Impact of the COVID-19 Pandemic on Gut Cancer Admissions and Management: A Comparative Study of Two Pandemic Years to a Similar Pre-Pandemic Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Main Characteristics of the Patients
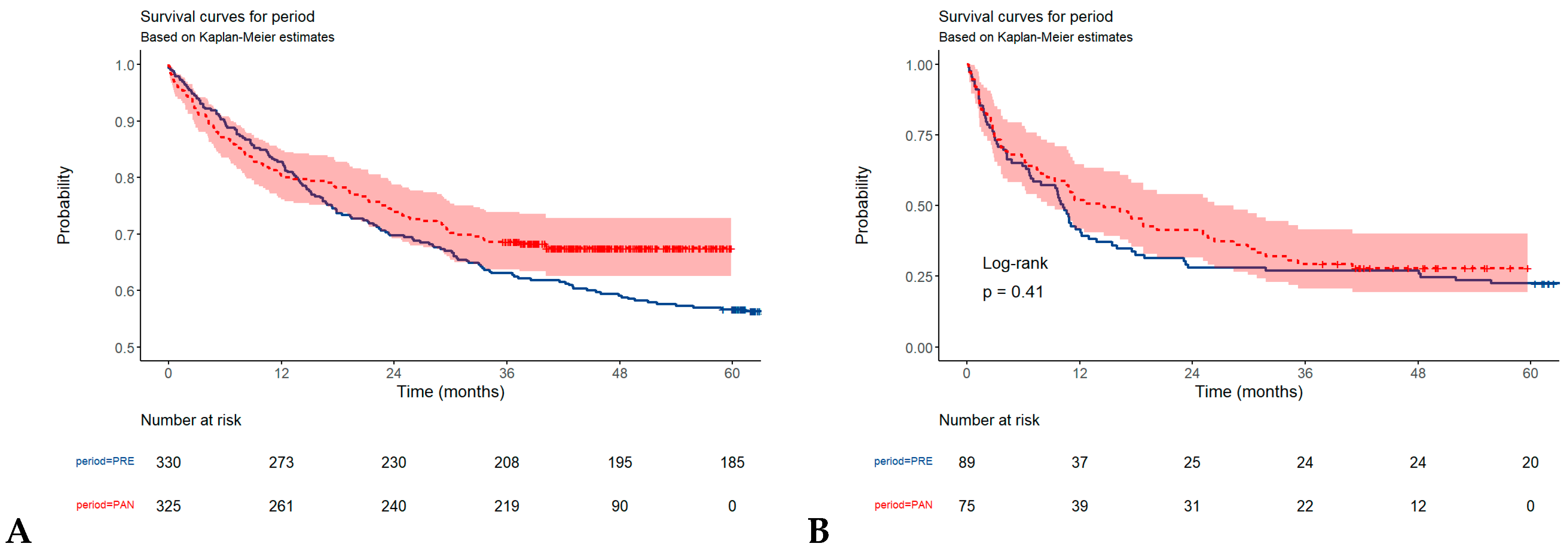
3.2. Overall Survival in Gastric, and Colorectal Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hannah Ritchie, E.M.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; Ortiz-Ospina, E.; Roser, M. Coronavirus Pandemic (COVID-19); Our World in Data: Oxford, UK, 2020; Available online: https://ourworldindata.org/coronavirus (accessed on 10 January 2024).
- Dhama, K.; Nainu, F.; Frediansyah, A.; Yatoo, M.I.; Mohapatra, R.K.; Chakraborty, S.; Zhou, H.; Islam, M.R.; Mamada, S.S.; Kusuma, H.I.; et al. Global emerging Omicron variant of SARS-CoV-2: Impacts, challenges and strategies. J. Infect. Public Health 2023, 16, 4–14. [Google Scholar] [PubMed]
- Bisen, A.C.; Agrawal, S.; Sanap, S.N.; Ravi Kumar, H.G.; Kumar, N.; Gupta, R.; Bhatta, R.S. COVID-19 retreats and world recovers: A silver lining in the dark cloud. Health Care Sci. 2023, 2, 264–285. [Google Scholar] [PubMed]
- Muka, T.; Li, J.J.X.; Farahani, S.J.; Ioannidis, J.P.A. An umbrella review of systematic reviews on the impact of the COVID-19 pandemic on cancer prevention and management, and patient needs. eLife 2023, 12, e85679. [Google Scholar] [CrossRef] [PubMed]
- Pennisi, F.; Odelli, S.; Borlini, S.; Morani, F.; Signorelli, C.; Renzi, C. Impact of the Covid pandemic on timely cancer diagnosis across European healthcare settings: A scoping review. Ann. Ig 2024, 36, 194–214. [Google Scholar]
- Marty, S.; Lamé, G.; Guével, E.; Priou, S.; Chatellier, G.; Tournigand, C.; Kempf, E.; A CRAB* Initiative. Impact of the Sars-Cov-2 outbreak on the initial clinical presentation of new solid cancer diagnoses: A systematic review and meta-analysis. BMC Cancer 2024, 24, 143. [Google Scholar]
- Okuyama, A.; Watabe, M.; Makoshi, R.; Takahashi, H.; Tsukada, Y.; Higashi, T. Impact of the COVID-19 pandemic on the diagnosis of cancer in Japan: Analysis of hospital-based cancer registries. Jpn. J. Clin. Oncol. 2022, 52, 1215–1224. [Google Scholar]
- Roderburg, C.; Loosen, S.H.; Leyh, C.; Joerdens, M.S.; Mohr, R.; Luedde, T.; Alymova, S.; Klein, I.; Kostev, K. Prevalence of and factors associated with a treatment delay due to the COVID-19 pandemic in patients with gastrointestinal cancer in Europe. J. Cancer Res. Clin. Oncol. 2023, 149, 11849–11856. [Google Scholar]
- Saeki, H.; Shirabe, K.; Miyazaki, T.; Ogawa, T.; Makita, F.; Shitara, Y.; Machida, M.; Yasuda, N.; Kato, H.; Ojima, H.; et al. Decreased numbers of gastric, colorectal, lung, and breast cancer surgeries performed in 17 cancer-designated hospitals in Gunma Prefecture of Japan during the COVID-19 pandemic. Surg. Today 2022, 52, 1714–1720. [Google Scholar]
- Mendonça, E.; Silva, D.R.; Fernandes, G.A.; França, E.; Silva, I.L.A.; Curado, M.P. Cancer stage and time from cancer diagnosis to first treatment during the COVID-19 pandemic. Semin. Oncol. 2023, 50, 60–65. [Google Scholar]
- Walker, E.; Fu, Y.; Sadowski, D.C.; Stewart, D.; Tang, P.; Kaposhi, B.; Chappell, H.; Robson, P.; Veldhuyzen van Zanten, S. Delayed Colorectal Cancer Diagnosis during the COVID-19 Pandemic in Alberta: A Framework for Analyzing Barriers to Diagnosis and Generating Evidence to Support Health System Changes Aimed at Reducing Time to Diagnosis. Int. J. Environ. Res. Public Health 2021, 18, 9098. [Google Scholar] [CrossRef]
- Reinacher-Schick, A.; Ebert, M.P.; Piso, P.; Hüppe, D.; Schmitt, J.; Schildmann, J. Effects of the Pandemic on the Care of Patients With Colorectal Cancer. Dtsch. Arztebl. Int. 2023, 120, 545–552. [Google Scholar] [PubMed]
- Kuzuu, K.; Misawa, N.; Ashikari, K.; Kessoku, T.; Kato, S.; Hosono, K.; Yoneda, M.; Nonaka, T.; Matsushima, S.; Komatsu, T.; et al. Gastrointestinal Cancer Stage at Diagnosis Before and During the COVID-19 Pandemic in Japan. JAMA Netw. Open 2021, 4, e2126334. [Google Scholar]
- Mouni, O.; Idrissi, A.; Bouziane, M.; Ahid, S.; Sair, K. Impact of the COVID-19 pandemic on digestive cancer staging, a case series. Ann. Med. Surg. 2022, 82, 104471. [Google Scholar]
- Berrian, J.; Liu, Y.; Ezenwajiaku, N.; Moreno-Aspitia, A.; Holton, S.J.; Toriola, A.T.; Colditz, G.A.; Housten, A.J.; Hall, L.; Fiala, M.A.; et al. Impact of the COVID-19 pandemic on breast, colorectal, lung, and prostate cancer stage at diagnosis according to race. Cancer Med. 2023, 12, 7381–7388. [Google Scholar]
- Braicu, V.; Fulger, L.; Nelluri, A.; Maganti, R.K.; Shetty, U.S.A.; Verdes, G.; Brebu, D.; Dumitru, C.; Toma, A.O.; Rosca, O.; et al. Three-Year Analysis of the Rectal Cancer Care Trajectory after the COVID-19 Pandemic. Diseases 2023, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Caldarella, C.; Cocciolillo, F.; Taralli, S.; Lorusso, M.; Scolozzi, V.; Pizzuto, D.A.; Calcagni, M.L.; Rufini, V.; Guido, D.; Palluzzi, F.; et al. The impact of the COVID-19 pandemic on oncological disease extent at FDG PET/CT staging: The ONCOVIPET study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1623–1629. [Google Scholar]
- Han, X.; Yang, N.N.; Nogueira, L.; Jiang, C.; Wagle, N.S.; Zhao, J.; Shi, K.S.; Fan, Q.; Schafer, E.; Yabroff, K.R.; et al. Changes in cancer diagnoses and stage distribution during the first year of the COVID-19 pandemic in the USA: A cross-sectional nationwide assessment. Lancet Oncol. 2023, 24, 855–867. [Google Scholar]
- Kulle, C.B.; Sengun, B.; Gok, A.F.K.; Ozgur, I.; Bayraktar, A.; Ertekin, C.; Deniz, A.B.; Keskin, M. Clinical outcomes of obstructive colorectal cancer patients during the coronavirus disease 2019 pandemic. Ulus Travma Acil Cerrahi. Derg. 2023, 29, 663–668. [Google Scholar]
- Meijer, J.; Elferink, M.A.G.; van Hoeve, J.C.; Buijsen, J.; van Erning, F.; Nagtegaal, I.D.; Tanis, P.J.; Vink, G.R.; Wumkes, M.L.; de Hingh, I.H.J.T.; et al. Impact of the COVID-19 Pandemic on Colorectal Cancer Care in the Netherlands: A Population-based Study. Clin. Colorectal Cancer 2022, 21, e171–e178. [Google Scholar]
- Mentrasti, G.; Cantini, L.; Zichi, C.; D’Ostilio, N.; Gelsomino, F.; Martinelli, E.; Chiari, R.; La Verde, N.; Bisonni, R.; Cognigni, V.; et al. Alarming Drop in Early Stage Colorectal Cancer Diagnoses After COVID-19 Outbreak: A Real-World Analysis from the Italian COVID-DELAY Study. Oncologist 2022, 27, e723–e730. [Google Scholar]
- Miyo, M.; Mizushima, T.; Nishimura, J.; Hata, T.; Tei, M.; Miyake, Y.; Kagawa, Y.; Noura, S.; Ikenaga, M.; Danno, K.; et al. Impact of the COVID-19 pandemic on colorectal cancer surgery in Japan: Clinical Study Group of Osaka University-A multicenter retrospective study. Ann. Gastroenterol. Surg. 2022, 7, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, R.S.; Cuk, V.V.; Juloski, J.T.; Arbutina, D.D.; Krdžic, I.D.; Milic, L.V.; Kenic, M.V.; Karamarkovic, A.R. Is Colorectal Cancer Stage Affected by COVID-19 Pandemic? Chirurgia 2021, 116, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Rottoli, M.; Gori, A.; Pellino, G.; Flacco, M.E.; Martellucci, C.; Spinelli, A.; Poggioli, G.; COVID–Colorectal Cancer (CRC) Study Group. Colorectal Cancer Stage at Diagnosis Before vs. During the COVID-19 Pandemic in Italy. JAMA Netw. Open 2022, 5, e2243119. [Google Scholar] [CrossRef] [PubMed]
- Shinkwin, M.; Silva, L.; Vogel, I.; Reeves, N.; Cornish, J.; Horwood, J.; Davies, M.M.; Torkington, J.; Ansell, J. COVID-19 and the emergency presentation of colorectal cancer. Colorectal Dis. 2021, 23, 2014–2019. [Google Scholar] [CrossRef]
- Terashima, T.; Konishi, H.; Sato, Y.; Igarashi, M.; Yanagibashi, T.; Konno, R.; Saya, H.; Doki, Y.; Kakizoe, T. Impact of coronavirus disease 2019 on the number of newly diagnosed cancer patients and examinations and surgeries performed for cancer in Japan: A nationwide study. BMC Cancer 2022, 22, 1303. [Google Scholar] [CrossRef]
- Cano-Valderrama, O.; Sánchez-Santos, R.; Vigorita, V.; Paniagua, M.; Flores, E.; Garrido, L.; Facal, C.; Ruano, A.; San-Ildefonso, A.; Moncada, E. Has the COVID-19 pandemic changed the clinical picture and tumour stage at the time of presentation of patients with colorectal cancer? A retrospective cohort study. Cir. Esp. 2023, 101, 90–96. [Google Scholar] [CrossRef]
- Violante, T.; Ferrari, D.; Day, C.N.; Mathis, K.L.; Dozois, E.J.; Larson, D.W. The effect of the pandemic on colorectal cancer in the United States: An increased disease burden. Surg. Oncol. Insight 2024, 1, 100014. [Google Scholar] [CrossRef]
- Abu-Freha, N.; Hizkiya, R.; Abu-Abed, M.; Michael, T.; Jacob, B.M.; Rouvinov, K.; Schwartz, D.; Reshef, A.; Netz, U.; Pinsk, I.; et al. The impact of the COVID-19 pandemic on colorectal and gastric cancer diagnosis, disease stage and mortality. Front. Med. 2022, 9, 954878. [Google Scholar] [CrossRef]
- Iijima, K.; Shimodaira, Y.; Watanabe, K.; Koizumi, S.; Matsuhashi, T.; Jin, M.; Miura, M.; Onochi, K.; Yamai, K.; Fujishima, Y.; et al. A Follow-up Report on the Diagnosis of Gastrointestinal Cancer during the COVID-19 Pandemic in Akita Prefecture, Japan in 2021. Tohoku J. Exp. Med. 2023, 259, 301–306. [Google Scholar] [CrossRef]
- Kanno, D.T.; Mattos, R.L.M.; Siqueira, R.M.; Pereira, J.A.; Campos, F.G.; Martinez, C.A.R. Impact of the COVID-19 pandemic on the emergency surgical treatment of colorectal cancer. Arq. Bras. Cir. Dig. 2024, 36, e1793. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, M.G.; Kang, M.R.; Yang, J.H.; Shin, M.H.; Kweon, S.S. No evidence of delay in colorectal cancer diagnosis during the COVID-19 pandemic in Gwangju and Jeonnam, Korea. Epidemiol. Health 2022, 44, e2022092. [Google Scholar]
- Solaini, L.; Bencivenga, M.; Rosa, F.; D’ignazio, A.; Marino, E.; Ministrini, S.; Sofia, S.; Sacco, M.; Mura, G.; Rausa, E.; et al. Consequences of the COVID-19 pandemic on the diagnosis and treatment of gastric cancer in referral centers in Italy. Tumori 2023, 109, 121–128. [Google Scholar]
- Toes-Zoutendijk, E.; Vink, G.; Nagtegaal, I.D.; Spaander, M.C.W.; Dekker, E.; van Leerdam, M.E.; Siesling, S.; Lansdorp-Vogelaar, I.; Elferink, M.A.G.; COVID and Cancer-NL consortium. Impact of COVID-19 and suspension of colorectal cancer screening on incidence and stage distribution of colorectal cancers in the Netherlands. Eur. J. Cancer 2022, 161, 38–43. [Google Scholar] [PubMed]
- Tran, C.; Cipriano, L.E.; Driman, D.K. Impact of COVID-19-related health care disruptions on pathologic cancer staging during the first pandemic year: A retrospective cohort study from March 2018 to March 2021. CMAJ Open 2023, 11, E475–E484. [Google Scholar] [PubMed]
- Alrahawy, M.; Johnson, C.; Aker, M.; Eltyeb, H.A.; Green, S. Impact of COVID-19 on the Mode of Presentation and Stage at Diagnosis of Colorectal Cancer. Cureus 2022, 14, e32037. [Google Scholar] [PubMed]
- Sutton, T.S.; Hao, S.; Suzuki, M.; Chua, A.; Ciarrocca, A.L.; Honaker, M.D. Rectal cancer presentation during the COVID-19 pandemic: Are decreasing screening rates leading to an increase in acute presentations? PLoS ONE 2023, 18, e0291447. [Google Scholar]
- Brugel, M.; Letrillart, L.; Evrard, C.; Thierry, A.; Tougeron, D.; El Amrani, M.; Piessen, G.; Truant, S.; Turpin, A.; d’Engremont, C.; et al. Impact of the COVID-19 pandemic on disease stage and treatment for patients with pancreatic adenocarcinoma: A French comprehensive multicentre ambispective observational cohort study (CAPANCOVID). Eur. J. Cancer 2022, 166, 8–20. [Google Scholar]
- Tejedor-Tejada, J.; Gómez-Díez, C.; Robles Gaitero, S.; Villar Caamaño, A.; Hermida Pérez, B.; Olmos, J.M.; Álvarez Álvarez, A.; Tojo González, R. Impact of the SARS-CoV-2 pandemic on pancreatic cancer: Diagnosis and short-term survival. Rev. Esp. Enferm. Dig. 2022, 114, 509–510. [Google Scholar]
- Bilican, G.; Özgül, S.; Ekmen, N.; Moral, K.; Küçük, H.; Dumanlı, S.; Abiyev, A.; Karakan, T.; Kekilli, M. Effect of COVID-19 Pandemic on Hepatocellular Carcinoma Diagnosis: Results from a Single Turkey Center Study. J. Gastrointestin. Liver Dis. 2023, 32, 367–370. [Google Scholar]
- Ribaldone, D.G.; Caviglia, G.P.; Gaia, S.; Rolle, E.; Risso, A.; Campion, D.; Brunocilla, P.R.; Saracco, G.M.; Carucci, P. Effect of COVID-19 Pandemic on Hepatocellular Carcinoma Diagnosis: Results from a Tertiary Care Center in North-West Italy. Curr. Oncol. 2022, 29, 1422–1429. [Google Scholar] [CrossRef]
- Urhuț, M.C.; Săndulescu, L.D.; Streba, C.T.; Mămuleanu, M.; Ciocâlteu, A.; Cazacu, S.M.; Dănoiu, S. Diagnostic Performance of an Artificial Intelligence Model Based on Contrast-Enhanced Ultrasound in Patients with Liver Lesions: A Comparative Study with Clinicians. Diagnostics 2023, 13, 3387. [Google Scholar] [CrossRef] [PubMed]
- Ciocalteu, A.; Iordache, S.; Cazacu, S.M.; Urhut, C.M.; Sandulescu, S.M.; Ciurea, A.M.; Saftoiu, A.; Sandulescu, L.D. Role of Contrast-Enhanced Ultrasonography in Hepatocellular Carcinoma by Using LI-RADS and Ancillary Features: A Single Tertiary Centre Experience. Diagnostics 2021, 11, 2232. [Google Scholar] [CrossRef] [PubMed]
- Minordi, L.M.; Binda, C.; Scaldaferri, F.; Holleran, G.; Larosa, L.; Belmonte, G.; Gasbarrini, A.; Colosimo, C.; Manfredi, R. Primary neoplasms of the small bowel at CT: A pictorial essay for the clinician. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 598–608. [Google Scholar]
- Vere, C.C.; Foarfă, C.; Streba, C.T.; Cazacu, S.; Pârvu, D.; Ciurea, T. Videocapsule endoscopy and single balloon enteroscopy: Novel diagnostic techniques in small bowel pathology. Rom. J. Morphol. Embryol. 2009, 50, 467–474. [Google Scholar]
- Tarta, C.; Marian, M.; Capitanio, M.; Faur, F.I.; Duta, C.; Diaconescu, R.; Oprescu-Macovei, A.M.; Totolici, B.; Dobrescu, A. The Challenges of Colorectal Cancer Surgery during the COVID-19 Pandemic in Romania: A Three-Year Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 14320. [Google Scholar] [CrossRef]
- Feier, C.V.I.; Santoro, R.R.; Faur, A.M.; Muntean, C.; Olariu, S. Assessing Changes in Colon Cancer Care during the COVID-19 Pandemic: A Four-Year Analysis at a Romanian University Hospital. J. Clin. Med. 2023, 12, 6558. [Google Scholar] [CrossRef]
- Kiss, B.I.; Sala, T.D.; Török, Á.; Dénes, M.I.; Borz, C.O.; Popescu, G.A.; Jr, T.B.; Muresan, M.G.; Darie, R.; Daniealopol, V.; et al. The Impact of the COVID-19 Pandemic on Colorectal Cancer Patients in a Tertiary Center in Romania. Single Center Retrospective Study. Chirurgia 2022, 117, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Aabed, H.; Bloanca, V.; Crainiceanu, Z.; Bratosin, F.; Citu, C.; Diaconu, M.M.; Ciorica, O.; Bratu, T. The Impact of SARS-CoV-2 Pandemic on Patients with Malignant Melanoma at a Romanian Academic Center: A Four-Year Retrospective Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8499. [Google Scholar] [CrossRef]
- Ungureanu, L.; Apostu, A.P.; Vesa, Ș.C.; Cășeriu, A.E.; Frățilă, S.; Iancu, G.; Bejinariu, N.; Munteanu, M.; Șenilă, S.C.; Vasilovici, A. Impact of the COVID-19 Pandemic on Melanoma Diagnosis in Romania-Data from Two University Centers. Int. J. Environ. Res. Public Health 2022, 19, 15129. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Rice, T.W.; Patil, D.T.; Blackstone, E.H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 2017, 6, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zheng, C.H.; Cao, L.L.; Li, P.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Lin, M.; Huang, C.M. The effectiveness of the 8th American Joint Committee on Cancer TNM classification in the prognosis evaluation of gastric cancer patients: A comparative study between the 7th and 8th editions. Eur. J. Surg. Oncol. 2017, 43, 2349–2356. [Google Scholar]
- López Sala, P.; Leturia Etxeberria, M.; Inchausti Iguíñiz, E.; Astiazaran Rodríguez, A.; Aguirre Oteiza, M.I.; Zubizarreta Etxaniz, M. Gastric adenocarcinoma: A review of the TNM classification system and ways of spreading. Radiologia 2023, 65, 66–80. [Google Scholar] [PubMed]
- Tong, G.J.; Zhang, G.Y.; Liu, J.; Zheng, Z.Z.; Chen, Y.; Niu, P.P.; Xu, X.T. Comparison of the eighth version of the American Joint Committee on Cancer manual to the seventh version for colorectal cancer: A retrospective review of our data. World J. Clin. Oncol. 2018, 9, 148–161. [Google Scholar]
- Therneau, T.M.; Grambsch, P.M. Modeling Survival Data: Extending the Cox Model; Springer: New York, NY, USA, 2020. [Google Scholar]
- Therneau, T.M. A Package for Survival Analysis in R. Available online: https://cran.r-project.org/package=survival (accessed on 11 December 2024).
- Perkons, N.R.; Kim, C.; Boedec, C.; Keele, L.J.; Schneider, C.; Teitelbaum, U.R.; Ben-Josef, E.; Gabriel, P.E.; Plastaras, J.P.; Shulman, L.N.; et al. Quantifying the impact of the COVID-19 pandemic on gastrointestinal cancer care delivery. Cancer Rep. 2022, 5, e1427. [Google Scholar] [CrossRef] [PubMed]
- Englum, B.R.; Prasad, N.K.; Lake, R.E.; Mayorga-Carlin, M.; Turner, D.J.; Siddiqui, T.; Sorkin, J.D.; Lal, B.K. Impact of the COVID-19 pandemic on diagnosis of new cancers: A national multicenter study of the Veterans Affairs Healthcare System. Cancer 2022, 128, 1048–1056. [Google Scholar]
- Ramanakumar, A.V.; Annie, B.; Frederic, L.; Christine, B.; Cathy, R.; Jean, L. Evaluating the impact of COVID-19 on cancer declarations in Quebec, Canada. Cancer Med. 2023, 12, 6260–6269. [Google Scholar]
- Kinslow, C.J.; DeStephano, D.M.; Neugut, A.I.; Taparra, K.; Horowitz, D.P.; Yu, J.B.; Cheng, S.K. Site-specific patterns of early-stage cancer diagnosis during the COVID-19 pandemic. JNCI Cancer. Spectr. 2024, 8, pkae022. [Google Scholar]
- Elamin, D.; Ozgur, I.; Steele, S.R.; Khorana, A.A.; Jia, X.; Gorgun, E. Impact of COVID-19 pandemic on treatment of colorectal cancer patients. Am. J. Surg. 2023, 225, 934–936. [Google Scholar]
- Minamimoto, R.; Hotta, M.; Okafuji, T.; Tsutui, S.; Tsukuda, M.; Nakayama, H.; Shida, Y.; Tajima, T. Change in cancer diagnosis during the COVID-19 pandemic: Trends estimated from FDG-PET/CT. Glob. Health Med. 2022, 4, 108–115. [Google Scholar] [CrossRef]
- Kodama, M.; Miyamori, D.; Kanno, K.; Ito, M. The impact of early-stage COVID-19 pandemic on the diagnosis and treatment of gastric cancer: A cross-sectional study using a large-scale cancer registry in Hiroshima, Japan. DEN Open 2022, 3, e180. [Google Scholar] [CrossRef] [PubMed]
- Haribhai, S.; Bhatia, K.; Shahmanesh, M. Global elective breast- and colorectal cancer surgery performance backlogs, attributable mortality and implemented health system responses during the COVID-19 pandemic: A scoping review. PLoS Glob. Public Health 2023, 3, e0001413. [Google Scholar]
- Ng, G.H.; Philip Ding, H.L.; Leow, Y.C.; Umasangar, R.; Ang, C.W. COVID-19 pandemic and its impact on emergency surgery in colorectal cancer: A single centre experience. Med. J. Malaysia 2023, 78, 32–34. [Google Scholar] [PubMed]
- Parisi, A.; Giampieri, R.; Villani, S.; Magnarini, A.; Gelsomino, F.; Traisci, D.; Barbin, F.; Salvatore, L.; Zichi, C.; Di Pietro, F.R.; et al. Changes in clinical presentation, management, and survival outcomes in patients affected by colorectal cancer following COVID-19 pandemic. Oncologist 2024, oyae310. [Google Scholar]
- Miyamori, D.; Kamitani, T.; Yoshida, S.; Shigenobu, Y.; Ikeda, K.; Kikuchi, Y.; Kashima, S.; Yamamoto, Y. Impact of the COVID-19 pandemic on the mortality among patients with colorectal cancer in Hiroshima, Japan: A large cancer registry study. Cancer Med. 2023, 12, 20554–20563. [Google Scholar] [PubMed]
- Guven, D.C.; Sahin, T.K.; Yildirim, H.C.; Cesmeci, E.; Incesu, F.G.G.; Tahillioglu, Y.; Ucgul, E.; Aksun, M.S.; Gurbuz, S.C.; Aktepe, O.H.; et al. Newly diagnosed cancer and the COVID-19 pandemic: Tumour stage migration and higher early mortality. BMJ Support Palliat. Care 2021, 14, e456–e461. [Google Scholar]
- Peacock, H.M.; Van Meensel, M.; Van Gool, B.; Silversmit, G.; Dekoninck, K.; Brierley, J.D.; Van Eycken, L. Cancer incidence, stage shift and survival during the 2020 COVID-19 pandemic: A population-based study in Belgium. Int. J. Cancer 2024, 155, 1212–1224. [Google Scholar]
- Barclay, N.L.; Burkard, T.; Burn, E.; Delmestri, A.; Miquel Dominguez, A.; Golozar, A.; Guarner-Argente, C.; Avilés-Jurado, F.X.; Man, W.Y.; Roselló Serrano, À.; et al. The Impact of the COVID-19 Pandemic on Incidence and Short-Term Survival for Common Solid Tumours in the United Kingdom: A Cohort Analysis. Clin. Epidemiol. 2024, 16, 417–429. [Google Scholar]
- García-Alfonso, P.; Jimenez-Fonseca, P.; Soto-Alsar, J.; Baraibar, I.; Santos, C.; La Casta, A.; Ghanem, I.; Pulido Cortijo, G.; Mariño Méndez, A.; Pazo-Cid, R.; et al. Three-year survival follow-up of patients with gastrointestinal cancer treated during the COVID-19 pandemic in Spain: Data from the PANDORA-TTD20 study. Oncologist 2024, oyae300. [Google Scholar]
- Upadhaya, S.; Yu, J.X.; Hodge, J.; Campbell, J. COVID-19 impact on oncology clinical trials: A 1-year analysis. Nat. Rev. Drug Discov. 2021, 20, 415. [Google Scholar]
- Dhada, S.; Stewart, D.; Cheema, E.; Hadi, M.A.; Paudyal, V. Cancer Services During the COVID-19 Pandemic: Systematic Review of Patient’s and Caregiver’s Experiences. Cancer Manag. Res. 2021, 13, 5875–5887. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, K.; Wu, Y.; Chakraborty, S.; Elhusseiny, G.; Gondhowiardjo, S.; Joseph, N.; Lee, A.W.M.; Loong, H.H.; Msadabwe-Chikuni, S.C.; Tan, B.F.; et al. Global Health System Resilience during Encounters with Stressors—Lessons Learnt from Cancer Services during the COVID-19 Pandemic. Clin. Oncol. 2023, 35, e289–e300. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Leung, J.L.; Romatoski, K.S.; Rasic, G.; Kobzeva-Herzog, A.J.; Tseng, J.F.; Kenzik, K.; Sachs, T.E. The COVID-19 Pandemic and Delays in Melanoma Treatment: A National Cancer Database Study. J. Surg. Res. 2025, 308, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Duma, N.; Lopes, G. COVID-19 and Global Oncology: A Year in Review. JCO Glob. Oncol. 2021, 7, 797–801. [Google Scholar] [CrossRef]


| PANDEMIC −46 pts- | PRE-PANDEMIC −66 pts- | p-Value | |
|---|---|---|---|
| -Age (mean ± stdev, Min–Max) | 61.8 ± 7.8 (35–77) | 61.8 ± 11.1 (32–86) | 0.9970 |
| -Gender Male/Female (%Male) | 35/11 (76.1) | 60/6 (90.9) | 0.0374 |
| -pathological type | |||
| Carcinoma | |||
| -adenocarcinoma | 7 (20.6) | 11 (20.7) | 0.9851 |
| -squamous | 27 (79.4) | 42 (79.3) | |
| -carcinoma NOS | 12 | 13 | |
| GIST | 1 | 2 | |
| Sarcoma | 1 | 0 | |
| -grading (carcinoma) | |||
| G1 | 0 (0) | 5 (12.2) | 0.1551 |
| G2 | 8 (32) | 12 (29.3) | 0.9144 |
| G3 | 15 (60) | 23 (56.1) | 0.8055 |
| G4 | 2 (8) | 1 (2.4) | 0.3824 |
| -staging (carcinoma) | |||
| I | 0 (0) | 2 (3.6) | 0.4114 |
| II | 4 (10) | 10 (18.2) | 0.3152 |
| III | 18 (45) | 21 (38.2) | 0.4249 |
| IV | 18 (45) | 22 (40) | 0.5291 |
| NA | 6 | 11 | 0.5999 |
| -T stage (carcinoma) | |||
| T1 | 0 (0) | 2 (4.8) | 0.4114 |
| T2 | 2 (5.6) | 4 (9.5) | 0.6934 |
| T3 | 17 (47.2) | 12 (57.1) | 0.0281 |
| T4 | 17 (47.2) | 24 (28.6) | 0.9489 |
| Tx | 10 | 24 | 0.1008 |
| -N stage (carcinoma) | |||
| N0 | 13 (35.1) | 17 (34) | 0.9123 |
| N+ | 24 (64.9) | 33 (66) | |
| Nx | 9 | 16 | 0.5593 |
| -M stage (carcinoma) | |||
| M0 | 24 (60) | 33 (60) | 1.0000 |
| M1 | 16 (40) | 22 (40) | |
| Mx | 6 | 11 | 0.5999 |
| -Location | |||
| Superior | 17 (42.5) | 20 (32.8) | 0.4620 |
| Middle | 10 (25) | 22 (36.1) | 0.1842 |
| Inferior | 5 (12.5) | 8 (13.1) | 0.8389 |
| Eso-cardial | 8 (20) | 11 (18) | 0.9199 |
| Unknown | 6 | 5 | 0.3441 |
| -Borrmann type | |||
| I | 10 (35.7) | 12 (26.1) | 0.6415 |
| II | 13 (46.4) | 31 (67.4) | 0.0481 |
| III | 0 (0) | 1 (2.2) | 0.6552 |
| IV | 5 (17.9) | 2 (4.3) | 0.1135 |
| Unknown | 20 | 20 | 0.1540 |
| -CT scan No (%) | 36 (76.6) | 42 (60) | 0.1008 |
| Visible tumor | 33 (91.7) | 32 (76.2) | 0.0792 |
| -Surgery | |||
| Radical | 2 (4.3) | 5 (7.6) | 0.4928 |
| Palliative gastrostomy | 18 (39.1) | 12 (18.2) | 0.0156 |
| -Esophageal stent | 4 (8.7) | 3 (4.5) | 0.3960 |
| -Complications | |||
| Stenosis | 35 (76.1) | 37 (56.1) | 0.0317 |
| Bleeding | 3 (6.5) | 4 (6.1) | 0.9210 |
| Perforation | 1 (2.2) | 0 (0) | 0.3687 |
| PANDEMIC −176 pts- | PRE-PANDEMIC −243 pts- | p-Value | |
|---|---|---|---|
| -Age (mean ± stdev, Min–Max) | 67.2 ± 12.4 (26–88) | 67.2 ± 11.7 (35–92) | 0.9709 |
| -Gender Male/Female (%Male) | 107/69 (60.8) | 153/90 (63) | 0.6518 |
| -pathological type | |||
| Carcinoma No (%) | |||
| -adenocarcinoma | 97 (91.5) | 126 (88.1) | 0.7891 |
| -adenosquamous | 1 | 1 | |
| -poorly cohesive | 25 | 31 | |
| -mixed | 14 | 15 | |
| -carcinoma NOS | 24 | 41 | |
| Lymphoma | 5 | 10 | |
| Neuroendocrine | 1 | 3 | |
| GIST | 7 | 16 | |
| Sarcoma | 2 | 0 | |
| -grading (carcinoma) | |||
| G1 | 10 (10.5) | 1 (0.8) | 0.0233 |
| G2 | 34 (35.8) | 48 (40) | 0.0411 |
| G3 | 34 (35.8) | 28 (23.3) | 0.3538 |
| G4 | 2 (2.1) | 5 (4.2) | 0.2640 |
| -staging (carcinoma) | |||
| I | 7 (4.4) | 7 (4.1) | 0.8982 |
| II | 3 (1.9) | 9 (5.3) | 0.1190 |
| III | 64 (40.3) | 53 (31.2) | 0.0300 |
| IV | 85 (53.5) | 101 (59.4) | 0.3365 |
| Unknown | 8 | 18 | 0.0901 |
| -T stage (carcinoma) | |||
| T1 | 3 (2.34) | 0 (0) | 0.2721 |
| T2 | 10 (7.81) | 16 (10.8) | 0.1381 |
| T3 | 63 (49.22) | 57 (38.5) | 0.5746 |
| T4 | 56 (43.75) | 47 (31.8) | 0.5994 |
| Tx | 44 | 78 | 0.0032 |
| -N stage (carcinoma) | |||
| N0 | 41 (30.1) | 33 (26.6) | 0.3053 |
| N1 | 33 (24.3) | 32 (25.8) | 0.7743 |
| N2 | 28 (20.6) | 28 (22.6) | 0.6964 |
| N3 | 34 (25) | 31 (25) | 1.0000 |
| N+ | 95 (69.9) | 91 (73.4) | 0.5284 |
| Nx | 40 | 74 | |
| -M stage (carcinoma) | |||
| M0 | 76 (48.4) | 76 (37.6) | 0.3166 |
| M1 | 81 (51.6) | 101 (50) | |
| Mx | 12 | 17 | 0.5718 |
| -Lymphatic/vascular/perineural ((carcinoma) | |||
| L1 | 20 (36.4) | 21 (48.8) | 0.2155 |
| V1 | 21 (38.2) | 29 (63) | 0.0138 |
| PN1 | 33 (60) | 27 (56.3) | 0.7003 |
| -Location | |||
| Antro-pyloric | 54 (34.4) | 48 (25.0) | 0.3623 |
| Corporeal | 71 (45.2) | 74 (38.5) | 0.9555 |
| Cardial | 40 (25.5) | 51 (26.6) | 0.2506 |
| Stump | 7 (4.5) | 5 (2.6) | 0.5193 |
| -Borrmann type | |||
| I | 19 (16.8) | 16 (10.7) | 0.2726 |
| II | 58 (51.3) | 74 (49.3) | 0.6388 |
| III | 30 (26.5) | 34 (22.7) | 0.8180 |
| IV | 18 (15.9) | 25 (16.7) | 0.5901 |
| -CT scan | 110 (62.5) | 123 (50.6) | 0.0160 |
| Visible tumor | 76 (69.1) | 70 (56.9) | 0.0559 |
| -Complications | |||
| Bleeding | 37 (23) | 46 (21.50) | 0.5959 |
| Stenosis | 32 (19.9) | 33 (15.42) | 0.2005 |
| Perforation | 2 (1.2) | 4 (1.87) | 0.6665 |
| -Surgery (%) | 96 (54.6) | 122 (50.2) | 0.3803 |
| Radical | 68 (38.6) | 85 (35) | 0.4430 |
| Palliative gastrectomy | 2 (1.1) | 4 (1.6) | 0.6665 |
| Complications | 20 (11.4) | 19 (7.8) | 0.2202 |
| Laparotomy | 6 (3.4) | 14 (5.8) | 0.2703 |
| -Perioperative mortality (%) | 12/96 (12.5) | 13/130 (10.7) | 0.5358 |
| -Invaded margins | 14/60 (23.3) | 26/77 (33.8) | 0.1847 |
| -Emergency surgery | 31/96 (32.3) | 32/122 (26.2) | 0.3276 |
| PANDEMIC −479 pts- | PRE-PANDEMIC −579 pts- | p-Value | |
|---|---|---|---|
| -Age (mean ± stdev, Min–Max) | 67.9 ± 10.4 (29–91) | 66.3 ± 10.6 (23–88) | 0.0141 |
| -Gender M/F (%M) | 286/193 (59.7) | 349/230 (60.3) | 0.8509 |
| -pathological type | |||
| Carcinoma No (%) | 476 (99.4) | 575 (99.3) | 0.8175 |
| -adenocarcinoma | 447 (93.3) | 558 (96.4) | |
| -adenosquamous | 3 (0.6) | 1 (0.2) | |
| -mucinous | 0 (0) | 2 (0.4) | |
| -mixed | 21 (4.4) | 77 (13.3) | |
| -carcinoma NOS | 29 (6) | 18 (3.1) | |
| Lymphoma | 0 (0) | 0 (0) | |
| Neuroendocrine | 1 (0.2) | 3 (0.5) | |
| Sarcoma | 0 (0) | 1 (0.2) | |
| GIST | 2 (0.4) | 0 (0) | |
| -grading (carcinoma) | |||
| G1 | 51 (13.8) | 45 (10.0) | 0.0904 |
| G2 | 240 (65.0) | 298 (65.9) | 0.7495 |
| G3 | 70 (19.0) | 99 (21.9) | 0.2927 |
| G4 | 7 (1.9) | 10 (2.2) | 0.7483 |
| -staging (carcinoma) | |||
| 0 | 4 (0.9) | 1 (0.2) | 0.1615 |
| I | 55 (12.1) | 64 (11.8) | 0.8937 |
| II | 118 (25.9) | 122 (22.5) | 0.0451 |
| III | 155 (34.0) | 193 (35.5) | 0.6081 |
| IV | 124 (27.2) | 163 (30) | 0.3257 |
| Unknown | 23 (4.8) | 36 (6.2) | 0.3190 |
| -T stage (carcinoma) | |||
| Tis | 1 (0.2) | 0 (0) | 0.4679 |
| T0 | 3 (0.7) | 1 (0.2) | 0.3039 |
| T1 | 18 (4) | 15 (3.1) | 0.4350 |
| T2 | 71 (15.9) | 84 (17.2) | 0.5755 |
| T3 | 232 (51.9) | 253 (51.8) | 0.9880 |
| T4 | 122 (27.3) | 134 (27.5) | 0.9393 |
| Tx | 32 (6.7) | 92 (15.9) | <0.0001 |
| -N stage (carcinoma) | |||
| N0 | 211 (50.2) | 231 (48) | 0.5270 |
| N1 | 138 (32.9) | 162 (33.7) | 0.7768 |
| N2 | 70 (16.7) | 86 (17.9) | 0.6212 |
| N3 | 1 (0.2) | 1 (0.2) | 0.9247 |
| N+ | 209 (48.8) | 249 (49.5) | 0.5270 |
| Nx | 59 (12.3) | 99 (17.1) | 0.0305 |
| -M stage (carcinoma) | |||
| M0 | 338 (73.5) | 376 (69) | 0.1291 |
| M1 | 122 (26.5) | 168 (30.8) | |
| Mx | 19 (4.1) | 35 (6.4) | 0.4990 |
| -Lymphatic/vascular/perineural ((carcinoma) | |||
| L1 | 25 (7.9) | 17 (4.8) | 0.0931 |
| V1 | 51 (16.2) | 91 (25.5) | 0.0034 |
| PN1 | 76 (24.1) | 83 (23.2) | 0.7894 |
| -Location | |||
| Rectum | 176(37.1) | 222 (38.6) | 0.6235 |
| Sigmoidum | 135 (28.5) | 158 (27.5) | 0.7187 |
| Descending | 49 (10.3) | 40 (7) | 0.0519 |
| Transverse | 23 (4.9) | 40 (7) | 0.1555 |
| Ascending | 61 (12.9) | 75 (13) | 0.9334 |
| Caecum | 30 (6.3) | 40 (7) | 0.6854 |
| Not Defined | 5 | 4 | 0.5366 |
| -Borrmann type | |||
| I | 104 (28.7) | 85 (44.3) | 0.0003 |
| II | 196 (54.1) | 94 (49) | 0.2452 |
| III | 36 (9.9) | 3 (1.6) | 0.0014 |
| IV | 26 (7.2) | 10 (5.2) | 0.3717 |
| Not Defined | 117 | 387 | <0.0001 |
| -CT scan | 236 (49.3) | 268 (46.3) | 0.3337 |
| Visible tumor | 196 (83.4) | 189 (72.7) | 0.0011 |
| -Complications No (%) | 249 (52.0) | 248 (42.8) | 0.0030 |
| Bleeding | 75 (15.7) | 104 (18) | 0.3200 |
| Stenosis | 96 (20) | 37 (6.4) | <0.0001 |
| Occlusion | 58 (12.1) | 70 (12.1) | 0.9926 |
| Perforation | 18 (3.8) | 23 (4) | 0.8572 |
| Fistula | 1 (0.2) | 9 (1.6) | 0.5556 |
| Abscess | 0 (0) | 4 (0.7) | 0.1769 |
| -Surgery/endoscopic resection | |||
| Polypectomy | 9 (1.9) | 1 (0.2) | 0.0173 |
| Radical | 312 (65.1) | 378 (65.2) | 0.3555 |
| Palliative | 51 (10.6) | 50 (8.6) | 0.1397 |
| Laparotomy | 0 (0) | 1 (0.2) | 0.6382 |
| Biopsy | 2 (0.4) | 3 (0.5) | 0.9344 |
| No | 105 (21.9) | 146 (25.2) | 0.2101 |
| -Perioperative mortality | 34/365 (9.3) | 23/432 (5.3) | 0.0189 |
| -Invaded margins | 13/299 (4.4) | 13/353 (3.7) | 0.6657 |
| -Emergency surgery | 128/363 (35.3) | 134/428 (31.3) | 0.2393 |
| Factor | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| Hazard Ratio (95%CI) | p-Value | Hazard Ratio (95%CI) | p-Value | |
| Age (years) ≥ 60 vs. <60 | 1.99 (1.39–2.84) | <0.001 | 1.95 (1.36–2.80) | <0.001 |
| Gender, Female vs. Male | 0.85 (0.65–1.09) | 0.201 | 0.72 (0.56–0.93) | 0.014 |
| TNM stage, III + IV vs. I + II | 3.80 (2.81–5.13) | <0.001 | 1.84 (1.19–2.86) | 0.006 |
| Surgery, radical vs. palliative | 0.43 (0.33–0.56) | <0.001 | 0.48 (0.37–0.63) | <0.001 |
| Occlusion, yes vs. no | 1.07 (0.74–1.55) | 0.711 | 1.12 (0.77–1.63) | 0.551 |
| Perforation, yes vs. no | 1.88 (1.17–3.04) | 0.01 | 2.13 (1.30–3.49) | 0.003 |
| Tumor, T3 + T4 vs. T1 + T2 | 2.42 (1.67–3.50) | <0.001 | 1.53 (1.02–2.31) | 0.041 |
| Lymph Nodes, N+ vs. N0 | 2.23 (1.73–2.88) | <0.001 | 1.24 (0.9–1.72) | 0.182 |
| Metastases, M1 vs. M0 | 4.34 (3.36–5.61) | <0.001 | 2.71 (2.02–3.63) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cazacu, S.M.; Rogoveanu, I.; Turcu-Stiolica, A.; Vieru, A.M.; Gabroveanu, A.; Popa, P.; Pirscoveanu, M.; Cartu, D.; Streba, L. Impact of the COVID-19 Pandemic on Gut Cancer Admissions and Management: A Comparative Study of Two Pandemic Years to a Similar Pre-Pandemic Period. Healthcare 2025, 13, 805. https://doi.org/10.3390/healthcare13070805
Cazacu SM, Rogoveanu I, Turcu-Stiolica A, Vieru AM, Gabroveanu A, Popa P, Pirscoveanu M, Cartu D, Streba L. Impact of the COVID-19 Pandemic on Gut Cancer Admissions and Management: A Comparative Study of Two Pandemic Years to a Similar Pre-Pandemic Period. Healthcare. 2025; 13(7):805. https://doi.org/10.3390/healthcare13070805
Chicago/Turabian StyleCazacu, Sergiu Marian, Ion Rogoveanu, Adina Turcu-Stiolica, Alexandru Marian Vieru, Anca Gabroveanu, Petrică Popa, Mircea Pirscoveanu, Dan Cartu, and Liliana Streba. 2025. "Impact of the COVID-19 Pandemic on Gut Cancer Admissions and Management: A Comparative Study of Two Pandemic Years to a Similar Pre-Pandemic Period" Healthcare 13, no. 7: 805. https://doi.org/10.3390/healthcare13070805
APA StyleCazacu, S. M., Rogoveanu, I., Turcu-Stiolica, A., Vieru, A. M., Gabroveanu, A., Popa, P., Pirscoveanu, M., Cartu, D., & Streba, L. (2025). Impact of the COVID-19 Pandemic on Gut Cancer Admissions and Management: A Comparative Study of Two Pandemic Years to a Similar Pre-Pandemic Period. Healthcare, 13(7), 805. https://doi.org/10.3390/healthcare13070805







