Time for Change: A 3-Year Prospective Study on Mediterranean Diet Adherence and Body Composition in Kidney Transplant Recipients
Abstract
1. Introduction
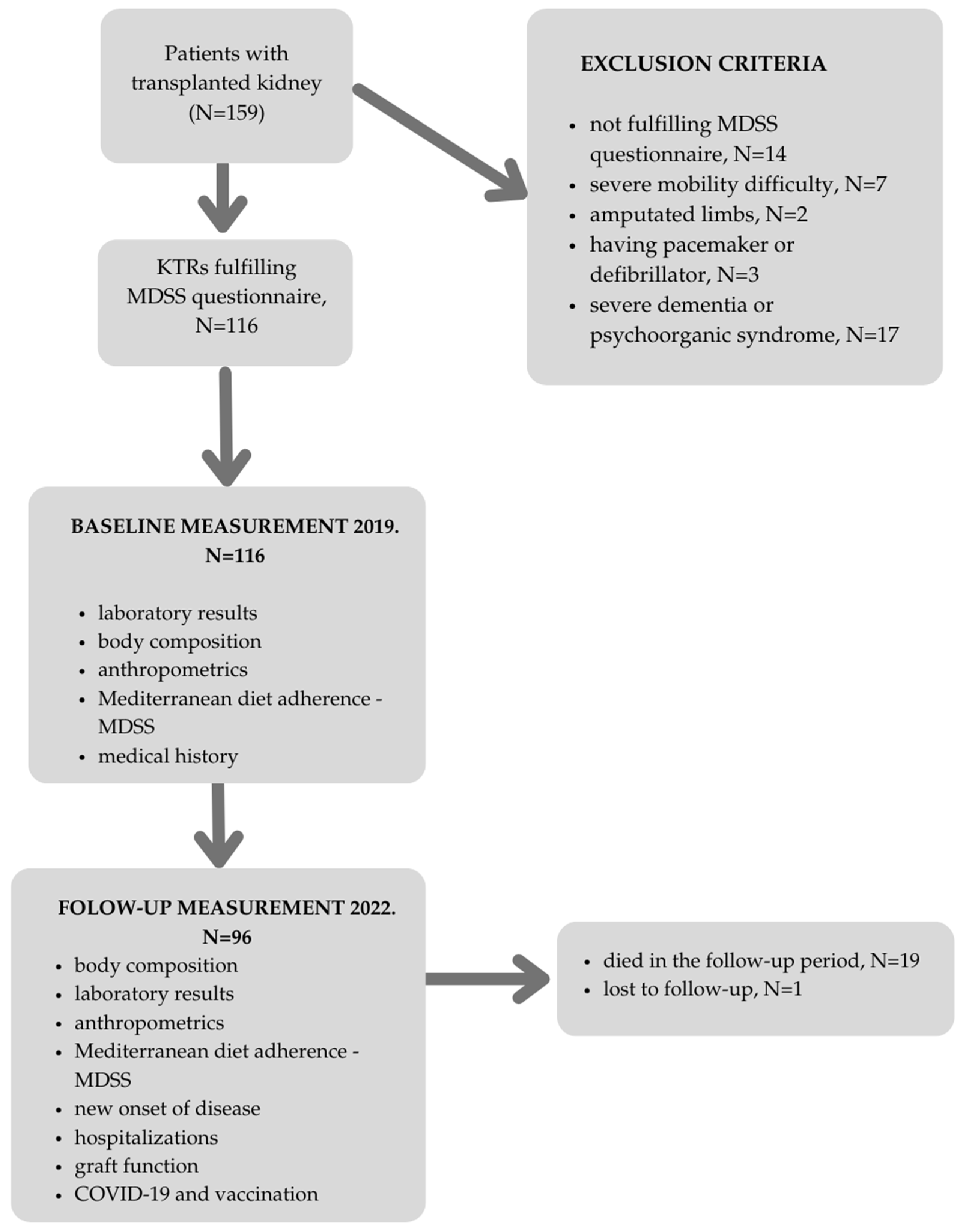
2. Materials and Methods
2.1. Follow-Up Study Measurements
2.1.1. Body Composition
2.1.2. Mediterranean Diet Adherence
2.1.3. Clinical Outcomes and Laboratory Parameters
2.2. Statistical Analysis
3. Results
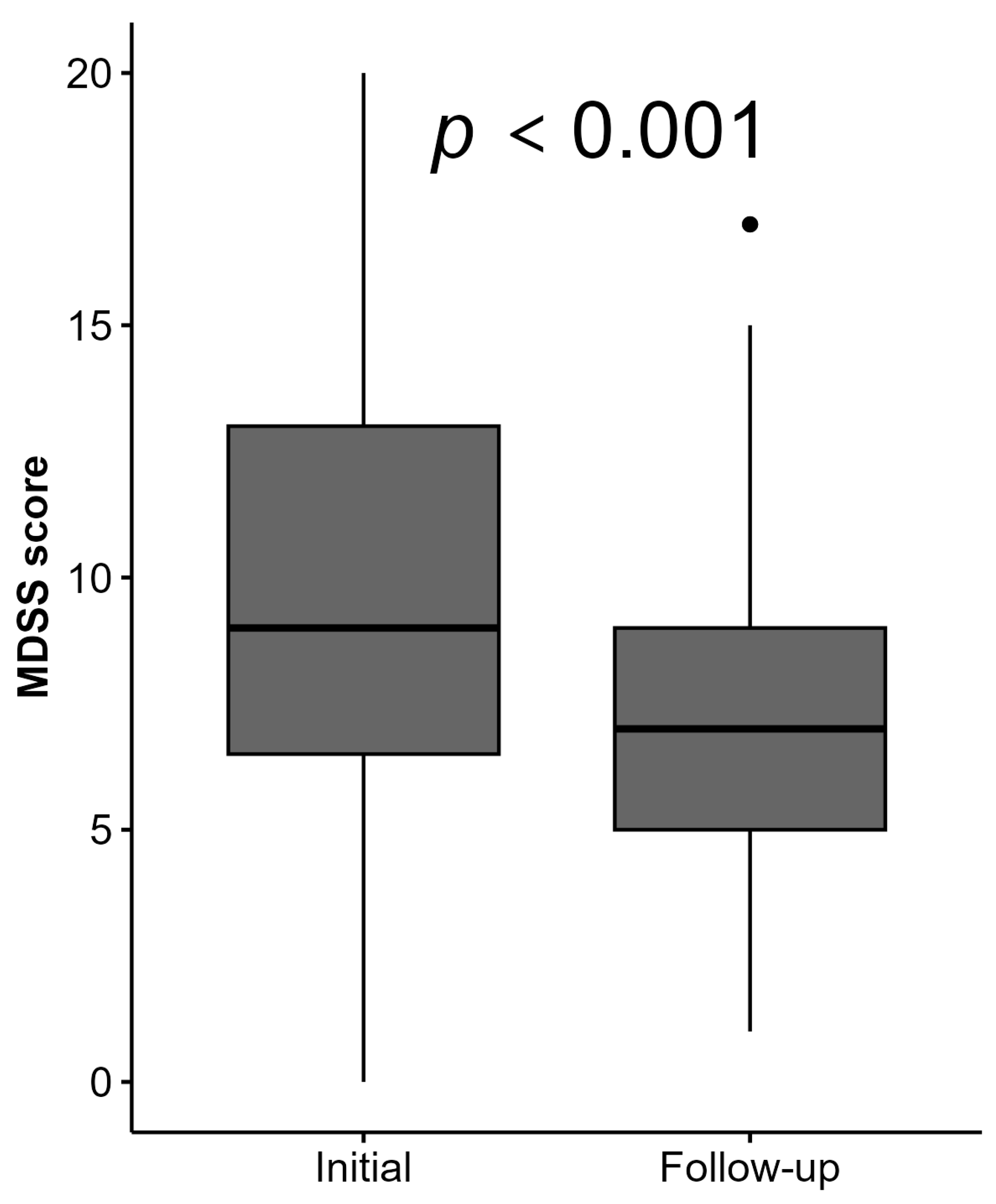
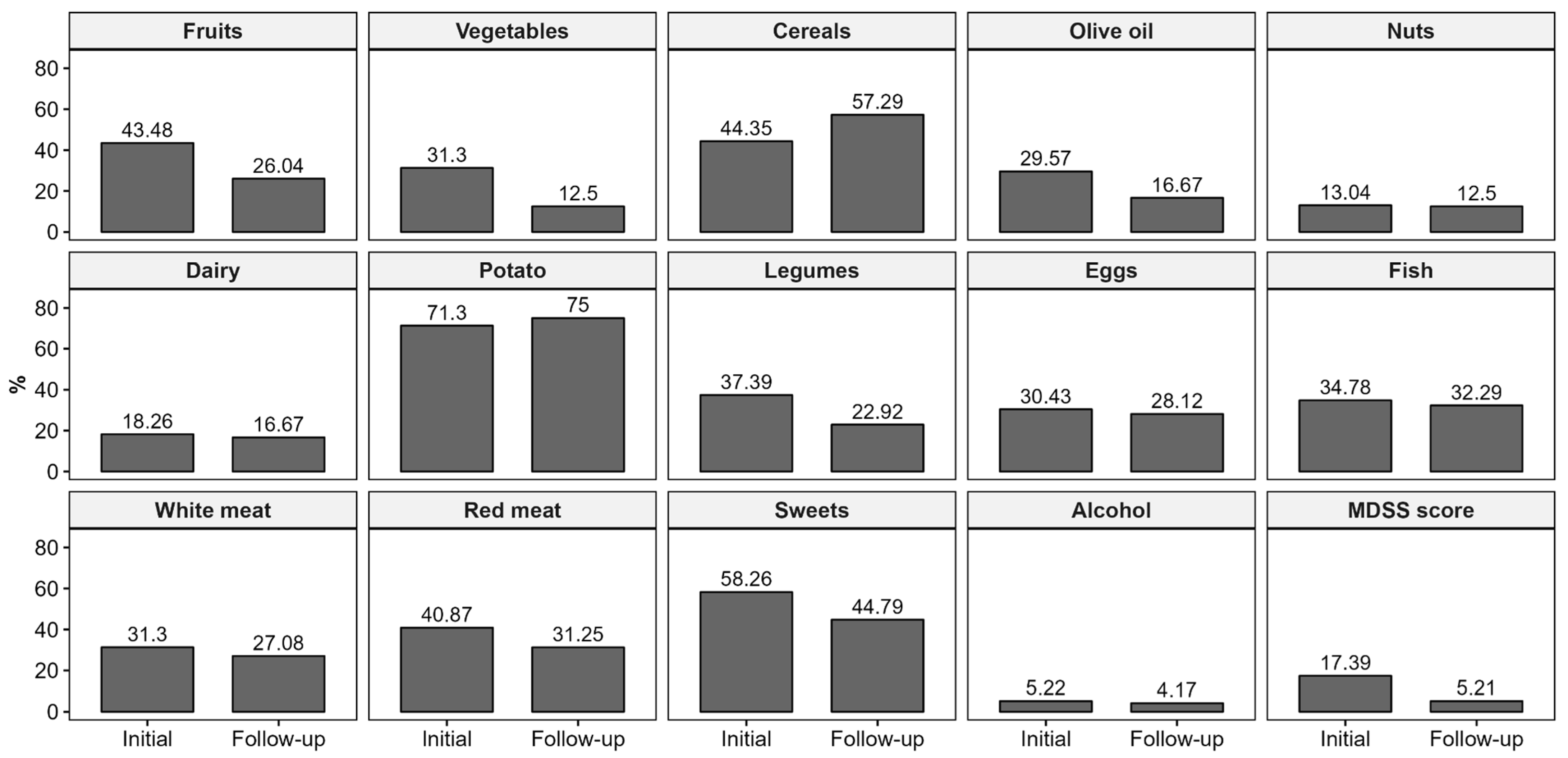
3.1. Changes in Mediterranean Diet
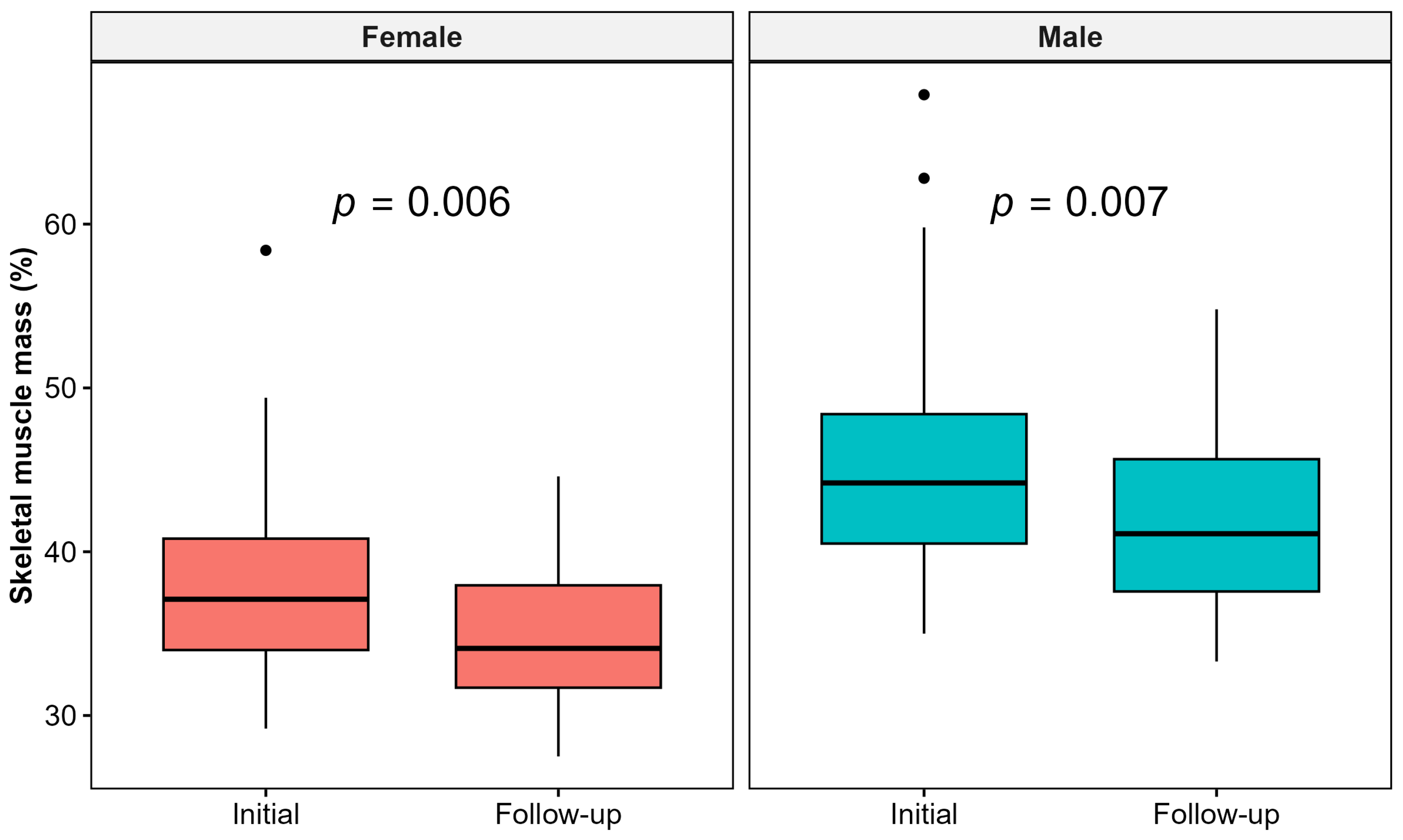
3.2. Changes in Body Composition Parameters
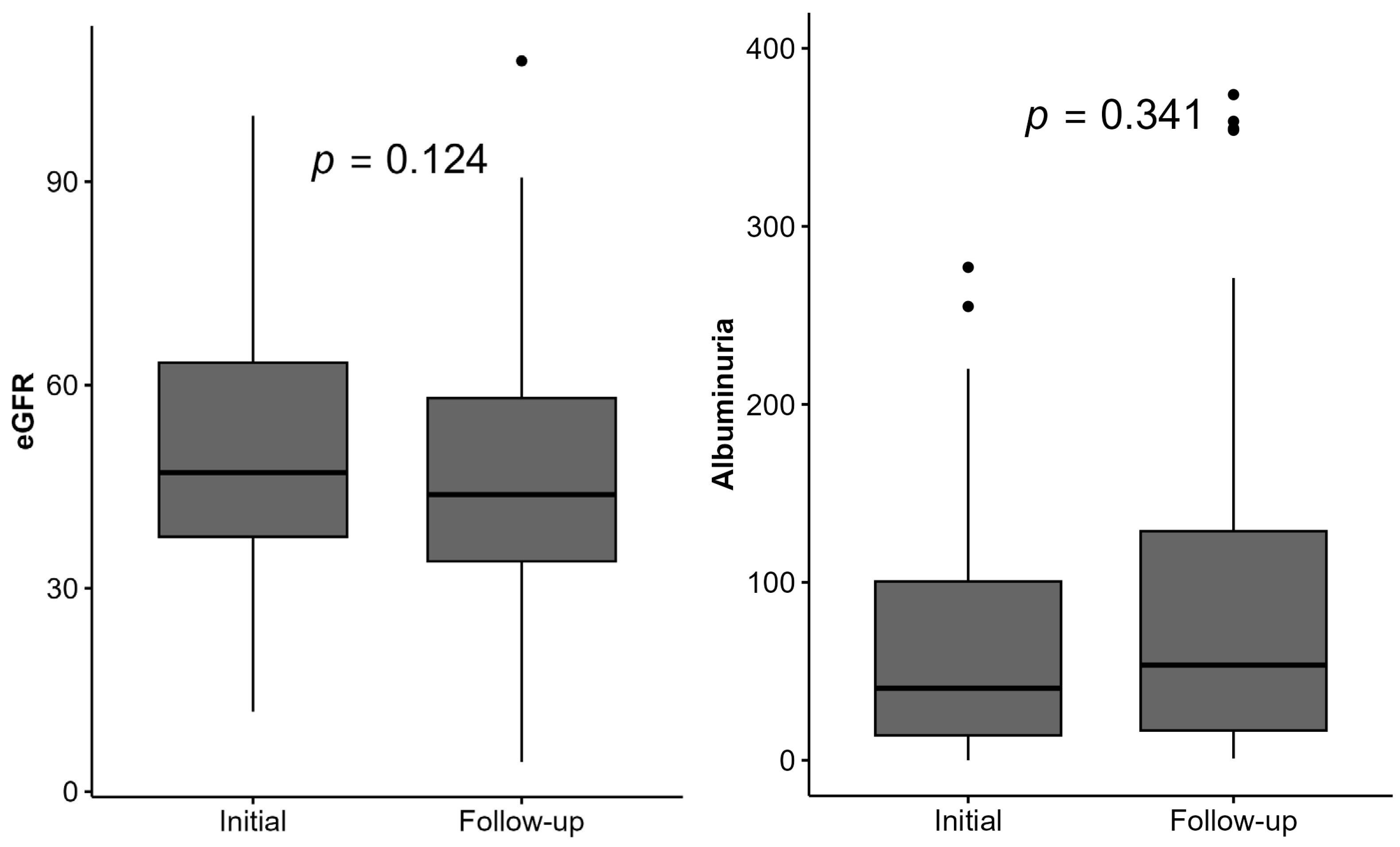
3.3. Changes in Laboratory Parameters
3.4. New-Onset Conditions and Clinical Outcomes
3.5. Associations Between MeDi Adherence, Body Composition, Laboratory Parameters, and Clinical Outcomes
4. Discussion
4.1. Mediterranean Diet
4.2. Body Composition Parameters
4.3. Clinical and Laboratory Parameters
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakis, H.; Chauveau, P.; Combe, C.; Pfirmann, P. Mediterranean Diet for Cardiovascular Risk Reduction in Chronic Kidney Disease. Adv. Kidney Dis. Health 2023, 30, 496–501. [Google Scholar] [CrossRef]
- Tantisattamo, E.; Kalantar-Zadeh, K.; Molnar, M.Z. Nutritional and Dietary Interventions to Prolong Renal Allograft Survival after Kidney Transplantation. Curr. Opin. Nephrol. Hypertens. 2022, 31, 6–17. [Google Scholar] [CrossRef]
- Cyrino, L.G.; Galpern, J.; Moore, L.; Borgi, L.; Riella, L.V. A Narrative Review of Dietary Approaches for Kidney Transplant Patients. Kidney Int. Rep. 2021, 6, 1764–1774. [Google Scholar] [CrossRef]
- Gomes-Neto, A.W.; Osté, M.C.J.; Sotomayor, C.G.; van den Berg, E.; Geleijnse, J.M.; Berger, S.P.; Gans, R.O.B.; Bakker, S.J.L.; Navis, G.J. Mediterranean Style Diet and Kidney Function Loss in Kidney Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2020, 15, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Ichikawa, K.; Morizawa, Y.; Gotoh, D.; Itami, Y.; Nakai, Y.; Miyake, M.; Yoneda, T.; Tanaka, N.; Yoshida, K.; et al. Clinical Significance of Postoperative Nutritional Status as a Prognostic Factor in Kidney Transplant Recipients. Transpl. Proc. 2019, 51, 6. [Google Scholar] [CrossRef]
- Stanfill, A.; Bloodworth, R.; Cashion, A. Lessons Learned: Experiences of Gaining Weight by Kidney Transplant Recipients. Prog. Transplant. 2012, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.M.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104, S11–S103. [Google Scholar] [CrossRef] [PubMed]
- Gurlek Demirci, B.; Karakan, M.S. Effect of Body Composition on Graft Function and Cardiovascular Outcomes in Normotensive Renal Transplant Recipients. Exp. Clin. Transplant. 2024, 22, 108–113. [Google Scholar] [CrossRef]
- Beetz, N.L.; Geisel, D.; Shnayien, S.; Auer, T.A.; Globke, B.; Öllinger, R.; Trippel, T.D.; Schachtner, T.; Fehrenbach, U. Effects of Artificial Intelligence-Derived Body Composition on Kidney Graft and Patient Survival in the Eurotransplant Senior Program. Biomedicines 2022, 10, 554. [Google Scholar] [CrossRef]
- Jiang, Y.; Villeneuve, P.J.; Schaubel, D.; Mao, Y.; Rao, P.; Morrison, H. Long-Term Follow-Up of Kidney Transplant Recipients: Comparison of Hospitalization Rates to the General Population. Transplant. Res. 2013, 2, 15. [Google Scholar] [CrossRef]
- Vučković, M.; Radić, J.; Gelemanović, A.; Raos, H.; Nenadić, D.B.; Kolak, E.; Radić, M. Mediterranean Diet Adherence and Nutritional Status in Dalmatian Kidney Transplant Recipients—Are They Related? Nutrients 2021, 13, 3246. [Google Scholar] [CrossRef]
- Tanita MC780—User Manual. Available online: https://tanita.eu/media/wysiwyg/manuals/medical-approved-body-composition-monitors/mc-780-portable-instruction-manual.pdf (accessed on 20 June 2018).
- Monteagudo, C.; Mariscal-Arcas, M.; Rivas, A.; Lorenzo-Tovar, M.L.; Tur, J.A.; Olea-Serrano, F. Proposal of a Mediterranean Diet Serving Score. PLoS ONE 2015, 10, e0128594. [Google Scholar] [CrossRef] [PubMed]
- van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: https://www.R-project.org/ (accessed on 3 February 2025).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. Ggpubr: ‘Ggplot2’ Based Publication Ready Plots, R Package Version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 3 February 2025).
- Nosrati-Oskouie, M.; Salavatizadeh, M.; Sabbagh, M.G.; Aghili-Moghaddam, N.S.; Tarighat-Esfanjani, A.; Sahebkar, A. Current Evidence on Dietary Factors and Kidney Allograft Function in Kidney Transplant Recipients: A Systematic Review. Curr. Med. Chem. 2024, 31, 5818–5836. [Google Scholar] [CrossRef] [PubMed]
- Bustos, N.I.; Benítez, J.M.; González-López, A.; López-Pedrera, C.; López-López, J.J.; Barbarroja, N. Polyphenols and Novel Insights Into Post-Kidney Transplant Complications and Cardiovascular Disease: A Narrative Review. Front. Cardiovasc. Med. 2021, 8, 751036. [Google Scholar] [CrossRef] [PubMed]
- Tsofliou, F.; Vlachos, D.; Hughes, C.; Appleton, K.M. Barriers and Facilitators Associated with the Adoption of and Adherence to a Mediterranean Style Diet in Adults: A Systematic Review of Published Observational and Qualitative Studies. Nutrients 2022, 14, 4314. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Pivari, F.; Soldati, L.; Attinà, A.; Cinelli, G.; Leggeri, C.; Caparello, G.; Barrea, L.; Scerbo, F.; et al. Eating Habits and Lifestyle Changes During COVID-19 Lockdown: An Italian Survey. Nutr. Metab. 2020, 17, 1–15. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Wasilewska, E.; Małgorzewicz, S. Dietary Habits Before and During the COVID-19 Epidemic in Selected European Countries. Nutrients 2021, 13, 1690. [Google Scholar] [CrossRef]
- Pfeifer, D.; Rešetar, J.; Gajdoš Kljusurić, J.; Panjkota Krbavčić, I.; Vranešić Bender, D.; Rodríguez-Pérez, C.; Ruíz-López, M.D.; Šatalić, Z. Cooking at Home and Adherence to the Mediterranean Diet During the COVID-19 Confinement: The Experience From the Croatian COVIDiet Study. Front. Nutr. 2021, 8, 617721. [Google Scholar] [CrossRef]
- Dienemann, T.; Ziolkowski, S.L.; Bender, S.; Goral, S.; Long, J.; Baker, J.F.; Shults, J.; Zemel, B.S.; Reese, P.P.; Wilson, F.P.; et al. Changes in Body Composition, Muscle Strength, and Fat Distribution Following Kidney Transplantation. Am. J. Kidney Dis. 2021, 78, 816–825. [Google Scholar] [CrossRef]
- Kosoku, A.; Iwai, T.; Ishihara, T.; Kabei, K.; Nishide, S.; Maeda, K.; Hanayama, Y.; Ishimura, E.; Uchida, J. Influence of Protein Intake on the Changes in Skeletal Muscle Mass After Kidney Transplantation. Clin. Nutr. 2022, 41, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Lima, P.S.; de Campos, A.S.; de Faria Neto, O.; Ferreira, T.C.A.; Amorim, C.E.N.; Stone, W.J. Prest of Combined Resistance Plus Aerobic Training on Body Composition, Muscle Strength, Aerobic Capacity, and Renal Function in Kidney Transplantation Subjects. J. Strength Cond. Res. 2021, 35, 3243–3250. [Google Scholar] [CrossRef]
- Fukuhara, H.; Nishida, H.; Takai, S.; Nawano, T.; Takehara, T.; Takai, Y.; Narisawa, T.; Kanno, H.; Yagi, M.; Yamagishi, A.; et al. Dialysis Duration, Time Interaction, and Visceral Fat Accumulation: A 6-Year Posttransplantation Study. Clin. Exp. Nephrol. 2024, 28, 943–952. [Google Scholar] [CrossRef]
- Marco, E.; Pérez-Sáez, M.J.; Muñoz-Redondo, E.; Curbelo, Y.G.; Ramírez-Fuentes, C.; Meza-Valderrama, D.; Acuña-Pardo, C.; Muns, M.D.; Vázquez-Ibar, O.; Chamoun, B.O.; et al. Phase Angle as Surrogate Marker of Muscle Weakness in Kidney Transplant Candidates Referred to Prehabilitation. Nutrients 2024, 16, 2245. [Google Scholar] [CrossRef]
- Kim, H.J.; Seong, E.Y.; Jung, H.J.; Song, S.H. The Phase Angle Before Transplantation Can Predict the Status of Low Muscle Mass After Kidney Transplantation. Clin. Exp. Nephrol. 2024, 28, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Kaya, E.; Bakir, A.; Koseoglu, Y.K.; Velidedeoglu, M.; Trabulus, S.; Seyahi, N. Association of Nutritional Assessment by Phase Angle With Mortality in Kidney Transplant Patients in an 8-Year Follow-Up. Prog. Transplant. 2019, 29, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Marcén, R.; Morales, J.M.; Fernández-Rodriguez, A.; Capdevila, L.; Pallardó, L.; Plaza, J.J.; Cubero, J.J.; Puig, J.M.; Sanchez-Fructuoso, A.; Arias, M.; et al. Long-Term Graft Function Changes in Kidney Transplant Recipients. NDT Plus 2010, 3 (Suppl. S2), ii2–ii8. [Google Scholar] [CrossRef]
- Devine, P.A.; Courtney, A.E.; Maxwell, A.P. Cardiovascular Risk in Renal Transplant Recipients. J. Nephrol. 2019, 32, 389–399. [Google Scholar] [CrossRef]
| Variable | All (N = 115) |
|---|---|
| Alive, N (%) | 96 (83.5) |
| Death outcome, N (%) | 19 (16.5) |
| Male sex, N (%) | 51 (44.4) |
| Age (years), median (IQR) | 63 (16) |
| pSBP (mmHg), median (IQR) | 140.0 (30.0) |
| pDBP (mmHg), mean (SD) | 82.9 (15.2) |
| Waist circumference (cm), median (IQR) | 99.0 (15.5) |
| Upper arm circumference (cm), median (IQR) | 31.0 (4.5) |
| Height (cm), mean (SD) | 173.8 (10.2) |
| Weight (kg), median (IQR) | 76.7 (21.2) |
| BMI (kg/m2), mean (SD) | 25.9 (4.0) |
| Body Composition | |
| Fat mass (kg), median (IQR) | 19.3 (12.1) |
| Fat mass (%), mean (SD) | 24.6 (9.4) |
| Fat free mass (kg), median (IQR) | 59.5 (19.2) |
| Visceral fat level, median (IQR) | 9.0 (5.0) |
| Muscle mass (kg), median (IQR) | 55.8 (18.3) |
| Skeletal muscle mass (kg), mean (SD) | 30.1 (8.0) |
| Skeletal muscle mass (%), mean (SD) | 39.0 (6.1) |
| Phase angle (°), median (IQR) | 5.0 (1.0) |
| Laboratory Parameters | |
| E (×1012/L), mean (SD) | 4.7 (0.7) |
| Hemoglobin (g/L), mean (SD) | 130.8 (18.2) |
| MCV (fL), median (IQR) | 87.5 (6.4) |
| Urea (mmol/L), median (IQR) | 9.9 (6.6) |
| Creatinine (µmol/L), median (IQR) | 130.0 (58.3) |
| eGFR using CKD-EPI (mL/min/1.73 m2), mean (SD) | 43.85 (24.1) |
| FBG (mmol/L), median (IQR) | 5.3 (1.6) |
| Albumin (g/L), mean (SD) | 42.3 (3.2) |
| CRP (mmol/L), median (IQR) | 2.2 (3.9) |
| Cholesterol (mmol/L), mean (SD) | 5.2 (1.0) |
| LDL (mmol/L), mean (SD) | 2.9 (0.9) |
| Triglycerides (mmol/L), median (IQR) | 1.8 (1.2) |
| Sodium (mmol/L), median (IQR) | 141.0 (5.0) |
| Potassium (mmol/L), median (IQR) | 4.2 (0.7) |
| Calcium (mmol/L), median (IQR) | 2.4 (0.2) |
| Phosphate (mmol/L), median (IQR) | 1.0 (0.3) |
| Uric acid (mmol/L), mean (SD) | 384.4 (88.1) |
| Albuminuria (mg/dU), median (IQR) | 65.0 (126.8) |
| New-onset cardiovascular disease, N (%) | 15 (16.3) |
| New-onset malignant disease, N (%) | 8 (8.7) |
| New-onset diabetes, N (%) | 1 (1.1) |
| New-onset disease—graft rejection, N (%) | 11 (12.1) |
| New-onset severe clinical infection, N (%) | 37 (40.2) |
| New-onset cerebrovascular disease, N (%) | 2 (2.17.0) |
| Number of hospitalizations in the follow-up period, median (IQR) | 1 (2) |
| Had COVID-19, N (%) | 37 (47.4) |
| Vaccinated for COVID-19, N (%) | 58 (76.3) |
| MDSS score, median (IQR) | 7 (4) |
| Adherence to the MeDi, N (%) | 5 (6.0) |
| Beta | OR (95% CI) | p | |
|---|---|---|---|
| Egg intake | −1.06 | 0.35 (0.14–0.87) | 0.023 |
| Phase angle (°) | −2.68 | 0.07 (0.01–0.43) | 0.004 |
| Cholesterol (mmol/L) | 0.95 | 2.60 (1.40–4.70) | 0.001 |
| Calcium (mmol/L) | −7.21 | 0.00 (0.00–1.50) | 0.063 |
| Beta | OR (95% CI) | p | |
|---|---|---|---|
| SBP (mmHg) | 0.04 | 1.04 (1.01–1.08) | 0.021 |
| Visceral fat | −0.18 | 0.84 (0.71–0.98) | 0.032 |
| Hemoglobin (g/L) | 0.06 | 1.06 (1.02–1.10) | 0.008 |
| Time since transplantation (years) | 0.16 | 1.17 (1.01–1.35) | 0.034 |
| Beta | OR (95% CI) | p | |
|---|---|---|---|
| Cereal intake | 1.24 | 3.46 (0.93–12.88) | 0.065 |
| FBG (mmol/L) | −0.45 | 0.64 (0.33–1.22) | 0.171 |
| Cholesterol (mmol/L) | 2.71 | 15.03 (1.23–183.95) | 0.034 |
| LDL (mmol/L) | −3.52 | 0.03 (0.00–0.55) | 0.018 |
| Triglycerides (mmol/L) | −0.96 | 0.38 (0.15–0.96) | 0.041 |
| Diabetes mellitus | −1.42 | 0.24 (0.05–1.18) | 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vučković, M.; Radić, J.; Gelemanović, A.; Belančić, A.; Đogaš, H.; Radić, M. Time for Change: A 3-Year Prospective Study on Mediterranean Diet Adherence and Body Composition in Kidney Transplant Recipients. Healthcare 2025, 13, 840. https://doi.org/10.3390/healthcare13070840
Vučković M, Radić J, Gelemanović A, Belančić A, Đogaš H, Radić M. Time for Change: A 3-Year Prospective Study on Mediterranean Diet Adherence and Body Composition in Kidney Transplant Recipients. Healthcare. 2025; 13(7):840. https://doi.org/10.3390/healthcare13070840
Chicago/Turabian StyleVučković, Marijana, Josipa Radić, Andrea Gelemanović, Andrej Belančić, Hana Đogaš, and Mislav Radić. 2025. "Time for Change: A 3-Year Prospective Study on Mediterranean Diet Adherence and Body Composition in Kidney Transplant Recipients" Healthcare 13, no. 7: 840. https://doi.org/10.3390/healthcare13070840
APA StyleVučković, M., Radić, J., Gelemanović, A., Belančić, A., Đogaš, H., & Radić, M. (2025). Time for Change: A 3-Year Prospective Study on Mediterranean Diet Adherence and Body Composition in Kidney Transplant Recipients. Healthcare, 13(7), 840. https://doi.org/10.3390/healthcare13070840








