Breaking the Habit: A Systematic Review and Meta-Analysis of Pregnancy-Related Smoking Cessation Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Searching Strategy
2.2. Selection Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Quality Assessment
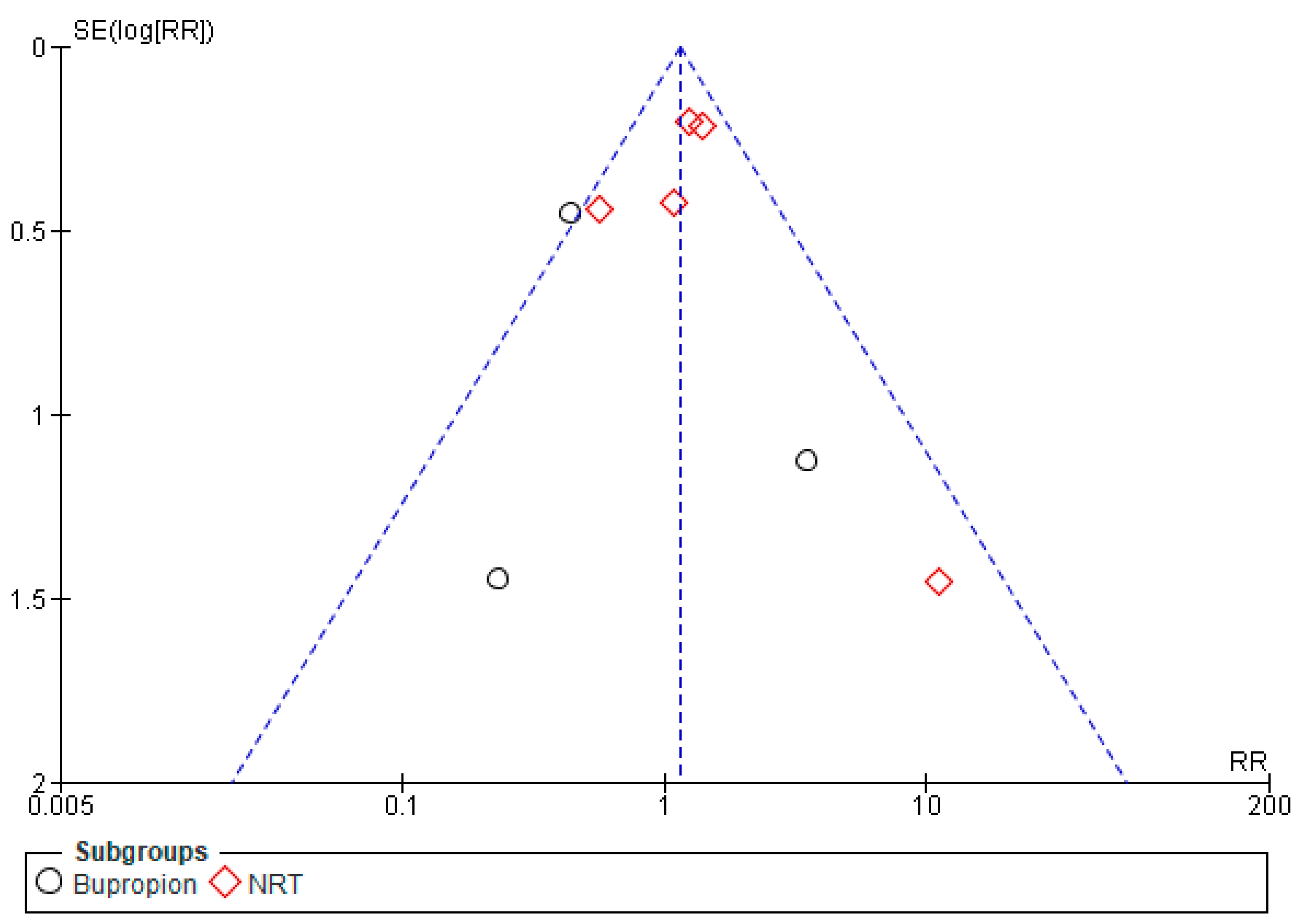
2.4. Risk of Bias
2.5. Statistical Analysis
3. Results
3.1. Features of the Research Involved
3.2. Pharmacologic Interventions
3.3. Behavioral Interventions
3.4. Pregnancy Outcomes
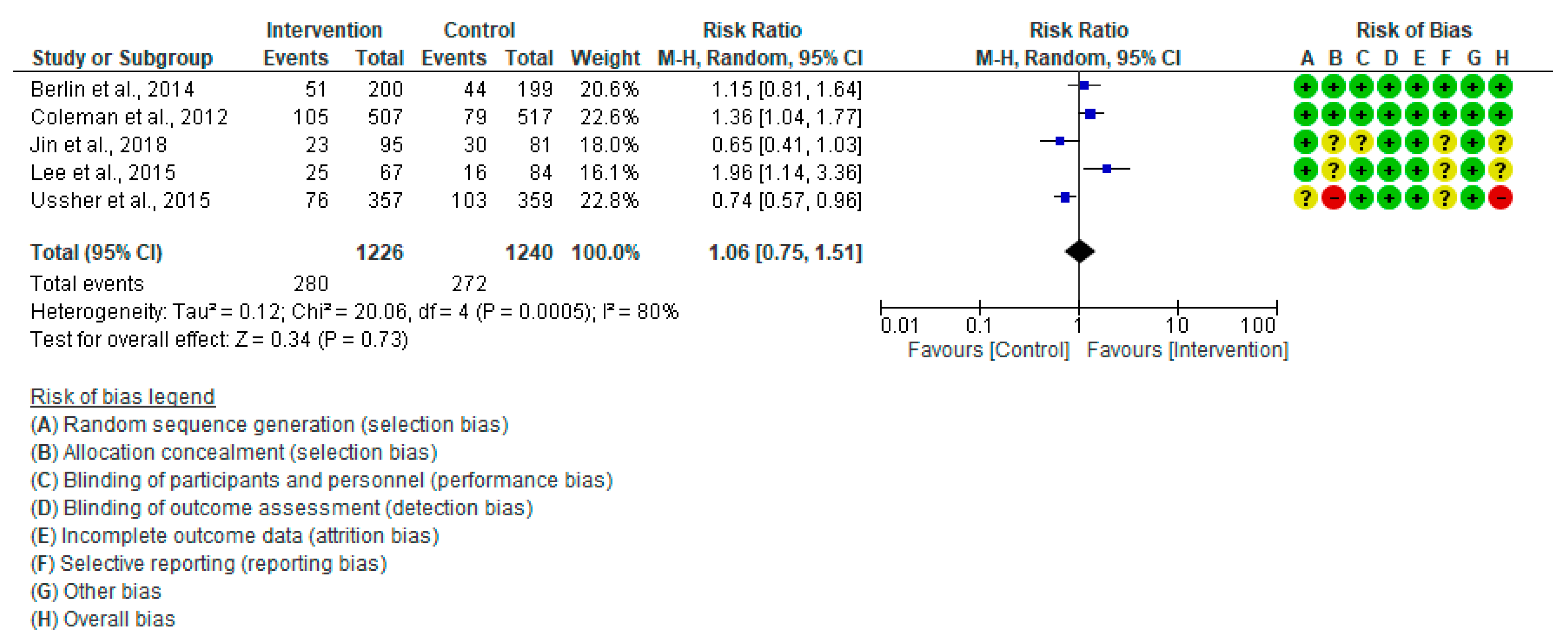
3.4.1. Cesarean Section (CS)
3.4.2. Birth Weight (gm)
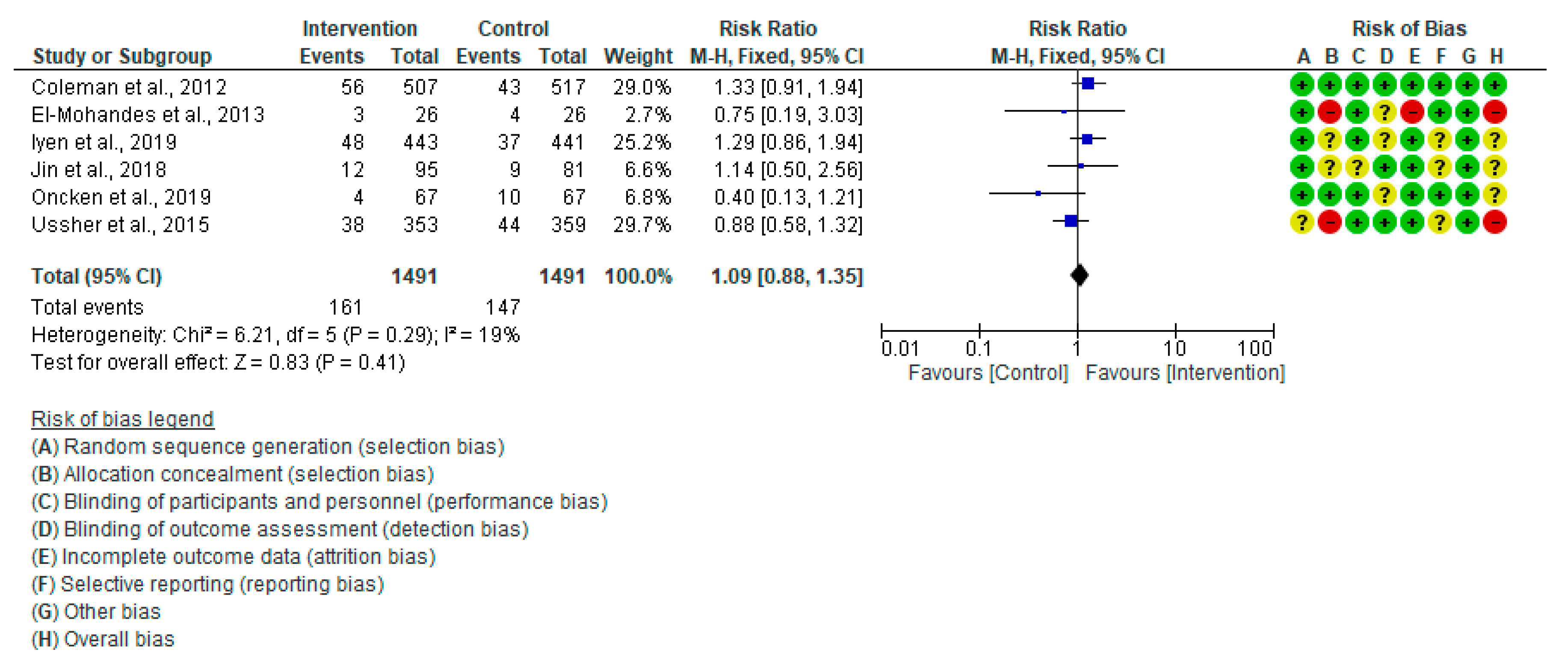
3.4.3. Low Birth Weight (<2500 gm)
3.4.4. Apgar Score < 7 (5 Min)
3.4.5. Preterm Birth
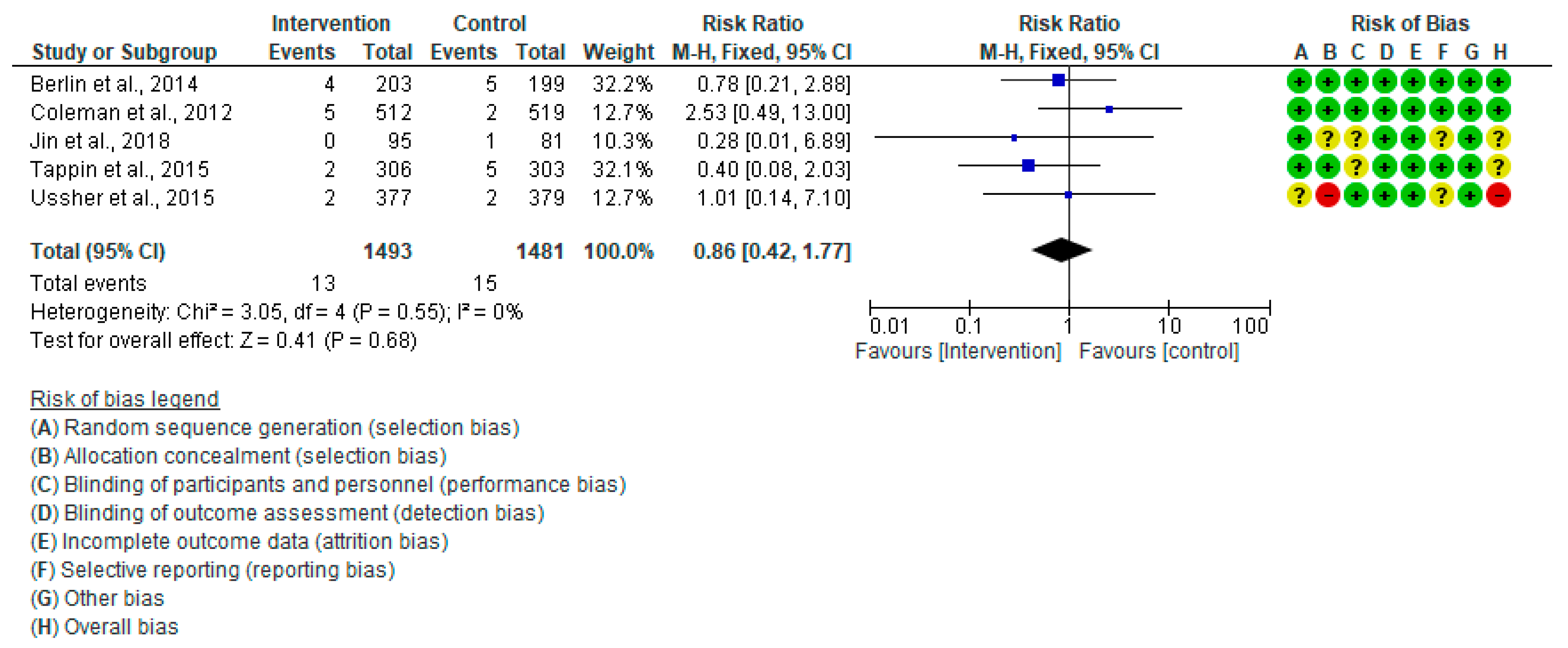
3.4.6. Stillbirth
4. Discussion
4.1. Pharmacological Interventions
4.2. Behavioral Interventions
4.3. Pregnancy Outcomes
4.4. Strengths and Limitations
4.5. Practical Implication
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Siddiqi, K.; Husain, S.; Vidyasagaran, A.; Readshaw, A.; Mishu, M.P.; Sheikh, A. Global burden of disease due to smokeless tobacco consumption in adults: An updated analysis of data from 127 countries. BMC Med. 2020, 18, 222. [Google Scholar] [CrossRef]
- Homa, D.M.; Neff, L.J.; King, B.A.; Caraballo, R.S.; Bunnell, R.E.; Babb, S.D.; Garrett, B.E.; Sosnoff, C.S.; Wang, L. Centers for Disease Control and Prevention (CDC). Vital signs: Disparities in nonsmokers’ exposure to secondhand smoke—United States, 1999–2012. MMWR Morb Mortal Wkly Rep. 2015, 64, 103–108. [Google Scholar]
- Pineles, B.L.; Hsu, S.; Park, E.; Samet, J.M. Systematic Review and Meta-Analyses of Perinatal Death and Maternal Exposure to Tobacco Smoke During Pregnancy. Am. J. Epidemiol. 2016, 184, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Rodriguez, B.; Gomez, A.R.; Jimenez Moreno, B.S.; de Alba, C.; Galindo, A.; Villalain, C.; Pallás, C.; Herraiz, I. Smoking influence on early and late fetal growth. J. Perinat. Med. 2021, 50, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, F.; Jenabi, E. Smoking and placenta previa: A meta-analysis. J. Matern. Fetal Neonatal Med. 2017, 30, 2985–2990. [Google Scholar] [CrossRef] [PubMed]
- Míguez, M.C.; Pereira, B. Effects of active and/or passive smoking during pregnancy and the postpartum period. Anales de Pediatría 2021, 95, 222–232. [Google Scholar] [CrossRef]
- Duan, P.; Wang, Y.; Lin, R.; Zeng, Y.; Chen, C.; Yang, L.; Yue, M.; Zhong, S.; Wang, Y.; Zhang, Q. Impact of early life exposures on COPD in adulthood: A systematic review and meta-analysis. Respirology 2021, 26, 1131–1151. [Google Scholar] [CrossRef]
- Holbrook, B.D. The effects of nicotine on human fetal development. Birth Defects Res. C Embryo Today 2016, 108, 181–192. [Google Scholar] [CrossRef]
- Clifford, A.; Lang, L.; Chen, R. Effects of maternal cigarette smoking during pregnancy on cognitive parameters of children and young adults: A literature review. Neurotoxicol. Teratol. 2012, 34, 560–570. [Google Scholar] [CrossRef]
- Wells, A.C.; Lotfipour, S. Prenatal nicotine exposure during pregnancy results in adverse neurodevelopmental alterations and neurobehavioral deficits. Adv. Drug Alcohol. Res. 2023, 3, 11628. [Google Scholar] [CrossRef]
- Connolly, N.; Kelly, D.; O’Donnell, P.; Hyde, S. Effectiveness of smoking cessation interventions in pregnant women attending primary care: A scoping review. BJGP Open 2024, 8, BJGPO.2023.0185. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Probst, C.; Rehm, J.; Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e769–e776. [Google Scholar] [CrossRef] [PubMed]
- Cressman, A.M.; Pupco, A.; Kim, E.; Koren, G.; Bozzo, P. Smoking cessation therapy during pregnancy. Can. Fam. Physician 2012, 58, 525–527. [Google Scholar]
- Taylor, L.; Claire, R.; Campbell, K.; Coleman-Haynes, T.; Leonardi-Bee, J.; Chamberlain, C.; Berlin, I.; Davey, M.A.; Cooper, S.; Coleman, T. Fetal safety of nicotine replacement therapy in pregnancy: Systematic review and meta-analysis. Addiction 2021, 116, 239–277. [Google Scholar] [CrossRef]
- Claire, R.; Chamberlain, C.; Davey, M.A.; Cooper, S.E.; Berlin, I.; Leonardi-Bee, J.; Coleman, T. Pharmacological interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst. Rev. 2020, 3, CD010078. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Committee Opinion No. 471. Smoking cessation during pregnancy. Obstet. Gynecol. 2010, 116, 1241–1244. [Google Scholar] [CrossRef]
- Filion, K.B.; Abenhaim, H.A.; Mottillo, S.; Joseph, L.; Gervais, A.; O’Loughlin, J.; Paradis, G.; Pihl, R.; Pilote, L.; Rinfret, S.; et al. The effect of smoking cessation counselling in pregnant women: A meta-analysis of randomised controlled trials. BJOG Int. J. Obstet. Gynaecol. 2011, 118, 1422–1428. [Google Scholar] [CrossRef]
- Vila-Farinas, A.; Pérez-Rios, M.; Montes-Martinez, A.; Ruano-Ravina, A.; Forray, A.; Rey-Brandariz, J.; Candal-Pedreira, C.; Fernández, E.; Casal-Acción, B.; Varela-Lema, L. Effectiveness of smoking cessation interventions among pregnant women: An updated systematic review and meta-analysis. Addict. Behav. 2024, 148, 107854. [Google Scholar] [CrossRef]
- Tombor, I.; Neale, J.; Shahab, L.; Ruiz, M.; West, R. Healthcare providers’ views on digital smoking cessation interventions for pregnant women. J. Smok. Cessat. 2015, 10, 116–123. [Google Scholar] [CrossRef]
- Ussher, M.; Lewis, S.; Aveyard, P.; Manyonda, I.; West, R.; Lewis, B.; Marcus, B.; Riaz, M.; Taylor, A.; Daley, A.; et al. Physical activity for smoking cessation in pregnancy: Randomised controlled trial. BMJ 2015, 350, h2145. [Google Scholar] [CrossRef]
- Higgins, S.T.; Washio, Y.; Lopez, A.A.; Heil, S.H.; Solomon, L.J.; Lynch, M.E.; Hanson, J.D.; Higgins, T.M.; Skelly, J.M.; Redner, R.; et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev. Med. 2014, 68, 51–57. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A.C. Chapter 8: Assessing risk of bias in a randomized trial [last updated October 2019]. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 15 December 2024).
- Deeks, J.; Higgins, J.; Altman, D. Analyzing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0; Higgins, J., Green, S., Eds.; Wiley: Chichester, UK, 2008. [Google Scholar]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. Clin. Res. Educ. 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Schünemann, H.J.; Higgins, J.P.T.; Vist, G.E.; Glasziou, P.; Akl, E.A.; Skoetz, N.; Guyatt, G.H. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence [last updated August 2023]. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 17 December 2024).
- Coleman, T.; Cooper, S.; Thornton, J.G.; Grainge, M.J.; Watts, K.; Britton, J.; Lewis, S. Smoking, Nicotine, and Pregnancy (SNAP) Trial Team. A randomized trial of nicotine-replacement therapy patches in pregnancy. N. Engl. J. Med. 2012, 366, 808–818. [Google Scholar] [CrossRef] [PubMed]
- El-Mohandes, A.A.; Windsor, R.; Tan, S.; Perry, D.C.; Gantz, M.G.; Kiely, M. A randomized clinical trial of trans-dermal nicotine replacement in pregnant African-American smokers. Matern. Child Health J. 2013, 17, 897–906. [Google Scholar] [CrossRef]
- Berlin, I.; Grangé, G.; Jacob, N.; Tanguy, M.L. Nicotine patches in pregnant smokers: Randomised, placebo controlled, multicentre trial of efficacy. BMJ 2014, 348, g1622. [Google Scholar] [CrossRef]
- Iyen, B.; Vaz, L.R.; Taggar, J.; Cooper, S.; Lewis, S.; Coleman, T. Is the Apparently Protective Effect of Maternal Nicotine Replacement Therapy (NRT) used in Pregnancy on Infant Development Explained by Smoking Cessation?: Secondary Analyses of a Randomised Controlled Trial. BMJ Open 2019, 9, e024923. [Google Scholar] [CrossRef]
- Oncken, C.; Dornelas, E.A.; Kuo, C.L.; Sankey, H.Z.; Kranzler, H.R.; Mead, E.L.; Thurlow, M.S.D. Randomized Trial of Nicotine Inhaler for Pregnant Smokers. Am. J. Obstet. Gynecol. MFM 2019, 1, 10–18. [Google Scholar] [CrossRef]
- Stotts, A.L.; Northrup, T.F.; Cinciripini, P.M.; Minnix, J.A.; Blalock, J.A.; Mullen, P.D.; Pedroza, C.; Blackwell, S. Randomized, controlled pilot trial of bupropion for pregnant smokers: Challenges and future directions. Am. J. Perinatol. 2015, 32, 351–356. [Google Scholar] [CrossRef]
- Nanovskaya, T.N.; Oncken, C.; Fokina, V.M.; Feinn, R.S.; Clark, S.M.; West, H.; Jain, S.K.; Ahmed, M.S.; Hankins, G.D.V. Bupropion sustained release for pregnant smokers: A randomized, placebo-controlled trial. Am. J. Obstet. Gynecol. 2017, 216, e1–e420. [Google Scholar] [CrossRef] [PubMed]
- Kranzler, H.R.; Washio, Y.; Zindel, L.R.; Wileyto, E.P.; Srinivas, S.; Hand, D.J.; Hoffman, M.; Oncken, C.; Schnoll, R.A. Placebo-controlled trial of bupropion for smoking cessation in pregnant women. Am. J. Obstet. Gynecol. MFM 2021, 3, 100315. [Google Scholar] [CrossRef]
- Emery, J.; Leonardi-Bee, J.; Coleman, T.; McDaid, L.; Naughton, F. The Effectiveness of Text Support for Stopping Smoking in Pregnancy (MiQuit): Multi-Trial Pooled Analysis Investigating Effect Moderators and Mechanisms of Action. Nicotine Tob. Res. 2024, 26, 1072–1080. [Google Scholar] [CrossRef]
- King, E.; Cheyne, H.; Abhyankar, P.; Elders, A.; Grindle, M.; Hapca, A.; Jones, C.; O’Carroll, R.; Steele, M.; Williams, B. Promoting smoking cessation during pregnancy: A feasibility and pilot trial of a digital storytelling intervention delivered via text-messaging. Patient Educ. Couns. 2022, 105, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Pollak, K.I.; Lyna, P.; Gao, X.; Noonan, D.; Bejarano Hernandez, S.; Subudhi, S.; Swamy, G.K.; Fish, L.J. Efficacy of a Texting Program to Promote Cessation Among Pregnant Smokers: A Randomized Control Trial. Nicotine Tob. Res. 2020, 22, 1187–1194. [Google Scholar] [CrossRef]
- Berlin, I.; Berlin, N.; Malecot, M.; Breton, M.; Jusot, F.; Goldzahl, L. Financial incentives for smoking cessation in pregnancy: Multicentre randomised controlled trial. BMJ 2021, 375, e065217. [Google Scholar] [CrossRef]
- Kurti, A.N.; Tang, K.; Bolivar, H.A.; Evemy, C.; Medina, N.; Skelly, J.; Nighbor, T.; Higgins, S.T. Smartphone-based financial incentives to promote smoking cessation during pregnancy: A pilot study. Prev. Med. 2020, 140, 106201. [Google Scholar] [CrossRef] [PubMed]
- Tappin, D.; Bauld, L.; Purves, D.; Boyd, K.; Sinclair, L.; MacAskill, S.; McKell, J.; Friel, B.; McConnachie, A.; de Caestecker, L.; et al. Cessation in Pregnancy Incentives Trial Team. Financial incentives for smoking cessation in pregnancy: Randomised controlled trial. BMJ 2015, 350, h134. [Google Scholar] [CrossRef]
- Tappin, D.; Sinclair, L.; Kee, F.; McFadden, M.; Robinson-Smith, L.; Mitchell, A.; Keding, A.; Watson, J.; Watson, S.; Dick, A.; et al. Effect of financial voucher incentives provided with UK stop smoking services on the cessation of smoking in pregnant women (CPIT III): Pragmatic, multicentre, single blinded, phase 3, randomised controlled trial. BMJ 2022, 379, e071522. [Google Scholar] [CrossRef]
- Bradizza, C.M.; Stasiewicz, P.R.; Zhuo, Y.; Ruszczyk, M.; Maisto, S.A.; Lucke, J.F.; Brandon, T.H.; Eiden, R.D.; Slosman, K.S.; Giarratano, P. Smoking Cessation for Pregnant Smokers: Development and Pilot Test of an Emotion Regulation Treatment Supplement to Standard Smoking Cessation for Negative Affect Smokers. Nicotine Tob. Res. 2017, 19, 578–584. [Google Scholar] [CrossRef]
- Jin, G.; Niu, Y.Y.; Yang, X.W.; Yang, Y. Effect of smoking cessation intervention for pregnant smokers. Medicine 2018, 97, e11988. [Google Scholar] [CrossRef]
- Lee, M.; Miller, S.M.; Wen, K.Y.; Hui, S.K.; Roussi, P.; Hernandez, E. Cognitive-behavioral intervention to promote smoking cessation for pregnant and postpartum inner-city women. J. Behav. Med. 2015, 38, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Loukopoulou, A.N.; Vardavas, C.I.; Farmakides, G.; Rosolymos, C.; Chrelias, C.; Tzatzarakis, M.; Tsatsakis, A.; Myridakis, A.; Lyberi, M.; Behrakis, P.K. Counselling for smoking cessation during pregnancy reduces tobacco-specific nitrosamine (NNAL) concentrations: A randomized controlled trial. Eur. J. Midwifery 2018, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Patten, C.A.; Lando, H.A.; Desnoyers, C.A.; Bock, M.J.; Alexie, L.; Decker, P.A.; Hughes, C.A.; Resnicow, K.; Burhansstipanov, L.; Boyer, R.; et al. Healthy Pregnancies Project: Cluster Randomized Controlled Trial of a Community Intervention to Reduce Tobacco Use among Alaska Native Women. Int. J. Environ. Res. Public Health 2020, 17, 9302. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.; Ju, W.; Jung, H.; Park, C.; Oh, S.; Seo, H.; Kim, H.; Korean Meta-Analysis (KORMA) Study Group. Efficacy and safety of pharmacotherapy for smoking cessation among pregnant smokers: A meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1029–1039. [Google Scholar] [CrossRef]
- Morales-Suárez-Varela, M.; Puig, B.M.; Kaerlev, L.; Peraita-Costa, I.; Perales-Marín, A. Safety of Nicotine Replacement Therapy during Pregnancy: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 20, 250. [Google Scholar] [CrossRef]
- Notley, C.; Gentry, S.; Livingstone-Banks, J.; Bauld, L.; Perera, R.; Conde, M.; Hartmann-Boyce, J. Incentives for smoking cessation. Cochrane Database Syst. Rev. 2025, 1, CD004307. [Google Scholar] [CrossRef]
- Kock, L.S.; Erath, T.G.; Coleman, S.R.M.; Higgins, S.T.; Heil, S.H. Contingency management interventions for abstinence from cigarette smoking in pregnancy and postpartum: A systematic review and meta-analysis. Prev. Med. 2023, 176, 107654. [Google Scholar] [CrossRef]
- Griffiths, S.E.; Parsons, J.; Naughton, F.; Fulton, E.A.; Tombor, I.; Brown, K.E. Are digital interventions for smoking cessation in pregnancy effective? A systematic review and meta-analysis. Health Psychol. Rev. 2018, 12, 333–356. [Google Scholar] [CrossRef]
- Lumley, J.; Chamberlain, C.; Dowswell, T.; Oliver, S.; Oakley, L.; Watson, L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst. Rev. 2009, 3, CD001055. [Google Scholar] [CrossRef]
- Turner, E.; Jones, M.; Vaz, L.R.; Coleman, T. Systematic Review and Meta-Analysis to Assess the Safety of Bupropion and Varenicline in Pregnancy. Nicotine Tob. Res. 2019, 21, 1001–1010. [Google Scholar] [CrossRef] [PubMed]







| The Research Included | Region | Total Participants | Intervention Participants | Controls | Type of Intervention for Smoking Cessation | Extracted Outcomes | Limitations |
|---|---|---|---|---|---|---|---|
| Jin et al., 2018 [44] | China | 176 | 95 | 81 | Cognitive-behavioral counseling | Smoking abstinence, preterm birth, low birth weight, birth weight (gm), CS, stillbirth, Apgar score < 7 (5 min.) |
|
| Loukopoulou et al., 2018 [46] | Greece | 84 | 42 | 42 | Cognitive-behavioral counseling | Smoking abstinence |
|
| Patten et al., 2020 [47] | USA | 352 | 188 | 164 | Behavioral counseling | Smoking abstinence |
|
| Ussher et al., 2015 [20] | UK | 784 | 391 | 393 | Physical activity | Smoking abstinence, preterm birth, low birth weight, CS, stillbirth, Apgar score < 7 (5 min.) |
|
| Kurti 2020 [40] | USA | 60 | 30 | 30 | Financial Incentives | Smoking abstinence |
|
| Berlin et al., 2021 [39] | France | 460 | 231 | 229 | Financial Incentives | Smoking abstinence |
|
| Berlin et al., 2014 [30] | France | 402 | 203 | 199 | NRT | Smoking abstinence, preterm birth, birth weight (gm), CS, stillbirth |
|
| Coleman et al., 2012 [28] | UK | 1050 | 521 | 529 | NRT | Smoking abstinence, preterm birth, low birth weight, CS, stillbirth, Apgar score < 7 (5 min.) | Difficult in the generalization for low-income nations. |
| El-Mohandes et al., 2013 [29] | USA | 52 | 26 | 26 | NRT | Smoking abstinence, preterm birth, low birth weight |
|
| Oncken et al., 2019 [32] | USA | 137 | 70 | 67 | NRT | Smoking abstinence, preterm birth, low birth weight |
|
| Nanovskaya et al., 2017 [34] | USA | 65 | 30 | 35 | Bupropion | Smoking abstinence, preterm birth, birth weight (gm) |
|
| Stotts et al., 2015 [33] | USA | 11 | 5 | 6 | Bupropion | Smoking abstinence, birth weight (gm), |
|
| Kranzler et al., 2021 [35] | USA | 129 | 64 | 65 | Bupropion | Smoking abstinence, birth weight (gm), Apgar score < 7 (5 min.) |
|
| Tappin et al., 2015 [41] | UK | 609 | 306 | 303 | Financial incentives | Smoking abstinence, preterm birth, birth weight (gm) |
|
| Lee et al., 2015 [45] | USA | 277 | 140 | 137 | Cognitive-behavioral counseling | Smoking abstinence, CS |
|
| King et al., 2022 [37] | UK | 28 | 15 | 13 | Text-messaging | Smoking abstinence | Difficult in the recruitment to low socioeconomic pregnant women. |
| Emery et al., 2024 [36] | UK | 1409 | 704 | 705 | Text-messaging | Smoking abstinence | This intervention was effective only for short-term response. |
| Bradizza et al., 2017 [43] | USA | 70 | 36 | 34 | Emotion with cognitive-behavioral | Smoking abstinence |
|
| Iyen et al., 2019 [31] | UK | 884 | 443 | 441 | NRT | Smoking abstinence, low birth weight, | Misattribution of smoking Status. |
| Pollak et al., 2020 [38] | USA | 314 | 154 | 160 | Text-messaging | Smoking abstinence |
|
| Tappin et al., 2022 [42] | UK | 941 | 471 | 470 | Financial incentives | Smoking abstinence, preterm birth | Difficult in the generalization for low-income nations. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elseifi, O.S.; Younis, F.E.; Mirza, I.M.; Alhewiti, A.; Abd-Elhady, N.M.S.; Mortada, E.M. Breaking the Habit: A Systematic Review and Meta-Analysis of Pregnancy-Related Smoking Cessation Randomized Controlled Trials. Healthcare 2025, 13, 732. https://doi.org/10.3390/healthcare13070732
Elseifi OS, Younis FE, Mirza IM, Alhewiti A, Abd-Elhady NMS, Mortada EM. Breaking the Habit: A Systematic Review and Meta-Analysis of Pregnancy-Related Smoking Cessation Randomized Controlled Trials. Healthcare. 2025; 13(7):732. https://doi.org/10.3390/healthcare13070732
Chicago/Turabian StyleElseifi, Omnia S., Faten Ezzelarab Younis, Iman M. Mirza, Abdullah Alhewiti, Nahla M. S. Abd-Elhady, and Eman M. Mortada. 2025. "Breaking the Habit: A Systematic Review and Meta-Analysis of Pregnancy-Related Smoking Cessation Randomized Controlled Trials" Healthcare 13, no. 7: 732. https://doi.org/10.3390/healthcare13070732
APA StyleElseifi, O. S., Younis, F. E., Mirza, I. M., Alhewiti, A., Abd-Elhady, N. M. S., & Mortada, E. M. (2025). Breaking the Habit: A Systematic Review and Meta-Analysis of Pregnancy-Related Smoking Cessation Randomized Controlled Trials. Healthcare, 13(7), 732. https://doi.org/10.3390/healthcare13070732


_Rachiotis.png)



