 36, 13–21. [Google Scholar] [CrossRef]
- Singla, N.; Kundu, R.; Dey, P. Artificial Intelligence: Exploring Utility in Detection and Typing of Fungus With Futuristic Application in Fungal Cytology. Cytopathology 2024, 35, 226–234. [Google Scholar] [CrossRef]
- Sunny, S.P.; DR, R.; Hariharan, A.; Mukhia, N.; Gurudath, S.; Raghavan, S.; Kolur, T.; Shetty, V.; Surolia, A.; Chandrashekhar, P.; et al. CD44-SNA1 Integrated Cytopathology for Delineation of High Grade Dysplastic and Neoplastic Oral Lesions. PLoS ONE. 2023, 18, e0291972. [Google Scholar] [CrossRef]
- Wong, C.M.; Kezlarian, B.E.; Lin, O. Current Status of Machine Learning in Thyroid Cytopathology. J. Pathol. Inform. 2023, 14, 100309. [Google Scholar] [CrossRef]
- Ludwig, M.; Ludwig, B.; Mikuła, A.; Biernat, S.; Rudnicki, J.; Kaliszewski, K. The Use of Artificial Intelligence in the Diagnosis and Classification of Thyroid Nodules: An Update. Cancers 2023, 15, 708. [Google Scholar] [CrossRef]
- Marletta, S.; L’Imperio, V.; Eccher, A.; Antonini, P.; Santonicco, N.; Girolami, I.; Dei Tos, A.P.; Sbaraglia, M.; Pagni, F.; Brunelli, M.; et al. Artificial Intelligence-Based Tools Applied to Pathological Diagnosis of Microbiological Diseases. Pathol. Res. Pract. 2023, 243, 154362. [Google Scholar] [CrossRef]
- Tessler, F.N.; Thomas, J. Artificial Intelligence for Evaluation of Thyroid Nodules: A Primer. Thyroid 2023, 33, 150–158. [Google Scholar] [CrossRef]
- Thakur, N.; Alam, M.R.; Abdul-Ghafar, J.; Chong, Y. Recent Application of Artificial Intelligence in Non-Gynecological Cancer Cytopathology: A Systematic Review. Cancers 2022, 14, 3529. [Google Scholar] [CrossRef]
- Alrafiah, A.R. Application and Performance of Artificial Intelligence Technology in Cytopathology. Acta Histochem. 2022, 124, 151890. [Google Scholar] [CrossRef]
- Vaickus, L.J.; Kerr, D.A.; Velez Torres, J.M.; Levy, J. Artificial Intelligence Applications in Cytopathology: Current State of the Art. Surg. Pathol. Clin. 2024, 17, 521–531. [Google Scholar] [CrossRef]
- Jorda, M.; Kryvenko, O.N.; Hanly, F.; Zuo, Y. Urinary Tract Cytopathology: Current and Future Impact on Patient Care. Surg. Pathol. Clin. 2024, 17, 383–394. [Google Scholar] [CrossRef]
- Velez Torres, J.M.; Vaickus, L.J.; Kerr, D.A. Thyroid Fine-Needle Aspiration: The Current and Future Landscape of Cytopathology. Surg. Pathol. Clin. 2024, 17, 371–381. [Google Scholar] [CrossRef]
- Atlante Delle Professioni. Tecnico Sanitario Di Laboratorio Biomedico, Tecnica Sanitaria Di Laboratorio Biomedico. Available online: https://atlantedelleprofessioni.it/professioni/tecnico-sanitario-di-laboratorio-biomedico-tecnica-sanitaria-di-laboratorio-biomedico (accessed on 28 February 2025).
- Atlante Delle Professioni. Biologo, Biologa. Available online: https://atlantedelleprofessioni.it/professioni/biologo-biologa (accessed on 28 February 2025).
- Scienze delle professioni sanitarie tecniche diagnostiche. Available online: https://corsidilaurea.uniroma1.it/it/corso/2021/30005/home (accessed on 28 February 2025).
- Kim, D.; Thrall, M.J.; Michelow, P.; Schmitt, F.C.; Vielh, P.R.; Siddiqui, M.T.; Sundling, K.E.; Virk, R.; Alperstein, S.; Bui, M.M.; et al. The Current State of Digital Cytology and Artificial Intelligence (AI): Global Survey Results From the American Society of Cytopathology Digital Cytology Task Force. J. Am. Soc. Cytopathol. 2024, 13, 319–328. [Google Scholar] [CrossRef] [PubMed]
- DICOM. Available online: https://www.dicomstandard.org/ (accessed on 28 February 2025).
- DICOM Whole Slide Imaging (WSI). Available online: https://dicom.nema.org/dicom/dicomwsi (accessed on 28 February 2025).
- Pubmed Search. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=%28%28Artificial+intelligence%5BTitle%2FAbstract%5D%29+AND+%28Radiology%5BTitle%2FAbstract%5D%29%29+AND+%28%28Questionnaire%5BTitle%2FAbstract%5D%29+OR+%28survey%5BTitle%2FAbstract%5D%29%29&sort=date&size=200 (accessed on 28 February 2025).
- Giansanti, D.; Di Basilio, F. The Artificial Intelligence in Digital Radiology: Part 1: The Challenges, Acceptance and Consensus. Healthcare 2022, 10, 509. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lastrucci, A.; Giarnieri, E.; Carico, E.; Giansanti, D. Revolutionizing Cytology and Cytopathology with Natural Language Processing and Chatbot Technologies: A Narrative Review on Current Trends and Future Directions. Bioengineering 2024, 11, 1134. [Google Scholar] [CrossRef]
- Giarnieri, E.; Scardapane, S. Towards Artificial Intelligence Applications in Next Generation Cytopathology. Biomedicines 2023, 11, 2225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, G.; Badal, A.; Jia, X.; Maltz, J.S.; Mueller, K.; Myers, K.J.; Niu, C.; Vannier, M.; Yan, P.; Yu, Z.; et al. Development of Metaverse for Intelligent Healthcare. Nat. Mach. Intell. 2022, 4, 922–929. [Google Scholar] [CrossRef]
- Ali, S.; Abdullah; Armand, T.P.T.; Athar, A.; Hussain, A.; Ali, M.; Yaseen, M.; Joo, M.-I.; Kim, H.-C. Metaverse in Healthcare Integrated with Explainable AI and Blockchain: Enabling Immersiveness, Ensuring Trust, and Providing Patient Data Security. Sensors 2023, 23, 565. [Google Scholar] [CrossRef]
- Yang, Y.; Xian, D.; Yu, L.; Kong, Y.; Lv, H.; Huang, L.; Liu, K.; Zhang, H.; Wei, W.; Tang, H. Integration of AI-Assisted in Digital Cervical Cytology Training: A Comparative Study. Cytopathology 2025, 36, 156–164. [Google Scholar] [CrossRef] [PubMed]
| Familiarity Level | Score Range | Percentage | Number of Participants (n) | Description |
|---|---|---|---|---|
| Low familiarity | 1–3 | 50% | 75 | Limited but existing exposure to digital cytology platforms. |
| High familiarity | 4–6 | 50% | 75 | Moderate to high comfort and experience with digital tools. |
| Familiarity Level | Score Range | Percentage | Number of Participants (n) | Description |
|---|---|---|---|---|
| Low familiarity | 1−3 | 75% | 113 | Limited or no exposure to AI technologies in clinical practice. |
| High familiarity | 4−6 | 25% | 37 | Little to no practical experience or understanding of AI’s role in diagnostic workflows. |
| AI Tool Usage | Percentage | Number of Participants (n) | Description |
|---|---|---|---|
| Yes | 35% | 52 | Moderate level of AI integration in diagnostic work, though still relatively low. |
| No | 65% | 98 | Significant barriers to AI adoption in cytology practice. |
| AI Application | Participants (n) | Percentage (%) |
|---|---|---|
| Automated Image Analysis | 26 | 50% |
| Support in Detecting Cellular Anomalies | 21 | 40% |
| Prediction of Diagnosis | 10 | 20% |
| Other | 5 | 10% |
| AI Benefit Category | Percentage (%) | n | Description |
|---|---|---|---|
| Improving Diagnostic Precision | 70% | 105 | AI is expected to enhance diagnostic accuracy, reduce human error, and provide consistent, reliable results. |
| Reducing Analysis and Reporting Time | 55% | 82 | AI could significantly speed up analysis and reporting, leading to more efficient clinical workflows. |
| Supporting the Detection of Hard-to-Identify Cellular Anomalies | 50% | 75 | AI can assist in identifying subtle anomalies, including early-stage malignancies and rare cytological features. |
| Optimizing Workflow and Sample Management | 45% | 68 | AI could streamline laboratory operations by automating tasks and improving sample management. |
| Enabling Remote Access and Faster Consultations | 30% | 45 | AI may facilitate remote access to cytological data, improving collaboration and consultation speed. |
| Other | 5% | 8 | Additional suggestions included enhancing multidisciplinary collaboration and supporting research in cytology. |
| Contribution to AI Integration | Percentage (%) | n | Description |
|---|---|---|---|
| Providing Training and Support for AI Tools | 50% | 75 | Assisting colleagues with training and guidance to ensure proper use and understanding of AI tools. |
| Collaborating to Improve Diagnostic Accuracy | 30% | 45 | Working alongside AI to refine algorithms and enhance diagnostic precision, potentially providing feedback to developers. |
| Adapting Workflows to Integrate AI Tools | 15% | 23 | Modifying existing workflows to effectively incorporate AI technologies. |
| Other | 5% | 7 | Involvement in AI development for cytology or advocating for AI adoption in professional settings. |
| View on AI’s Impact | Percentage (%) | n | Description |
|---|---|---|---|
| Complementary to Human Work | 80% | 120 | Strong belief that AI will complement human efforts by supporting more accurate and timely diagnoses. |
| A Replacement for Human Work | 10% | 15 | Concerns that AI may replace some human tasks, reflecting apprehension about job security and the future of clinical expertise. |
| Unnecessary | 4% | 6 | Belief that AI would not be useful in digital cytology, possibly due to skepticism about or unfamiliarity with its capabilities. |
| Difficult to Integrate into the Workflow | 4% | 6 | View that AI might face challenges in integration due to technical, procedural, or logistical issues. |
| Other | 2% | 3 | Various views suggesting AI might lead to more automated systems or new collaborations between clinicians and machines. |
| Obstacle | Percentage (%) | n | Description |
|---|---|---|---|
| Resistance to Change from Professionals | 60% | 90 | Concerns about job displacement, reluctance to adopt new technologies, or discomfort with AI’s role in decision-making. |
| High Costs for Implementation and Maintenance | 55% | 82 | Financial challenges in implementing and maintaining AI tools, particularly in resource-limited settings. |
| Concerns Over Image Quality and Scanning | 40% | 60 | Worries about the quality of images produced by AI systems, potentially leading to inaccurate diagnoses due to low-quality scans or insufficient data. |
| Difficulty Integrating with Existing Systems | 35% | 52 | Technical difficulties in integrating AI tools with existing laboratory and hospital systems, especially outdated or incompatible systems. |
| Need for Continuous Staff Training and Updates | 30% | 45 | Ongoing staff training required to keep up with evolving AI tools, necessitating a sustained investment in education and professional development. |
| Data Management and Privacy Concerns | 25% | 38 | Concerns about security and privacy of patient data when using AI tools, particularly with stringent data protection regulations like GDPR. |
| Lack of Sufficient Clinical Evidence Supporting AI Effectiveness | 20% | 30 | Limited clinical evidence on AI’s effectiveness in improving cytological diagnostics, which may slow adoption due to need for robust supporting data. |
| Comment Theme | Frequency |
|---|---|
| Need for hands-on training | 8 (23.5%) |
| Ongoing support and guidance | 5 (14.7%) |
| Desire for more comprehensive training materials | 4 (11.8%) |
| Integration of AI in existing workflows | 3 (8.8%) |
| Concerns about AI’s reliability and usability | 3 (8.8%) |
| Need for continuous updates on new AI developments | 2 (5.9%) |
| General satisfaction | 3 (8.8%) |
| Desire for more clarity on AI’s role | 1 (2.9%) |
| Concerns about AI replacing jobs | 1 (2.9%) |
| Lack of technical support | 1 (2.9%) |
| Need for clearer guidelines on AI’s implementation | 1 (2.9%) |
| Preference for more practical experience | 1 (2.9%) |
| Concerns about AI’s reliability and usability | 3 (8.8%) |
| Need for continuous updates on new AI developments | 2 (5.9%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giansanti, D.; Carico, E.; Lastrucci, A.; Giarnieri, E. Surveying the Digital Cytology Workflow in Italy: An Initial Report on AI Integration Across Key Professional Roles. Healthcare 2025, 13, 903. https://doi.org/10.3390/healthcare13080903
Giansanti D, Carico E, Lastrucci A, Giarnieri E. Surveying the Digital Cytology Workflow in Italy: An Initial Report on AI Integration Across Key Professional Roles. Healthcare. 2025; 13(8):903. https://doi.org/10.3390/healthcare13080903
Chicago/Turabian StyleGiansanti, Daniele, Elisabetta Carico, Andrea Lastrucci, and Enrico Giarnieri. 2025. "Surveying the Digital Cytology Workflow in Italy: An Initial Report on AI Integration Across Key Professional Roles" Healthcare 13, no. 8: 903. https://doi.org/10.3390/healthcare13080903
APA StyleGiansanti, D., Carico, E., Lastrucci, A., & Giarnieri, E. (2025). Surveying the Digital Cytology Workflow in Italy: An Initial Report on AI Integration Across Key Professional Roles. Healthcare, 13(8), 903. https://doi.org/10.3390/healthcare13080903







