Transfusion Thresholds and Risk Factors of Acute Kidney Injury in Gastrointestinal Oncology Surgery: Insights from a Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Diagnostic Criteria
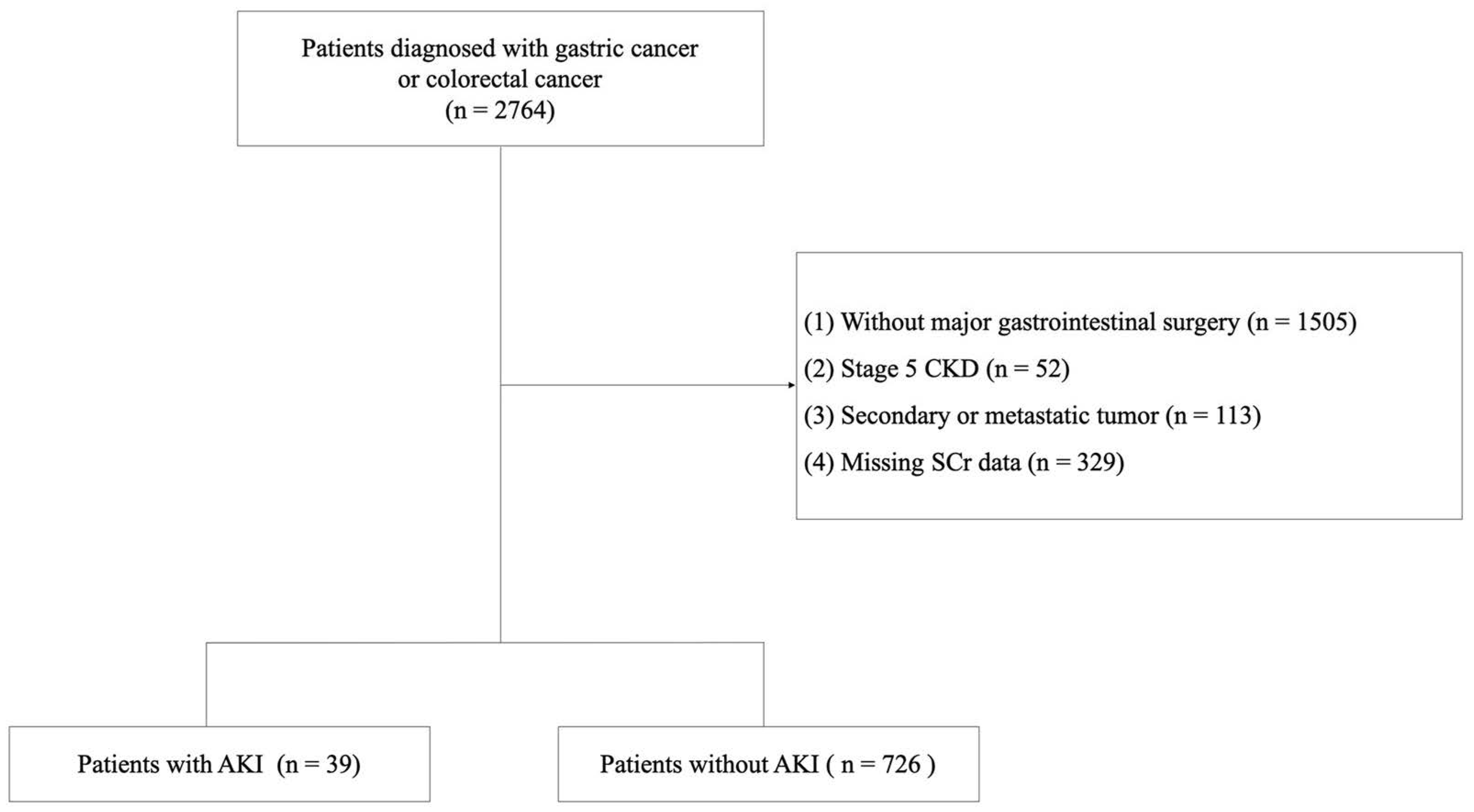
2.3. Inclusion and Exclusion Criteria
2.4. Surgical Procedure and Perioperative Management
2.5. Data Collection
2.6. Statistical Analysis
3. Results
3.1. Baseline Clinicopathological Characteristics
3.2. Short-Term Outcomes
3.3. Risk Factors for Postoperative AKI
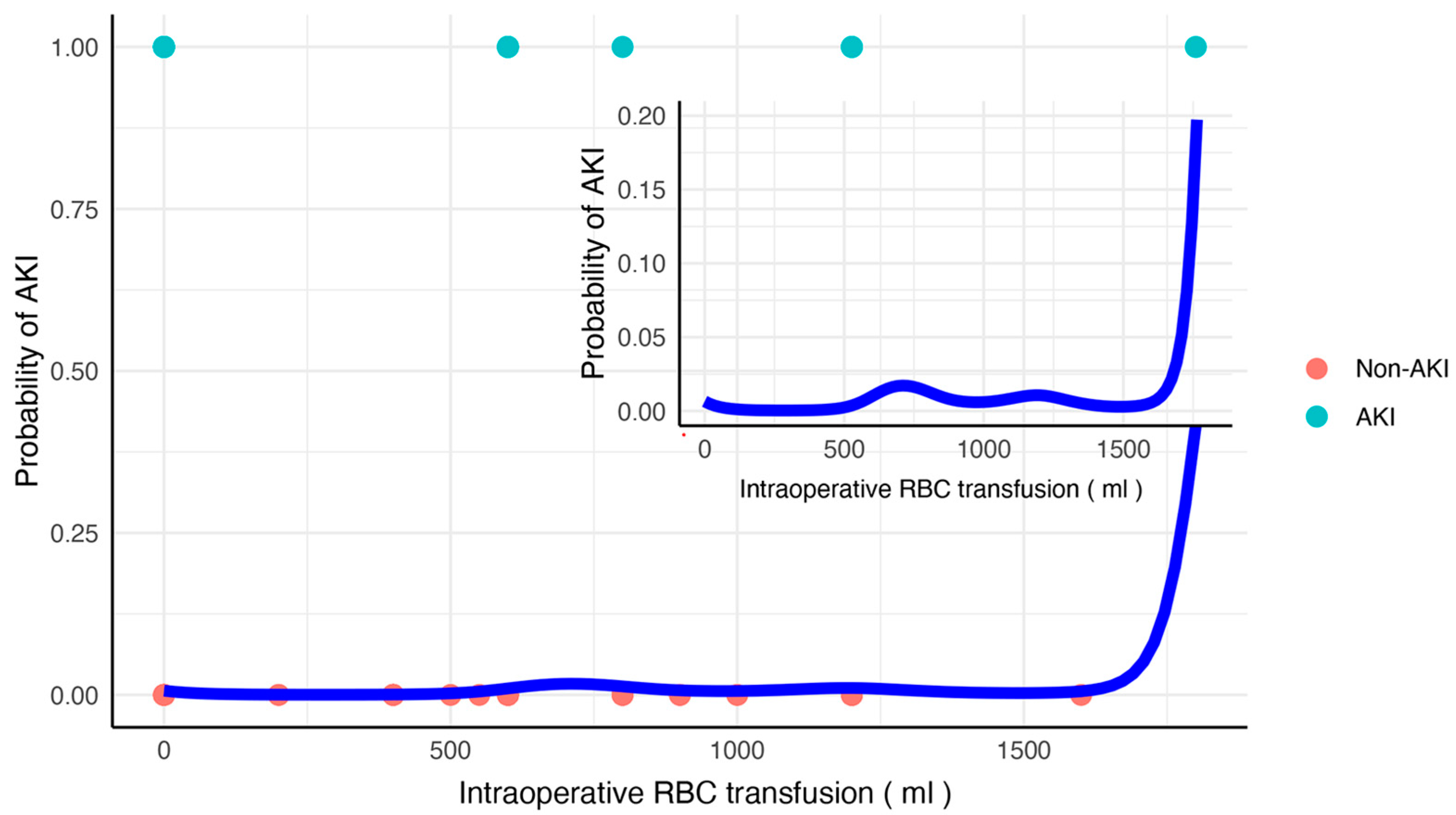
3.4. Dose–Effect Relationship Between Intraoperative RBC Transfusion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grams, M.E.; Sang, Y.; Coresh, J.; Ballew, S.; Matsushita, K.; Molnar, M.Z.; Szabo, Z.; Kalantar-Zadeh, K.; Kovesdy, C.P. Acute Kidney Injury After Major Surgery: A Retrospective Analysis of Veterans Health Administration Data. Am. J. Kidney Dis. 2016, 67, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Long, T.E.; Helgason, D.; Helgadottir, S.; Palsson, R.; Gudbjartsson, T.; Sigurdsson, G.H.; Indridason, O.S.; Sigurdsson, M.I. Acute Kidney Injury After Abdominal Surgery: Incidence, Risk Factors, and Outcome. Anesth. Analg. 2016, 122, 1912–1920. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Kim, S.C.; Kim, M.G.; Jo, S.K.; Cho, W.Y.; Kim, H.K. The incidence and risk factors of acute kidney injury after hepatobiliary surgery: A prospective observational study. BMC Nephrol. 2014, 15, 169. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Bhave, N.; Bragg-Gresham, J.; Balkrishnan, R.; Dietrich, X.; Eckard, A.; Eggers, P.W.; et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2018, 71 (Suppl. 1), A7, Erratum in Am. J. Kidney Dis. 2018, 71, 501. [Google Scholar] [CrossRef]
- Biteker, M.; Dayan, A.; Tekkeşin, A.İ.; Can, M.M.; Taycı, İ.; İlhan, E.; Şahin, G. Incidence, risk factors, and outcomes of perioperative acute kidney injury in noncardiac and nonvascular surgery. Am. J. Surg. 2014, 207, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.B.; Suneja, M. Cardiopulmonary bypass-associated acute kidney injury. Anesthesiology 2011, 114, 964–970. [Google Scholar] [CrossRef]
- Rosner, M.H.; Okusa, M.D. Acute kidney injury associated with cardiac surgery. Clin. J. Am. Soc. Nephrol. 2006, 1, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.R.; Kandler, K.; Nielsen, R.V.; Cornelius Jakobsen, P.; Knudsen, N.N.; Ranucci, M.; Christian Nilsson, J.; Ravn, H.B. Duration of critically low oxygen delivery is associated with acute kidney injury after cardiac surgery. Acta Anaesthesiol. Scand. 2019, 63, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Aloisio, T.; Carboni, G.; Ballotta, A.; Pistuddi, V.; Menicanti, L.; Frigiola, A.; Surgical and Clinical Outcome REsearch (SCORE) Group. Acute Kidney Injury and Hemodilution During Cardiopulmonary Bypass: A Changing Scenario. Ann. Thorac. Surg. 2015, 100, 95–100. [Google Scholar] [CrossRef]
- Ranucci, M.; Romitti, F.; Isgrò, G.; Cotza, M.; Brozzi, S.; Boncilli, A.; Ditta, A. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann. Thorac. Surg. 2005, 80, 2213–2220. [Google Scholar] [CrossRef]
- Mazzone, A.L.; Baker, R.A.; Gleadle, J.M. Mending a broken heart but breaking the kidney. Nephrology 2016, 21, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Lucy, M.; Krokhin, O.; Hayglass, K.; Pascoe, E.; Darroch, G.; Rush, D.; Nickerson, P.; Rigatto, C.; Reslerova, M. Mass spectrometry-based proteomic analysis of urine in acute kidney injury following cardiopulmonary bypass: A nested case-control study. Am. J. Kidney Dis. 2009, 53, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Rabb, H.; Griffin, M.D.; McKay, D.B.; Swaminathan, S.; Pickkers, P.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; Acute Dialysis Quality Initiative Consensus XIII Work Group. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J. Am. Soc. Nephrol. 2016, 27, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Karkouti, K. Transfusion and risk of acute kidney injury in cardiac surgery. Br. J. Anaesth. 2012, 109 (Suppl. 1), i29–i38. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Gameiro, J.; Agapito Fonseca, J.; Jorge, S.; Lopes, J.A. Acute Kidney Injury Definition and Diagnosis: A Narrative Review. J. Clin. Med. 2018, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Lenet, T.; McIsaac, D.I.; Hallet, J.H.; Jerath, A.; Lalu, M.M.; Nicholls, S.G.; Presseau, J.; Tinmouth, A.; Verret, M.; Wherrett, C.G.; et al. Intraoperative Blood Management Strategies for Patients Undergoing Noncardiac Surgery: The Ottawa Intraoperative Transfusion Consensus. JAMA Netw. Open 2023, 6, e2349559. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.R.; Kandler, K.; Nielsen, R.V.; Jakobsen, P.C.; Ranucci, M.; Ravn, H.B. Association between transfusion of blood products and acute kidney injury following cardiac surgery. Acta Anaesthesiol. Scand. 2020, 64, 1397–1404. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Spanou, L. Acute Kidney Injury: Diagnostic Approaches and Controversies. Clin. Biochem. Rev. 2016, 37, 153–175. [Google Scholar] [PubMed]
- Fowler, A.J.; Ahmad, T.; Phull, M.K.; Allard, S.; Gillies, M.A.; Pearse, R.M. Meta-analysis of the association between preoperative anaemia and mortality after surgery. Br. J. Surg. 2015, 102, 1314–1324. [Google Scholar] [CrossRef] [PubMed]
- Mathis, M.R.; Naik, B.I.; Freundlich, R.E.; Shanks, A.M.; Heung, M.; Kim, M.; Burns, M.L.; Colquhoun, D.A.; Rangrass, G.; Janda, A.; et al. Preoperative Risk and the Association between Hypotension and Postoperative Acute Kidney Injury. Anesthesiology 2020, 132, 461–475, Erratum in Anesthesiology 2020, 132, 937. [Google Scholar] [CrossRef] [PubMed]
- Han, S.S.; Baek, S.H.; Ahn, S.Y.; Chin, H.J.; Na, K.Y.; Chae, D.W.; Kim, S. Anemia Is a Risk Factor for Acute Kidney Injury and Long-Term Mortality in Critically Ill Patients. Tohoku J. Exp. Med. 2015, 237, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Ru, W.; Hu, C.; Shen, Y. Interaction of hemoglobin, transfusion, and acute kidney injury in patients undergoing cardiopulmonary bypass: A group-based trajectory analysis. Ren. Fail. 2022, 44, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Messmer, A.S.; Zingg, C.; Müller, M.; Gerber, J.L.; Schefold, J.C.; Pfortmueller, C.A. Fluid Overload and Mortality in Adult Critical Care Patients-A Systematic Review and Meta-Analysis of Observational Studies. Crit. Care Med. 2020, 48, 1862–1870. [Google Scholar] [CrossRef]
- Isaka, Y.; Hayashi, H.; Aonuma, K.; Horio, M.; Terada, Y.; Doi, K.; Fujigaki, Y.; Yasuda, H.; Sato, T.; Fujikura, T.; et al. Guideline on the use of iodinated contrast media in patients with kidney disease 2018. Clin Exp Nephrol 2020, 24, 1–44. [Google Scholar] [CrossRef]
- Romano, G.; Mastroianni, C.; Bancone, C.; Della Corte, A.; Galdieri, N.; Nappi, G.; De Santo, L.S. Leukoreduction program for red blood cell transfusions in coronary surgery: Association with reduced acute kidney injury and in-hospital mortality. J. Thorac. Cardiovasc. Surg. 2010, 140, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Messina, A.; Calatroni, M.; Castellani, G.; De Rosa, S.; Ostermann, M.; Cecconi, M. Understanding fluid dynamics and renal perfusion in acute kidney injury management. J. Clin. Monit. Comput. 2024. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, T.; Pugashetti, N.; Durbin-Johnson, B.; Dall’Era, M.A.; Evans, C.P.; deVere White, R.W.; Yap, S.A. Effect of Neoadjuvant Chemotherapy on Renal Function following Radical Cystectomy: Is there a Meaningful Impact? Bladder Cancer 2016, 2, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Stille, K.; Kribben, A.; Herget-Rosenthal, S. Incidence, severity, risk factors and outcomes of acute kidney injury in older adults: Systematic review and meta-analysis. J. Nephrol. 2022, 35, 2237–2250. [Google Scholar] [CrossRef]
- Marrelli, D.; Piccioni, S.A.; Carbone, L.; Petrioli, R.; Costantini, M.; Malagnino, V.; Bagnacci, G.; Rizzoli, G.; Calomino, N.; Piagnerelli, R.; et al. Posterior and Para-Aortic (D2plus) Lymphadenectomy after Neoadjuvant/Conversion Therapy for Locally Advanced/Oligometastatic Gastric Cancer. Cancers 2024, 16, 1376. [Google Scholar] [CrossRef]
- Zarbock, A.; Weiss, R.; Albert, F.; Rutledge, K.; Kellum, J.A.; Bellomo, R.; Grigoryev, E.; Candela-Toha, A.M.; Demir, Z.A.; Legros, V.; et al. Epidemiology of surgery associated acute kidney injury (EPIS-AKI): A prospective international observational multi-center clinical study. Intensive Care Med. 2023, 49, 1441–1455. [Google Scholar] [CrossRef]
- De La Vega-Méndez, F.M.; Estrada, M.I.; Zuno-Reyes, E.E.; Gutierrez-Rivera, C.A.; Oliva-Martinez, A.E.; Díaz-Villavicencio, B.; Calderon-Garcia, C.E.; González-Barajas, J.D.; Arizaga-Nápoles, M.; García-Peña, F.; et al. Blood transfusion reactions and risk of acute kidney injury and major adverse kidney events. J. Nephrol. 2024, 37, 951–960. [Google Scholar] [CrossRef]
- Kinnunen, E.M.; Zanobini, M.; Onorati, F.; Brascia, D.; Mariscalco, G.; Franzese, I.; Ruggieri, V.G.; Bounader, K.; Perrotti, A.; Musumeci, F.; et al. The impact of minor blood transfusion on the outcome after coronary artery bypass grafting. J. Crit. Care 2017, 40, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Klanderman, R.B.; Wijnberge, M.; Bosboom, J.J.; Roelofs, J.J.T.H.; de Korte, D.; van Bruggen, R.; Hollmann, M.W.; Vroom, M.B.; Veelo, D.P.; Juffermans, N.P.; et al. Differential effects of speed and volume on transfusion-associated circulatory overload: A randomized study in rats. Vox Sang. 2022, 117, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Marano, L.; Carbone, L.; Poto, G.E.; Calomino, N.; Neri, A.; Piagnerelli, R.; Fontani, A.; Verre, L.; Savelli, V.; Roviello, F.; et al. Antimicrobial Prophylaxis Reduces the Rate of Surgical Site Infection in Upper Gastrointestinal Surgery: A Systematic Review. Antibiotics 2022, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Walder, B.; Schafer, M.; Henzi, I.; Tramèr, M.R. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol. Scand. 2001, 45, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Brown, C.R.; Mazzulla, J.P.; Mok, M.S.; Nussdorf, R.T.; Rubin, P.D.; Schwesinger, W.H. Comparison of repeat doses of intramuscular ketorolac tromethamine and morphine sulfate for analgesia after major surgery. Pharmacotherapy. 1990, 10 Pt. 2, 45S–50S. [Google Scholar] [CrossRef]
- Ghai, B.; Jafra, A.; Bhatia, N.; Chanana, N.; Bansal, D.; Mehta, V. Opioid sparing strategies for perioperative pain management other than regional anaesthesia: A narrative review. J. Anaesthesiol. Clin. Pharmacol. 2022, 38, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Whelton, A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. Am. J. Med. 1999, 106, 13S–24S. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jeon, Y.T.; Kim, H.G.; Oh, A.Y.; Ryu, J.H.; Bae, Y.K.; Koo, C.H. Comparison between ketorolac- and fentanyl-based patient-controlled analgesia for acute kidney injury after robot-assisted radical prostatectomy: A retrospective propensity score-matched analysis. World J. Urol. 2023, 41, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jeon, Y.T.; Oh, A.Y.; Koo, C.H.; Bae, Y.K.; Ryu, J.H. Comparison of Postoperative Renal Function between Non-Steroidal Anti-Inflammatory Drug and Opioids for Patient-Controlled Analgesia after Laparoscopic Nephrectomy: A Retrospective Cohort Study. J. Clin. Med. 2020, 9, 2959. [Google Scholar] [CrossRef] [PubMed]
- Zager, R.A.; Gamelin, L.M. Pathogenetic mechanisms in experimental hemoglobinuric acute renal failure. Am. J. Physiol. 1989, 256 Pt 2, F446–F655. [Google Scholar] [CrossRef] [PubMed]
- Libório, A.B.; Barbosa, M.L.; Sá, V.B.; Leite, T.T. Impact of loop diuretics on critically ill patients with a positive fluid balance. Anaesthesia 2020, 75 (Suppl. 1), e134–e142. [Google Scholar] [CrossRef] [PubMed]
| AKI (n = 39) | Non-AKI (n = 726) | p | |
|---|---|---|---|
| Sex (Male, %) | 26 (66.7%) | 457 (62.9%) | 0.639 |
| Age (years) | 75.2 ± 11.8 | 64.8 ± 11.8 | <0.001 |
| Height (cm) | 162.7 ± 9.4 | 164.1 ± 7.7 | 0.511 |
| Weight (kg) | 67.8 ± 15.5 | 63.7 ± 10.7 | 0.114 |
| Weight loss (kg) | 0.8 ± 1.8 | 1.1 ± 2.9 | 0.459 |
| Comorbidity (%) | 29 (74.4%) | 394 (54.3%) | 0.014 |
| Diabetes (%) | 9 (23.1%) | 117 (16.1%) | 0.254 |
| Hypertension (%) | 19 (48.7%) | 271 (37.3%) | 0.153 |
| Cerebrovascular (%) | 8 (20.5%) | 56 (7.7%) | 0.012 |
| Cardiovascular (%) | 9 (23.1%) | 78 (10.7%) | 0.033 |
| Smoking (%) | 3 (7.7%) | 94 (13.0%) | 0.461 |
| Alcohol use (%) | 1 (2.6%) | 62 (8.6%) | 0.242 |
| Site | |||
| Gastric/colon/rectum | 8/18/13 | 238/254/234 | 0.218 |
| Preoperative laboratory tests | |||
| WBC (×109/L) | 7.0 ± 2.2 | 6.4 ± 2.3 | 0.110 * |
| Hb (g/L) | 109.9 ± 23.0 | 119.9 ± 21.5 | 0.003 * |
| Alb (g/L) | 37.0 ± 3.8 | 39.5 ± 4.4 | <0.001 * |
| Alt (U/L) | 14.5 ± 7.4 | 19.0 ± 16.9 | 0.055 * |
| BUN (mmol/L) | 6.7 ± 3.4 | 5.4 ± 2.1 | 0.021 * |
| SCr (μmol/L) | 95.7 ± 45.0 | 68.8 ± 20.7 | <0.001 * |
| Glu (mmol/L) | 6.2 ± 2.2 | 5.6 ± 1.6 | 0.250 * |
| Minimal invasive surgery (%) | 18 (46.2%) | 432 (59.5%) | 0.113 |
| Intraoperative RBC transfusion (mL) | 374.4 ± 472.8 | 180.7 ± 375.0 | 0.001 * |
| Intraoperative colloid (mL) | 243.6 ± 378.2 | 247.9 ± 307.1 | 0.654 * |
| Postoperative RBC transfusion (mL) | 1394.9 ± 2293.6 | 136.0 ± 460.2 | <0.001 * |
| Postoperative colloid (mL) | 65.8 ± 171.3 | 32.4 ± 180.0 | 0.016 * |
| Albumin intravenous infusion (mL) | 236.6 ± 373.7 | 51.9 ± 90.0 | <0.001 * |
| PCA (%) | 17 (43.6%) | 554 (76.4%) | <0.001 |
| Diuretics (%) | 35 (89.7%) | 247 (34.0%) | <0.001 |
| Vasopressor (%) | 35 (89.7%) | 504 (69.4%) | 0.007 |
| Aminoglycoside antibiotics (%) | 7 (17.9%) | 182 (25.1%) | 0.315 |
| Non-selective COX-2 inhibitors (%) | 36 (92.3%) | 657 (90.5%) | 0.706 |
| Selective COX-2 inhibitors (%) | 10 (25.6%) | 133 (18.3%) | 0.253 |
| AKI (n = 39) | Non-AKI (n = 726) | p | |
|---|---|---|---|
| Postoperative LOS (d) | 25.4 ± 22.5 | 12.3 ± 7.9 | <0.001 |
| First oral feeding (d) | 8.8 ± 12.4 | 5.3 ± 4.4 | 0.255 |
| ICU stay (d) | 21 (53.8%) | 163 (22.5%) | <0.001 |
| Length of ICU stay (d) | 10.5 ± 11.2 | 4.3 ± 3.9 | 0.002 |
| Ventilator (%) | 18 (46.2%) | 49 (6.7%) | <0.001 |
| RRT (%) | 2 (5.1%) | 1 (0.1%) | 0.007 |
| Complications (%) | 19 (48.3%) | 103 (14.2%) | <0.001 |
| Infection (%) | 10 (25.6%) | 59 (8.1%) | <0.001 |
| Leakage (%) | 3 (7.7%) | 16 (2.2%) | 0.067 |
| Obstruction (%) | 1 (2.6%) | 13 (1.8%) | 0.522 |
| Hemorrhage (%) | 5 (12.8%) | 17 (2.3%) | 0.004 |
| 30 d re-admission (%) | 5 (13.9%) | 16 (2.4%) | 0.003 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Sex | ||||||
| Female | Ref | |||||
| Male | 1.177 | 0.595–2.330 | 0.639 | |||
| Age (per 10 years) | 2.501 | 1.767–3.540 | <0.001 | 1.567 | 1.103–2.423 | 0.043 |
| Weight loss | 0.940 | 0.800–1.106 | 0.458 | |||
| Comorbidity | ||||||
| Diabetes | 1.562 | 0.722–3.375 | 0.257 | |||
| Hypertension | 1.595 | 0.836–3.042 | 0.156 | |||
| Cerebrovascular | 3.088 | 1.355–7.036 | 0.007 | |||
| Cardiovascular | 2.492 | 1.141–5.443 | 0.022 | |||
| Site | ||||||
| Gastric | Ref | |||||
| Colon | 2.108 | 0.900–4.939 | 0.086 | |||
| Rectum | 1.653 | 0.673–4.061 | 0.273 | |||
| Minimal invasive surgery | 0.531 | 0.276–1.023 | 0.059 | |||
| Intraoperative RBC transfusion (per 1000 mL) | 2.331 | 1.296–4.195 | 0.005 | 1.992 | 1.311–3.027 | 0.001 |
| Intraoperative colloid (per 1000 mL) | 2.176 | 0.919–5.149 | 0.077 | |||
| Postoperative RBC transfusion (per 1000 mL) | 2.938 | 2.088–4.134 | <0.001 | |||
| Postoperative colloid (per 1000 mL) | 1.561 | 1.249–1.949 | <0.001 | |||
| Albumin intravenous infusion (per 100 g) | 1.781 | 1.457–2.178 | <0.001 | |||
| PCA | 0.239 | 0.124–0.460 | <0.001 | 0.388 | 0.163–0.928 | 0.033 |
| Diuretics | 16.969 | 5.963–48.287 | <0.001 | 5.495 | 1.720–17.557 | 0.004 |
| Vasopressor | 3.854 | 1.354–10.975 | 0.012 | |||
| Aminoglycoside antibiotics | 0.654 | 0.284–1.507 | 0.319 | |||
| Non-selective COX-2 inhibitors | 1.260 | 0.378–4.199 | 0.706 | |||
| Selective COX-2 inhibitors | 1.537 | 0.731–3.232 | 0.256 | |||
| Complications | ||||||
| Infection | 3.898 | 1.811–8.390 | 0.001 | |||
| Leakage | 3.698 | 1.030–13.270 | 0.045 | |||
| Obstruction | 1.443 | 0.184–11.324 | 0.727 | |||
| Hemorrhage | 6.133 | 2.136–17.611 | 0.001 | |||
| Preoperative laboratory tests | ||||||
| WBC (per 10 × 109/L) | 2.363 | 0.686–8.146 | 0.173 | |||
| Hb (per 10 g/L) | 0.809 | 0.697–0.940 | 0.006 | |||
| Alb (per 10 g/L) | 0.272 | 0.123–0.600 | 0.001 | |||
| Alt (per 10 U/L) | 0.679 | 0.433–1.065 | 0.092 | |||
| BUN (per 10 mmol/L) | 4.640 | 1.650–1.466 | <0.001 | |||
| SCr (per 10 μmol/L) | 1.325 | 1.197–1.466 | <0.001 | 1.173 | 1.044–1.319 | 0.007 |
| Glu (per 5 mmol/L) | 2.226 | 1.049–4.721 | 0.037 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; He, Q.; Yang, C.; Zhou, Z.; He, Y.; Yu, C.; Yao, D.; Zheng, L.; Huang, Y.; Li, Y. Transfusion Thresholds and Risk Factors of Acute Kidney Injury in Gastrointestinal Oncology Surgery: Insights from a Retrospective Study. Healthcare 2025, 13, 525. https://doi.org/10.3390/healthcare13050525
Ma S, He Q, Yang C, Zhou Z, He Y, Yu C, Yao D, Zheng L, Huang Y, Li Y. Transfusion Thresholds and Risk Factors of Acute Kidney Injury in Gastrointestinal Oncology Surgery: Insights from a Retrospective Study. Healthcare. 2025; 13(5):525. https://doi.org/10.3390/healthcare13050525
Chicago/Turabian StyleMa, Shuai, Qi He, Chengcan Yang, Zhiyuan Zhou, Yining He, Chaoran Yu, Danhua Yao, Lei Zheng, Yuhua Huang, and Yousheng Li. 2025. "Transfusion Thresholds and Risk Factors of Acute Kidney Injury in Gastrointestinal Oncology Surgery: Insights from a Retrospective Study" Healthcare 13, no. 5: 525. https://doi.org/10.3390/healthcare13050525
APA StyleMa, S., He, Q., Yang, C., Zhou, Z., He, Y., Yu, C., Yao, D., Zheng, L., Huang, Y., & Li, Y. (2025). Transfusion Thresholds and Risk Factors of Acute Kidney Injury in Gastrointestinal Oncology Surgery: Insights from a Retrospective Study. Healthcare, 13(5), 525. https://doi.org/10.3390/healthcare13050525






