Moderating Effect of Muscular Strength in the Association Between Cancer and Depressive Symptomatology
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Procedures
2.2. Measures
2.3. Covariates
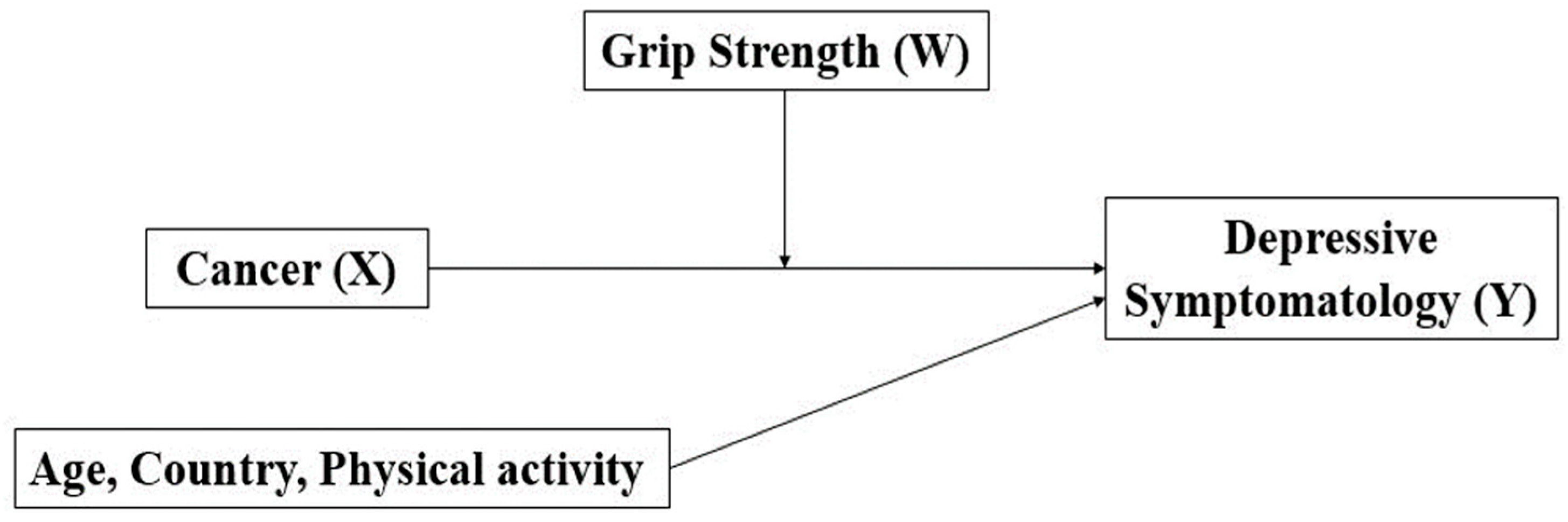
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SHARE | Survey of Health, Aging, and Retirement in Europe |
| CAPI | Computer-assisted personal interviewing |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar]
- Mayer, D.K.; Nasso, S.F.; Earp, J.A. Defining Cancer Survivors, Their Needs, and Perspectives on Survivorship Health Care in the USA. Lancet Oncol. 2017, 18, e11–e18. [Google Scholar] [PubMed]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar]
- Tan, S.Y.C.; Tsoukalas, T.; Javier, K.; Fazon, T.; Singh, S.; Vardy, J. Recommendations on the Surveillance and Supplementation of Vitamins and Minerals for Upper Gastrointestinal Cancer Survivors: A Scoping Review. J. Cancer Surviv. 2024. [Google Scholar] [CrossRef]
- Gillies, R.J.; Gatenby, R.A. Metabolism and Its Sequelae in Cancer Evolution and Therapy. Cancer J. 2015, 21, 88–96. [Google Scholar]
- de PL Oliveira, M.; Siqueira, J.M.; Santos, A.N.; Lemos, E.B.; Lemos, E.B.; Soares, E.M.; Pimentel, G.D. Low Handgrip Strength Is a Risk Factor for Symptoms of Anxiety and Depression in Survivors Breast Cancer Patients. Pers. Med. Psychiatry 2024, 47–48, 100143. [Google Scholar]
- Xie, H.; Ruan, G.; Deng, L.; Zhang, H.; Ge, Y.; Zhang, Q.; Lin, S.; Song, M.; Zhang, X.; Liu, X.; et al. Comparison of Absolute and Relative Handgrip Strength To Predict Cancer Prognosis: A Prospective Multicenter Cohort Study. Clin. Nutr. 2022, 41, 1636–1643. [Google Scholar]
- Yi, J.C.; Syrjala, K.L. Anxiety and Depression in Cancer Survivors. Med. Clin. N. Am. 2017, 101, 1099–1113. [Google Scholar]
- WHO. Depressive Disorder (Depression). 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 16 December 2024).
- Mejareh, Z.N.; Abdollahi, B.; Hoseinipalangi, Z.; Jeze, M.S.; Hosseinifard, H.; Rafiei, S.; Aghajani, F.; Dehnad, A.; Ardakani, M.F.; Ahmadi, S.; et al. Global, Regional, and National Prevalence of Depression Among Cancer Patients: A Systematic Review and Meta-Analysis. Indian J. Psychiatry 2021, 63, 527–535. [Google Scholar] [PubMed]
- Riedl, D.; Schuessler, G. Prevalence of Depression and Cancer—A systematic review. Z. Psychosom. Med. Psychother. 2022, 68, 74–86. [Google Scholar]
- Zhou, L.; Sun, H. The Longitudinal Changes of Anxiety and Depression, Their Related Risk Factors and Prognostic Value in Colorectal Cancer Survivors: A 36-Month Follow-Up Study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101511. [Google Scholar]
- Vaishya, R.; Misra, A.; Vaish, A.; Ursino, N.; D’Ambrosi, R. Hand Grip Strength as a Proposed New Vital Sign of Health: A Narrative Review of Evidences. J. Health Popul. Nutr. 2024, 43, 7. [Google Scholar]
- Parra-Soto, S.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Absolute and Relative Grip Strength as Predictors of Cancer: Prospective Cohort Study of 445 552 Participants in UK Biobank. J. Cachexia Sarcopenia Muscle 2022, 13, 325–332. [Google Scholar] [PubMed]
- Zhang, X.M.; Zhang, Z.B.; Chen, W.; Wu, X. The Association Between Handgrip Strength and Depression in Cancer Survivors: A Cross-Sectional Study. BMC Geriatrics 2022, 22, 111. [Google Scholar]
- Börsch-Supan, A.; Brandt, M.; Hunkler, C.; Kneip, T.; Korbmacher, J.; Malter, F.; Schaan, B.; Stuck, S.; Zuber, S.; SHARE Central Coordination Team. Data Resource Profile: The Survey of Health, Ageing and Retirement in Europe (SHARE). Int. J. Epidemiol. 2013, 42, 992–1001. [Google Scholar]
- Gallagher, D.; Savva, G.M.; Kenny, R.; Lawlor, B.A. What Predicts Persistent Depression in Older Adults Across Europe? Utility of Clinical and Neuropsychological Predictors From the SHARE Study. J. Affect. Disord. 2013, 147, 192–197. [Google Scholar] [PubMed]
- Prince, M.J.; Reischies, F.; Beekman, A.T.; Fuhrer, R.; Jonker, C.; Kivela, S.L.; Lawlor, B.A.; Lobo, A.; Magnusson, H.; Fichter, M.; et al. Development of the EURO-D Scale—A European, Union Initiative To Compare Symptoms of Depression in 14 European Centres. Br. J. Psychiatry J. Ment. Sci. 1999, 174, 330–338. [Google Scholar]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A Review of the Measurement of Grip Strength in Clinical and Epidemiological Studies: Towards a Standardised Approach. Age Ageing 2011, 40, 423–429. [Google Scholar]
- Janosky, J.E. Pearson Correlation Coefficients vs. Reliability Coefficients. J. Am. Diet. Assoc. 1991, 91, 912–913. [Google Scholar] [PubMed]
- Baron, R.M.; Kenny, D.A. The Moderator-Mediator Variable Distinction in Social Psychological Research: Conceptual, Strategic, and Statistical Considerations. J. Pers. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Albert, P.R. Why Is Depression More Prevalent in Women? J. Psychiatry Neurosci. 2015, 40, 219–221. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Yang, A.; Zhao, Q.; Chen, Z.; Ren, X.; Dai, Q. A Hypothesis of Gender Differences in Self-Reporting Symptom of Depression: Implications to Solve Under-Diagnosis and Under-Treatment of Depression in Males. Front. Psychiatry 2021, 12, 589687. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, J.; Song, X.; Lai, S.; Zhong, S.; Jia, Y. The Effect of Exogenous Estrogen on Depressive Mood in Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Psychiatr. Res. 2023, 162, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.M.; Sun, P.Z. Gender Differences in the Interaction Effect of Cumulative Risk and Problem-Focused Coping on Depression Among Adult Employees. PLoS ONE 2019, 14, e0226036. [Google Scholar] [CrossRef]
- Kang, H.J.; Park, Y.; Yoo, K.H.; Kim, K.T.; Kim, E.S.; Kim, J.W.; Kim, S.W.; Shin, I.S.; Yoon, J.S.; Kim, J.H.; et al. Sex Differences in the Genetic Architecture of Depression. Sci. Rep. 2020, 10, 9927. [Google Scholar] [CrossRef]
- Marques, A.; Henriques-Neto, D.; Peralta, M.; Marconcin, P.; Gouveia, É.R.; Ferrari, G.; Martins, J.; Ihle, A. Exploring Grip Strength as a Predictor of Depression in Middle-Aged and Older Adults. Sci. Rep. 2021, 11, 15946. [Google Scholar] [CrossRef] [PubMed]
- Ganipineni, V.D.P.; Idavalapati, A.S.K.K.; Tamalapakula, S.S.; Moparthi, V.; Potru, M.; Owolabi, O.J. Depression and Hand-Grip: Unraveling the Association. Cureus 2023, 15, e38632. [Google Scholar] [CrossRef]
- Bohannon, R.W. Grip Strength: An Indispensable Biomarker For Older Adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef]
- Blanchet, C.; Peralta, M.; de Maio Nascimento, M.; Gouveia, É.R.; Ferrari, G.; Ribeiro, T.D.; Marques, A. Grip Strength Buffers the Harmful Association Between Multimorbidity and Depression Among Middle-Aged and Older Adults. Arch. Gerontol. Geriatr. 2024, 122, 105391. [Google Scholar] [PubMed]
- Gu, W.; Xu, Y.M.; Zhu, J.H.; Zhong, B.L. Depression and Its Impact on Health-Related Quality of Life Among Chinese Inpatients With Lung Cancer. Oncotarget 2017, 8, 104806–104812. [Google Scholar] [CrossRef] [PubMed]
- Vucic, V.; Radovanovic, S.; Radevic, S.; Savkovic, Z.; Mihailovic, N.; Mihaljevic, O.; Macuzic, I.Z.; Djordjic, M.; Gavrilovic, A.; Matic, T.B. Mental Health Assessment of Cancer Patients: Prevalence and Predictive Factors of Depression and Anxiety. Iran. J. Public. Health 2021, 50, 2017–2027. [Google Scholar] [PubMed]
- Wang, Y.H.; Li, J.Q.; Shi, J.F.; Que, J.Y.; Liu, J.J.; Lappin, J.M.; Leung, J.; Ravindran, A.V.; Chen, W.Q.; Qiao, Y.L.; et al. Depression and Anxiety in Relation to Cancer Incidence and Mortality: A Systematic Review and Meta-Analysis of Cohort Studies. Mol. Psychiatry 2020, 25, 1487–1499. [Google Scholar]
- Zhang, L. Anxiety and Depression in Recurrent Gastric Cancer: Their Prevalence and Independent Risk Factors Analyses. Medicine 2021, 100, e28358. [Google Scholar]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major Depressive Disorder: Hypothesis, Mechanism, Prevention and Treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar]
- Parish, A.L.; Gillis, B.; Anthamatten, A. Pharmacotherapy for Depression and Anxiety in the Primary Care Setting. J. Nurse Pract. 2023, 19, 104556. [Google Scholar]
- Nascimento, W.; Ferrari, G.; Martins, C.B.; Rey-Lopez, J.P.; Izquierdo, M.; Lee, D.H.; Giovannucci, E.L.; Rezende, L.F.M. Muscle-Strengthening Activities and Cancer Incidence and Mortality: A Systematic Review and Meta-Analysis of Observational Studies. Int. J. Behav. Nutr. Phys. Act. 2021, 18, 69. [Google Scholar] [CrossRef]
- Tavares, V.D.O.; Cuthbert, C.; Teychenne, M.; Schuch, F.B.; Cabral, D.; Menezes de Sousa, G.; Prado, C.M.; Patten, S.; Galvão-Coelho, N.L.; Hallgren, M. The Effects of Exercise on Anxiety and Depression in Adults With Cancer: A Meta-Review of Meta-Analyses. J. Psychosoc. Oncol. 2024, Epub ahead of print, 1–24. [Google Scholar] [CrossRef]
- Xu, N.; An, Q. Correlation Between Dietary Score and Depression in Cancer Patients: Data From the 2005–2018 National Health and Nutrition Examination Surveys. Front. Psychol. 2022, 13, 978913. [Google Scholar] [CrossRef]
- Trudel-Fitzgerald, C.; Zevon, E.S.; Kawachi, I.; Tucker-Seeley, R.D.; Kubzansky, L.D. Depression, Smoking, and Lung Cancer Risk Over 24 Years Among Women. Psychol. Med. 2022, 52, 2510–2519. [Google Scholar] [PubMed]
- Révész, D.; Bours, M.J.L.; Wegdam, J.A.; Keulen, E.T.P.; Breukink, S.O.; Slooter, G.D.; Vogelaar, F.J.; Weijenberg, M.P.; Mols, F. Associations Between Alcohol Consumption and Anxiety, Depression, and Health-Related Quality of Life in Colorectal Cancer Survivors. J. Cancer Surviv. 2022, 16, 988–997. [Google Scholar] [PubMed]
- Olds, T.S.; Gomersall, S.R.; Olds, S.T.; Ridley, K. A Source of Systematic Bias in Self-Reported Physical Activity: The Cutpoint Bias Hypothesis. J. Sci. Med. Sport. 2019, 22, 924–928. [Google Scholar] [PubMed]
- Zhou, J.; Liu, X.; Liang, X.; Wei, S. Association Between Depressive Symptoms and Second Primary Cancer in Cancer Survivors: Insights From a Nationally Representative Study. Gen. Hosp. Psychiatry 2024, 90, 150–156. [Google Scholar]
| Mean (SD) or n (%) | ||||
|---|---|---|---|---|
| Total (n = 41,666) | Male (n = 17,986) | Female (n = 23,680) | p | |
| Age (years) | 70.65 (9.14) | 71.12 (8.77) | 70.29 (9.40) | <0.001 |
| Grip strength (kg) | 32.04 (11.18) | 40.68 (9.93) | 25.47 (6.72) | <0.001 |
| EURO-D score | 2.32 (2.16) | 1.90 (1.95) | 2.63 (2.25) | <0.001 |
| EURO-D ≥ 4 Yes [n (%)] No [n (%)] | 10,523 (25.3) 31,143 (74.7) | 3295 (18.3) 14,691 (81.7) | 7228 (30.5) 16,452 (69.5) | <0.001 |
| MPA [n (%)] <1x/week 1/week >1/week | 7696 (18.5) 6215 (14.9) 27,755 (66.6) | 3166 (17.6) 2695 (15.0) 12,125 (67.4) | 4530 (19.1) 3520 (14.9) 15,630 (66.0) | <0.001 |
| VPA [n (%)] <1x/week 1/week >1/week | 21,788 (52.3) 6326 (15.2) 13,552 (32.5) | 8719 (48.5) 2699 (14.5, 15.0) 6568 (36.5) | 13,069 (55.2) 3627 (15.3) 6984 (29.5) | <0.001 |
| Cancer Yes [n (%)] No [n (%)] | 2117 (5.1) 39,549 (94.9) | 1032 (5.7) 16,954 (94.3) | 1085 (4.6) 22,595 (95.4) | <0.001 |
| Depressive Symptoms (EURO-D 12 Score) | ||||||
|---|---|---|---|---|---|---|
| Total Sample | Male | Female | ||||
| r | p | r | p | r | p | |
| Grip strength | −0.254 | <0.001 | −0.193 | <0.001 | −0.210 | <0.001 |
| Depressive Symptoms (EURO-D 12 Score) | ||||||
|---|---|---|---|---|---|---|
| Total | Male | Female | ||||
| Mean (SD) | p | Mean (SD) | p | Mean (SD) | p | |
| With Cancer | 3.03 (2.31) | <0.001 | 2.66 (2.2) | <0.001 | 3.39 (2.24) | <0.001 |
| Without Cancer | 2.28 (2.14) | 1.86 (1.9) | 2.59 (2.40) | |||
| Depressive Symptoms (EURO-D 12 Score) | ||||||
|---|---|---|---|---|---|---|
| Total Sample | Male | Female | ||||
| B | 95% CI | B | 95% CI | B | 95% CI | |
| Cancer (X) | 1.09 | 0.81, 1.37 | 1.65 | 1.13, 2.17 | 1.17 | 0.66, 1.68 |
| Grip Strength (W) | −0.04 | −0.04, −0.04 | −0.02 | −0.03, −0.02 | −0.05 | −0.06, −0.05 |
| Cancer Grip Strength | −0.01 | −0.02, −0.01 | −0.03 | −0.04, −0.01 | −0.02 | −0.04, 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veiga, D.; de Maio Nascimento, M.; Peralta, M.; R. Gouveia, É.; Marques, A. Moderating Effect of Muscular Strength in the Association Between Cancer and Depressive Symptomatology. Healthcare 2025, 13, 715. https://doi.org/10.3390/healthcare13070715
Veiga D, de Maio Nascimento M, Peralta M, R. Gouveia É, Marques A. Moderating Effect of Muscular Strength in the Association Between Cancer and Depressive Symptomatology. Healthcare. 2025; 13(7):715. https://doi.org/10.3390/healthcare13070715
Chicago/Turabian StyleVeiga, Diogo, Marcelo de Maio Nascimento, Miguel Peralta, Élvio R. Gouveia, and Adilson Marques. 2025. "Moderating Effect of Muscular Strength in the Association Between Cancer and Depressive Symptomatology" Healthcare 13, no. 7: 715. https://doi.org/10.3390/healthcare13070715
APA StyleVeiga, D., de Maio Nascimento, M., Peralta, M., R. Gouveia, É., & Marques, A. (2025). Moderating Effect of Muscular Strength in the Association Between Cancer and Depressive Symptomatology. Healthcare, 13(7), 715. https://doi.org/10.3390/healthcare13070715









