Association of Hormone Replacement Therapy with Inflammatory Bowel Disease Risk in Women with Menopausal Disorders: A Population-Based Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
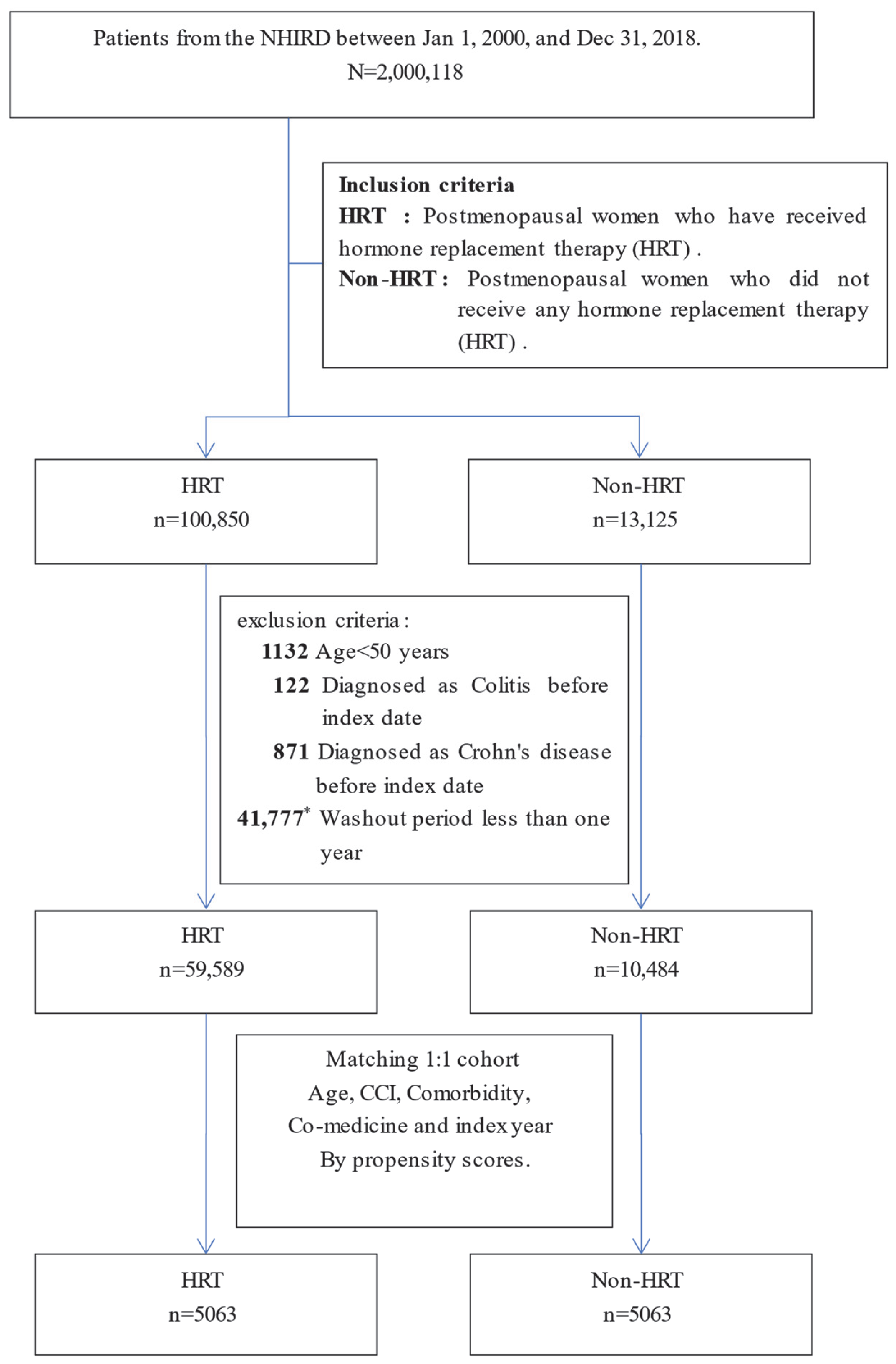
2.2. Study Design and Definition of Study Cohort
2.3. Definition of Study Periods and Bias Mitigation Methods
2.4. Main Outcome and Covariate Measurements
2.5. Statistical Method
3. Results
3.1. Patient Characteristics and Balanced Groups
3.2. Comparison of Incidence Rates of UC and CD
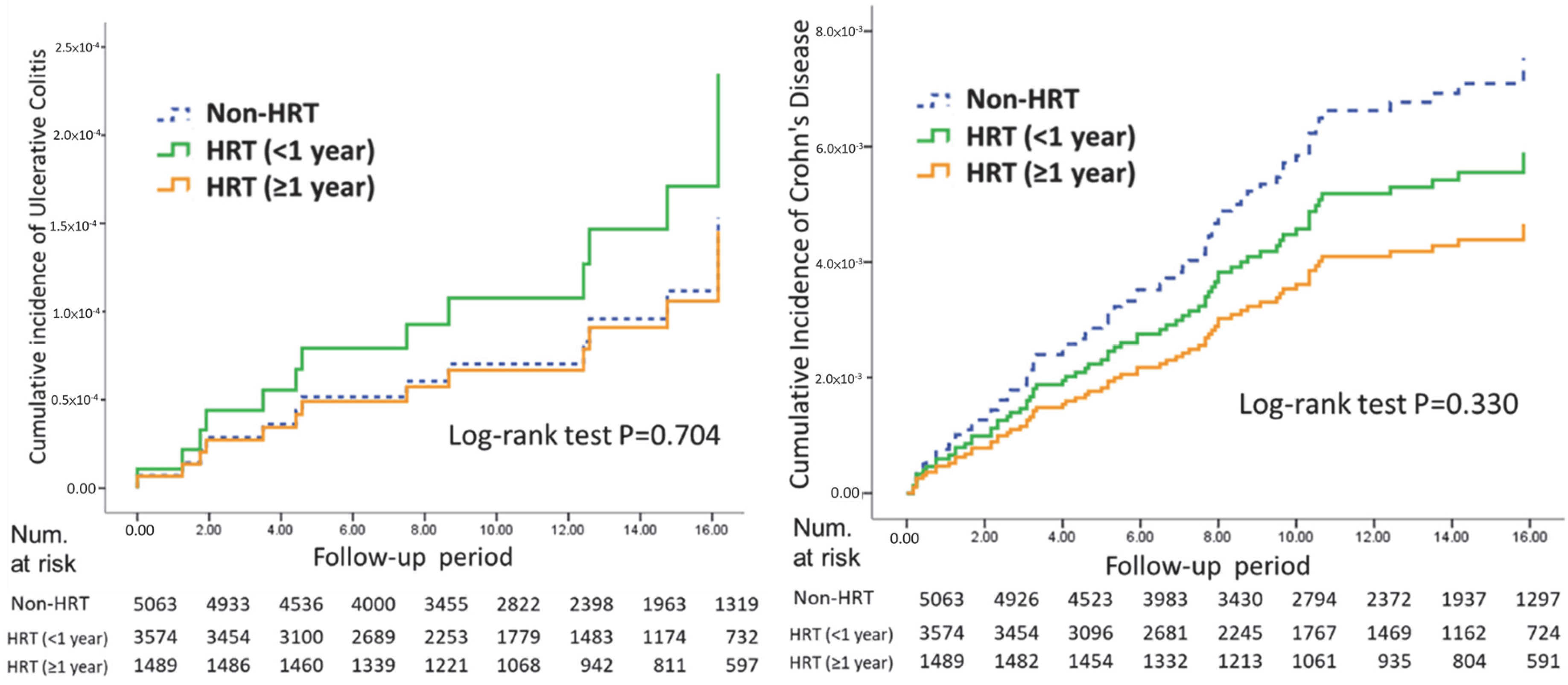
3.3. UC and CD Incidence by HRT Duration
3.4. Associations with UC and CD
4. Discussion
4.1. Comparison with Existing Literature
4.2. Biological Mechanisms and Study Implications
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HRT | Hormone replacement therapy |
| IBD | Inflammatory bowel disease |
| UC | Ulcerative colitis |
| CD | Crohn’s disease |
| NHI | National Health Insurance |
| ICD | International Classification of Disease |
| ICD-9-CM | International Classification of Disease, Ninth Revision, Clinical Modification |
| ICD-10-CM | International Classification of Disease, Tenth Revision, Clinical Modification |
| CKD | Chronic kidney disease |
| DM | Diabetes mellitus |
| CCI | Charlson Comorbidity Index |
| HR | Hazard ratio |
| CI | Confidence interval |
| PSM | Propensity score matching |
| IRB | Institutional Review Board |
| SSRI | Selective serotonin reuptake inhibitor |
| ACEI | Angiotensin-converting enzyme inhibitor |
References
- Garcia-Villatoro, E.L.; Allred, C.D. Estrogen receptor actions in colitis. Essays Biochem. 2021, 65, 1003–1013. [Google Scholar] [CrossRef]
- Estevinho, M.; Midya, V.; Cohen-Mekelburg, S.; Allin, K.; Fumery, M.; Pinho, S.; Colombel, J.; Agrawal, M. Emerging role of environmental pollutants in inflammatory bowel disease risk, outcomes and underlying mechanisms. Gut 2025, 74, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Greuter, T.; Manser, C.; Pittet, V.; Vavricka, S.R.; Biedermann, L. Gender differences in inflammatory bowel disease. Digestion 2020, 101 (Suppl. S1), 98–104. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Hu, Y.; Xu, M.; Wang, S.; Wu, Z.; Deng, F. Associations between sex hormones, receptors, binding proteins and inflammatory bowel disease: A Mendelian randomization study. Front. Endocrinol. 2024, 15, 1272746. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Jiang, W.; Xin, L.; Wu, J.; Zhu, S.; Liu, Z.; Shen, Z. The Potential Role of Female Sex Hormones in Patients With Inflammatory Bowel Disease: A 2-Sample Mendelian Randomization Study. Clin. Transl. Gastroenterol. 2024, 15, e00748. [Google Scholar] [CrossRef]
- Xu, L.; Huang, G.; Cong, Y.; Yu, Y.; Li, Y. Sex-related Differences in Inflammatory Bowel Diseases: The Potential Role of Sex Hormones. Inflamm. Bowel Dis. 2022, 28, 1766–1775. [Google Scholar] [CrossRef]
- Fuss, I.J.; Heller, F.; Boirivant, M.; Leon, F.; Yoshida, M.; Fichtner-Feigl, S.; Yang, Z.; Exley, M.; Kitani, A.; Blumberg, R.S. Nonclassical CD1d-restricted NK T cells that produce IL-13 characterize an atypical Th2 response in ulcerative colitis. J. Clin. Investig. 2004, 113, 1490–1497. [Google Scholar] [CrossRef]
- Yang, X.; Tian, Y.; Zheng, L.; Luu, T.; Kwak-Kim, J. The Update Immune-Regulatory Role of Pro- and Anti-Inflammatory Cytokines in Recurrent Pregnancy Losses. Int. J. Mol. Sci. 2022, 24, 132. [Google Scholar] [CrossRef]
- Freeman, M.; Lally, L.; Teigen, L.; Graziano, E.; Shivashankar, R.; Shmidt, E. Hormone Replacement Therapy Is Associated with Disease Activity Improvement among Post-Menopausal Women with Inflammatory Bowel Disease. J. Clin. Med. 2023, 13, 88. [Google Scholar] [CrossRef]
- Jovani, M.; Ma, W.; Joshi, A.D.; Liu, P.-H.; Nguyen, L.H.; Cao, Y.; Tam, I.; Wu, K.; Giovannucci, E.L.; Chan, A.T. Menopausal hormone therapy and risk of diverticulitis. Am. J. Gastroenterol. 2019, 114, 315. [Google Scholar] [CrossRef]
- Moleski, S.M.; Choudhary, C. Special considerations for women with IBD. Gastroenterol. Clin. 2011, 40, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.L.; Kao, Y.H.Y.; Lin, S.J.; Lee, C.H.; Lai, M.L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol. Drug Saf. 2011, 20, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Su, C.-C.; Shao, S.; Sung, S.; Lin, S.-J.; Kao, Y.-H.Y.; Lai, E. Taiwan’s National Health Insurance Research Database: Past and future. Clin. Epidemiol. 2019, 11, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.T.; Chen, C.Y.; Bair, M.J. Epidemiology, Clinical Features, and Prescribing Patterns of Irritable Bowel Syndrome in Taiwan. Front. Pharmacol. 2021, 12, 788795. [Google Scholar] [CrossRef]
- Webster-Clark, M.; Stürmer, T.; Wang, T.; Man, K.; Marinac-Dabic, D.; Rothman, K.J.; Ellis, A.R.; Gokhale, M.; Lunt, M.; Girman, C. Using propensity scores to estimate effects of treatment initiation decisions: State of the science. Stat. Med. 2021, 40, 1718–1735. [Google Scholar] [CrossRef]
- ElHafeez, S.A.; D’Arrigo, G.; Leonardis, D.; Fusaro, M.; Tripepi, G.; Roumeliotis, S. Methods to Analyze Time-to-Event Data: The Cox Regression Analysis. Oxidative Med. Cell. Longev. 2021, 2021, 1302811. [Google Scholar] [CrossRef]
- Jacenik, D.; Krajewska, W.M. Significance of G protein-coupled estrogen receptor in the pathophysiology of irritable bowel syndrome, inflammatory bowel diseases and colorectal cancer. Front. Endocrinol. 2020, 11, 390. [Google Scholar] [CrossRef]
- Zhu, Z.; Jia, Y.; Li, F.-R.; Li, Y.; Chen, L.-H.; Yang, H.-H.; Guo, D.; Sun, L.; Shi, M.; Wang, T.; et al. Inflammatory Bowel Disease and Risk of Global Cardiovascular Diseases and Type 2 Diabetes. Inflamm. Bowel Dis. 2024, 30, 1130–1137. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, M.; Liu, Y.; Ge, C.; Lu, Y.; Shen, H.; Zhu, L. Prevalence of metabolic syndrome in patients with inflammatory bowel disease: A systematic review and meta-analysis. BMJ Open 2024, 14, e074659. [Google Scholar] [CrossRef]
- Kane, S.V.; Reddy, D. Hormonal replacement therapy after menopause is protective of disease activity in women with inflammatory bowel disease. Off. J. Am. Coll. Gastroenterol.|ACG 2008, 103, 1193–1196. [Google Scholar] [CrossRef]
- Wang, J.; Hou, Y.-S.; Zhang, L.; Liu, M.; Zhao, J.; Zhang, Z.; Ma, Y.; Hou, W. Estrogen Attenuates Traumatic Brain Injury by Inhibiting the Activation of Microglia and Astrocyte-Mediated Neuroinflammatory Responses. Mol. Neurobiol. 2020, 58, 1052–1061. [Google Scholar] [CrossRef]
- Zdrojkowski, Ł.; Jasiński, T.; Ferreira-Dias, G.; Pawliński, B.; Domino, M. The Role of NF-κB in Endometrial Diseases in Humans and Animals: A Review. Int. J. Mol. Sci. 2023, 24, 2901. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Leveque, M.; Buisson-Brenac, C.; Bueno, L.; Fioramonti, J.; Houdeau, E. Oestradiol decreases colonic permeability through oestrogen receptor β-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J. Physiol. 2009, 587, 3317–3328. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, B.; Byemerwa, J.; Krebs, T.; Lim, F.; Chang, C.-Y.; McDonnell, D.P. Estrogen Receptor Signaling in the Immune System. Endocr. Rev. 2022, 44, 117–141. [Google Scholar] [CrossRef] [PubMed]
- Sleutjes, J.A.M.; van der Woude, C.J.; Verploegh, P.J.P.; Aribas, E.; Kavousi, M.; Roeters van Lennep, J.E.; de Vries, A.C. Cardiovascular risk profiles in patients with inflammatory bowel disease differ from matched controls from the general population. Eur. J. Prev. Cardiol. 2023, 30, 1615–1622. [Google Scholar] [CrossRef]
- Hyun, C. Molecular and Pathophysiological Links between Metabolic Disorders and Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 9139. [Google Scholar] [CrossRef]

| Characteristic | Non-HRT (n = 5063) | HRT (n = 5063) |
|---|---|---|
| Demographics | ||
| Age, years ‡ | 62.5 ± 8.7 | 62.5 ± 8.7 |
| CCI ‡ | 1.74 ± 2.0 | 1.74 ± 2.0 |
| Comorbidities, n (%) | ||
| Hypertension | 1818 (35.9) | 1818 (35.9) |
| Hyperlipidemia | 1957 (38.7) | 1957 (38.7) |
| DM | 887 (17.5) | 887 (17.5) |
| CKD | 100 (2.0) | 100 (2.0) |
| Medications, n (%) | ||
| Aspirin | 1509 (29.8) | 1509 (29.8) |
| Statins | 1531 (30.2) | 1531 (30.2) |
| ACEIs | 1142 (22.6) | 1142 (22.6) |
| β-blockers | 2915 (57.6) | 2915 (57.6) |
| Spironolactone | 176 (3.5) | 176 (3.5) |
| Glucocorticoids | 4494 (88.8) | 4494 (88.8) |
| SSRIs | 674 (13.3) | 674 (13.3) |
| End Point | HRT | IR † | (95% CI) | Non-HRT | IR † | (95% CI) |
|---|---|---|---|---|---|---|
| Ulcerative colitis | 8 | 0.14 | (0.07–0.28) | 6 | 0.11 | (0.04–0.24) |
| Crohn’s disease | 30 | 0.54 | (0.37–0.76) | 42 | 0.75 | (0.56–1.02) |
| HRT Duration | UC | CD | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Non-HRT (Reference) | 1.00 | - | 1.00 | - |
| Any duration | 1.33 (0.46–3.83) | 0.600 | 0.72 (0.45–1.16) | 0.177 |
| <1 year | 1.53 (0.49–4.76) | 0.461 | 0.78 (0.46–1.32) | 0.359 |
| ≥1 year | 0.95 (0.19–4.73) | 0.949 | 0.62 (0.30–1.27) | 0.190 |
| Factors | UC | p | CD | p |
|---|---|---|---|---|
| Age | 1.02 (0.95–1.09) | 0.621 | 1.04 (1.01–1.07) | 0.010 |
| CCI | 1.19 (0.89–1.60) | 0.240 | 1.00 (0.87–1.16) | 0.957 |
| Hypertension | 3.19 (0.66–15.5) | 0.150 | 2.03 (0.99–4.17) | 0.053 |
| Hyperlipidemia | 0.43 (0.07–2.80) | 0.380 | 0.87 (0.42–1.82) | 0.711 |
| DM | 2.90 (0.63–13.4) | 0.174 | 0.63 (0.33–1.23) | 0.179 |
| CKD | 2.06 (0.33–12.9) | 0.440 | 0.52 (0.12–2.28) | 0.385 |
| Aspirin | 0.24 (0.05–1.10) | 0.066 | 1.31 (0.73–2.35) | 0.366 |
| Statins | 0.88 (0.15–5.10) | 0.882 | 0.95 (0.46–1.96) | 0.888 |
| ACEIs | 0.48 (0.11–2.05) | 0.319 | 0.89 (0.48–1.65) | 0.701 |
| β-blockers | 1.42 (0.26–7.87) | 0.689 | 1.33 (0.66–2.70) | 0.426 |
| Spironolactone | 1.41 (0.24–8.16) | 0.699 | 1.86 (0.86–4.03) | 0.113 |
| Glucocorticoids | n/a | 0.983 | 2.91 (0.7–12.09) | 0.142 |
| SSRIs | 2.21 (0.69–7.07) | 0.180 | 0.97 (0.50–1.87) | 0.928 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, Y.-T.; Chen, I.-I.; Wang, C.-H. Association of Hormone Replacement Therapy with Inflammatory Bowel Disease Risk in Women with Menopausal Disorders: A Population-Based Retrospective Cohort Study. Healthcare 2025, 13, 578. https://doi.org/10.3390/healthcare13050578
Tseng Y-T, Chen I-I, Wang C-H. Association of Hormone Replacement Therapy with Inflammatory Bowel Disease Risk in Women with Menopausal Disorders: A Population-Based Retrospective Cohort Study. Healthcare. 2025; 13(5):578. https://doi.org/10.3390/healthcare13050578
Chicago/Turabian StyleTseng, Yuan-Tsung, I-I Chen, and Chun-Hsiang Wang. 2025. "Association of Hormone Replacement Therapy with Inflammatory Bowel Disease Risk in Women with Menopausal Disorders: A Population-Based Retrospective Cohort Study" Healthcare 13, no. 5: 578. https://doi.org/10.3390/healthcare13050578
APA StyleTseng, Y.-T., Chen, I.-I., & Wang, C.-H. (2025). Association of Hormone Replacement Therapy with Inflammatory Bowel Disease Risk in Women with Menopausal Disorders: A Population-Based Retrospective Cohort Study. Healthcare, 13(5), 578. https://doi.org/10.3390/healthcare13050578





