Effects of a Self-Management Program on Adults with Stroke: A Quasi-Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
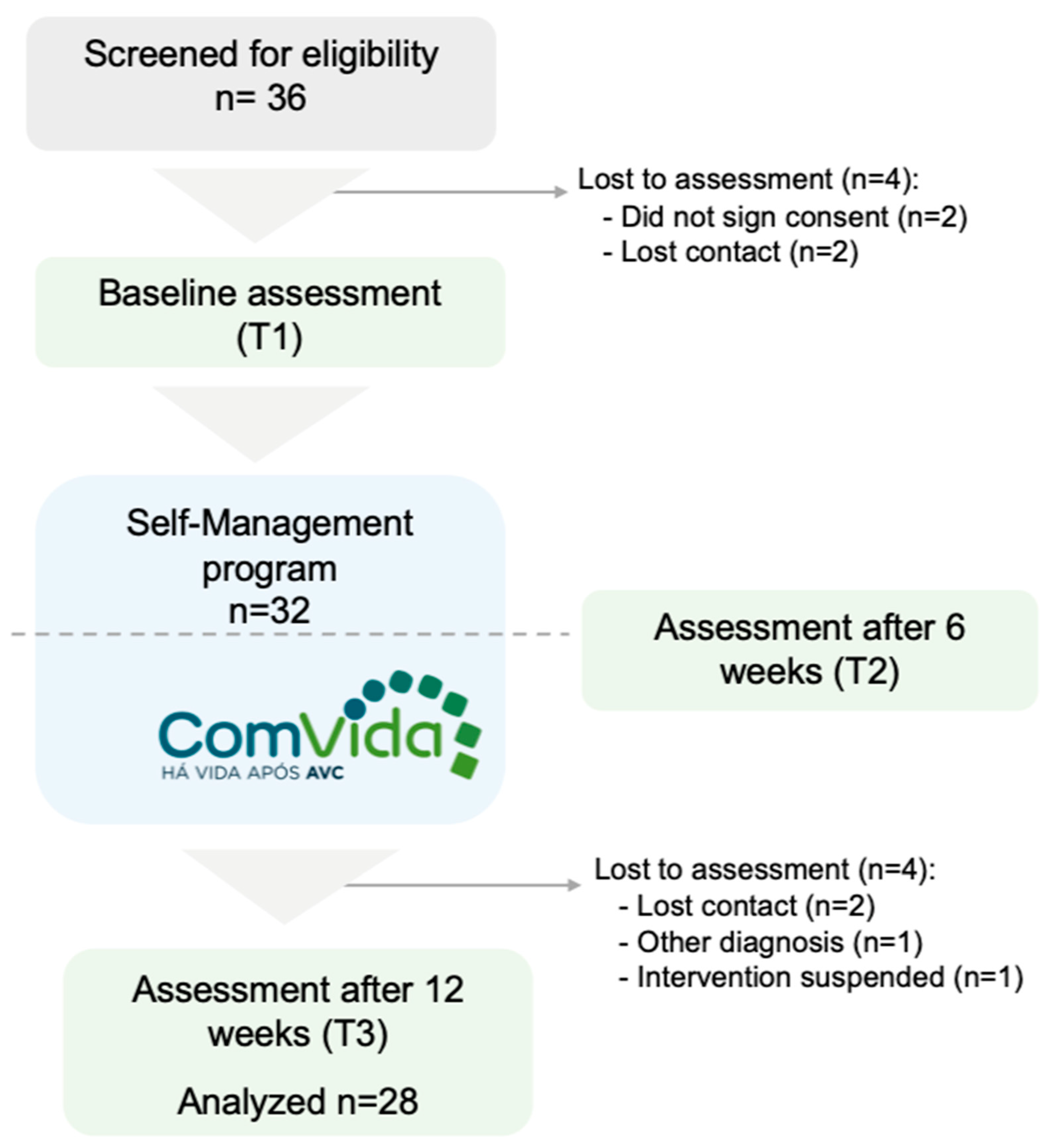
2.2. Setting and Participants
2.3. Intervention
2.4. Instruments
2.5. Data Collection
2.6. Data Analysis
2.7. Ethical Considerations
3. Results
3.1. Effect on Self-Efficacy
3.2. Effect on Physical Function
3.3. Effect on Emotional State
3.4. Effect on Health-Related Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, S.J.; Pinnock, H.; Epiphaniou, E.; Pearce, G.; Parke, H.L.; Schwappach, A.; Purushotham, N.; Jacob, S.; Griffiths, C.J.; Greenhalgh, T.; et al. A rapid synthesis of the evidence on interventions supporting self-management for people with long-term conditions: PRISMS—Practical systematic RevIew of Self-Management Support for long-term conditions. Health Soc. Care Deliv. Res. 2014, 2, 1–580. [Google Scholar] [CrossRef] [PubMed]
- Audulv, Å.; Hutchinson, S.; Warner, G.; Kephart, G.; Versnel, J.; Packer, T.L. Managing everyday life: Self-management strategies people use to live well with neurological conditions. Patient Educ. Couns. 2021, 104, 413–421. [Google Scholar] [CrossRef] [PubMed]
- NICE. National Clinical Guidelines for Stroke for the United Kingdom and Ireland; King’s College London: London, UK, 2023. [Google Scholar]
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A system. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef] [PubMed]
- DGS. Plano Nacional de Saúde 2021–2030. Saúde Sustentável: De Tod@s para Tod@s [National Health Plan 2021–2030. Sustainable Health: From Everyone for Everyone]; DGS: Lisboa, Portugal, 2021. (In Portuguese) [Google Scholar]
- Abbafati, C.; Machado, D.B.; Cislaghi, B.; Salman, O.M.; Karanikolos, M.; McKee, M.; Abbas, K.M.; Brady, O.J.; Larson, H.J.; Trias-Llimós, S.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Mountain, A.; Patrice Lindsay, M.; Teasell, R.; Salbach, N.M.; de Jong, A.; Foley, N.; Bhogal, S.; Bains, N.; Bowes, R.; Cheung, D.; et al. Canadian Stroke Best Practice Recommendations: Rehabilitation, Recovery, and Community Participation following Stroke. Part Two: Transitions and Community Participation Following Stroke. Int. J. Stroke 2020, 15, 789–806. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Owolabi, M.O.; Feigin, V.L.; Abd-Allah, F.; Akinyemi, R.O.; Bhattacharjee, N.V.; Brainin, M.; Cao, J.; Caso, V.; Dalton, B.; et al. Pragmatic solutions to reduce the global burden of stroke: A World Stroke Organization–Lancet Neurology Commission. Lancet Neurol. 2023, 22, 1160–1206. [Google Scholar] [CrossRef] [PubMed]
- Fryer, C.E.; Luker, J.A.; McDonnell, M.N.; Hillier, S.L. Self-Management Programs for Quality of Life in People with Stroke. Stroke 2016, 47, e266–e267. [Google Scholar] [CrossRef]
- Warner, G.; Packer, T.; Villeneuve, M.; Audulv, A.; Versnel, J. A systematic review of the effectiveness of stroke self-management programs for improving function and participation outcomes: Self-management programs for stroke survivors. Disabil. Rehabil. 2015, 37, 2141–2163. [Google Scholar] [CrossRef]
- Parke, H.L.; Epiphaniou, E.; Pearce, G.; Taylor, S.J.C.; Sheikh, A.; Griffiths, C.J.; Greenhalgh, T.; Pinnock, H. Self-management support interventions for stroke survivors: A systematic meta-review. PLoS ONE 2015, 10, e0131448. [Google Scholar] [CrossRef]
- Prados-Román, E.; Cabrera-Martos, I.; Martín-Nuñez, J.; Valenza-Peña, G.; Granados-Santiago, M.; Valenza, M.C. Effectiveness of self-management interventions during the peri-hospitalization period in patients with stroke: A systematic review and meta-analysis. Clin. Rehabil. 2024, 38, 34–46. [Google Scholar] [CrossRef]
- Murphy, M. Helping people help themselves. J. AWWA 2000, 92, 139–148. [Google Scholar] [CrossRef]
- Lau, S.C.L.; Judycki, S.; Mix, M.; DePaul, O.; Tomazin, R.; Hardi, A.; Wong, A.W.K.; Baum, C. Theory-Based Self-Management Interventions for Community-Dwelling Stroke Survivors: A Systematic Review and Meta-Analysis. Am. J. Occup. Ther. 2022, 76, 7604205010. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Self-efficacy: Toward a unifying theory of behavioral change. Psychol. Rev. 1977, 84, 191–215. [Google Scholar] [CrossRef]
- Gangwani, R.; Cain, A.; Collins, A.; Cassidy, J.M. Leveraging Factors of Self-Efficacy and Motivation to Optimize Stroke Recovery. Front. Neurol. 2022, 13, 823202. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.H.S.; Chau, J.P.C.; Lam, S.K.Y.; Saran, R.; Choi, K.C.; Zhao, J.; Thompson, D.R. Association between participation self-efficacy and participation in stroke survivors. BMC Neurol. 2022, 22, 361. [Google Scholar] [CrossRef]
- Szczepańska-Gieracha, J.; Mazurek, J. The role of self-efficacy in the recovery process of stroke survivors. Psychol. Res. Behav. Manag. 2020, 13, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.S.; Abolhassani, S.; Alimohammadi, N.; Roghani, T. Investigating the effect of self-management program on stroke’s patients’ self-efficacy. BMC Neurol. 2022, 22, 360. [Google Scholar] [CrossRef]
- Pereira, C.M.; Matos, M.; Carvalho, D.; Macedo, P.; Calheiros, J.M.; Alves, J.; Ferreira, L.P.; Dias, T.L.; Madeira, R.N.; Jones, F. Building Bridges between People with Stroke, Families, and Health Professionals: Development of a Blended Care Program for Self-Management. J. Clin. Med. 2024, 13, 300. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.; Gage, H.; Drummond, A.; Bhalla, A.; Grant, R.; Lennon, S.; McKevitt, C.; Riazi, A.; Liston, M. Feasibility study of an integrated stroke self-management programme: A cluster-randomised controlled trial. BMJ Open 2016, 6, e008900. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.; Jones, F.; Glenfield, P.; Lennon, S. Bridges self-management program for people with stroke in the community: A feasibility randomized controlled trial. Int. J. Stroke 2015, 10, 697–704. [Google Scholar] [CrossRef]
- Hale, L.; Jones, F.; Mulligan, H.; Levack, W.; Smith, C.; Claydon, L.; Milosavljevic, S.; Taylor, D.; Allan, J.; MacKenzie, N.; et al. Developing the bridges self-management programme for New Zealand stroke survivors: A case study. Int. J. Ther. Rehabil. 2014, 21, 381–388. [Google Scholar] [CrossRef]
- Hale, L.; McCulloch, M.; De Ruiter, S.; Wihongia, E.; Norlinga, E.M.; Gorczynski, D.; Kennedy, P.; Jones, F. Implementing and evaluating the bridges stroke self-management programme into a new zealand district health board stroke service: A case study. N. Z. J. Physiother. 2021, 49, 58–69. [Google Scholar] [CrossRef]
- Singer, B.; Jones, F.; Lennon, S.; Singer, B. Adapting the Bridges stroke self-management programme for use in Australia. Int. J. Ther. Rehabil. 2018, 25, 414–423. [Google Scholar] [CrossRef]
- Jones, F.; Partridge, C.; Reid, F. The Stroke Self-Efficacy Questionnaire: Measuring individual confidence in functional performance after stroke. J. Clin. Nurs. 2008, 17, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Riazi, A.; Aspden, T.; Jones, F. Stroke self-efifcacy questionnaire: A Rasch-refined measure of confidence post stroke. J. Rehabil. Med. 2014, 46, 406–412. [Google Scholar] [CrossRef]
- Figueira, C. Adaptação Cultural e Contributo Para a Validação do Stroke Self-Efficacy Questionnaire (SSEQ) [Cultural Adaptation and Contribution to the Validation of the Portuguese Version of the Stroke Self-Efficacy Questionnaire (SSEQ)]; Escola Superior de Saúde do Instituto Politécnico de Setúbal: Setúbal, Portugal, 2023. (In Portuguese) [Google Scholar]
- Duncan, P.W.; Lai, S.M.; Bode, R.K.; Perera, S.; DeRosa, J. Stroke impact scale-16: A brief assessment of physical function. Neurology 2003, 60, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Bode, R.K.; Lai, S.M.; Perera, S. Rasch analysis of a new stroke-specific outcome scale: The stroke impact scale. Arch. Phys. Med. Rehabil. 2003, 84, 950–963. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, M.; Pereira, C.M. Contributo Para a Adaptação e Validação da Versão Portuguesa do Questionário de Impacto do AVC-Versão 3.0 e Versão Proxy [Contribution to the Adaptation and Validation of the Portuguese Version of the Stroke Impact Questionnaire—Version 3.0 and Proxy]; Escola Superior de Saúde do Instituto Politécnico de Setúbal: Setúbal, Portugal, 2009. (In Portuguese) [Google Scholar]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review Ingvar. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Pais-Ribeiro, J.; Silva, I.; Ferreira, T.; Martins, A.; Meneses, R.; Baltar, M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol. Health Med. 2007, 2, 225–237. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Pais-Ribeiro, J.L. O importante é a Saúde: Estudo de Adaptação de um Instrumento para Avaliar o Estado de Saúde; Fundação Merck Sharp & Dohme: Lisboa, Portugal, 2005. [Google Scholar]
- Soh, S.E.; Morello, R.; Ayton, D.; Ahern, S.; Scarborough, R.; Zammit, C.; Brand, M.; Stirling, R.G.; Zalcberg, J. Measurement properties of the 12-item Short Form Health Survey version 2 in Australians with lung cancer: A Rasch analysis. Health Qual. Life Outcomes 2021, 19, 157. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Guo, Y.; Shenkman, E.; Muller, K. Assessing the reliability of the short form 12 (SF-12) health survey in adults with mental health conditions: A report from the wellness incentive and navigation (WIN) study. Health Qual. Life Outcomes 2018, 16, 34. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.J.; Hocking, C.; Theadom, A.; McPherson, K.M. Exploring challenges at 6 months after stroke: What is important to patients for self-management? Int. J. Ther. Rehabil. 2018, 25, 565–575. [Google Scholar] [CrossRef]
- O’Callaghan, G.; Fahy, M.; Murphy, P.; Langhorne, P.; Galvin, R.; Horgan, F. Effectiveness of interventions to support the transition home after acute stroke: A systematic review and meta-analysis. BMC Health Serv. Res. 2022, 22, 1095. [Google Scholar] [CrossRef] [PubMed]
- Roesner, K.; Scheffler, B.; Kaehler, M.; Schmidt-Maciejewski, B.; Boettger, T.; Saal, S. Effects of physical therapy modalities for motor function, functional recovery, and post-stroke complications in patients with severe stroke: A systematic review update. Syst. Rev. 2024, 13, 270. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. Health promotion by social cognitive means. Health Educ. Behav. 2004, 31, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Lo, S.H.S.; Chang, A.M.; Chau, J.P.C. Stroke self-management support improves survivors’ self-efficacy and outcome expectation of self-management behaviors. Stroke 2018, 49, 758–760. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.L.; Apps, L.; Kreit, E.; Mullis, R.; Mant, J.; Davies, M.J. The feasibility of a self-management programme (My Life After Stroke; MLAS) for stroke survivors. Disabil. Rehabil. 2023, 45, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Nott, M.; Wiseman, L.; Seymour, T.; Pike, S.; Cuming, T.; Wall, G. Stroke self-management and the role of self-efficacy. Disabil. Rehabil. 2021, 43, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
| Reflection: | Supporting people to reflect on their progress and useful strategies, helping them to attribute changes and improvements to personal effort, not the skills of the health professional. |
| Problem-solving: | Supporting people to think through problems together and come up with different ideas, strategies, and ways to adjust, rather than relying on suggestions from health professionals. |
| Self-discovery: | Supporting people to try new ways of doing things and try out different activities and strategies, which may involve taking risks. |
| Goal setting: | Avoiding clinician-led goals, focusing on patient priorities, and what is meaningful and relevant. Encouraging small steps to promote feelings of success and working towards longer-term aspirational goals. |
| Taking action: | Supporting people to do more, even small things, and appraising their efforts. |
| Support: | Supporting people to access their support network, and available resources in the community. |
| Knowledge: | Supporting people to develop greater self-awareness about what works for their own situation and challenges, and giving them meaningful information. |
| Demographic Characteristics | N | % | Mean | SD | Min–Max |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 19 | 67.9 | |||
| Male | 9 | 32.1 | |||
| Age (years) | 66.4 | 12 | 34–86 | ||
| Marital status | |||||
| Single | 4 | 14.3 | |||
| Married | 20 | 71.4 | |||
| Divorced | 3 | 10.7 | |||
| Widow | 1 | 3.6 | |||
| Educational level | |||||
| Primary school | 11 | 39.3 | |||
| High school diploma | 11 | 39.3 | |||
| University degree | 6 | 21.4 | |||
| Professional status | |||||
| Unemployed | 3 | 10.7 | |||
| Retired | 17 | 60.7 | |||
| Medical leave/unable to work | 8 | 28.6 | |||
| Recruitment context | |||||
| Hospital | 21 | 75 | |||
| Community | 7 | 25 | |||
| Type of stroke | |||||
| Ischemic | 24 | 85.7 | |||
| Hemorrhagic | 4 | 14.3 | |||
| Time after stroke (months) | 1.2 | 1.5 | 0.1–5 | ||
| Length of hospital stay (days) | 19.9 | 18.3 | 2–90 | ||
| In-patient rehabilitation (yes) | 8 | 28.4 | |||
| Length of rehabilitation stay (months) | 1.3 | 1.4 | 0.23–4 | ||
| Outcome | Baseline Mean (SD) | 6 Weeks Mean (SD) | 12 Weeks Mean (SD) | Effect Size (6 Weeks) | Effect Size (12 Weeks) |
|---|---|---|---|---|---|
| Self-Efficacy (SSEQ Total) | 23.3 (7.7) | 29.6 (10.5) | 33.3 (6.1) | 0.67 (p < 0.001) | 1.57 (p < 0.001) |
| Physical Function (SIS-16) | 47.5 (12.2) | 61.2 (13.6) | 67.2 (12.9) | 1.31 (p < 0.001) | 1.81 (p < 0.001) |
| Emotional State (HADS Total) | 12.6 (6.9) | 8.6 (5.9) | 5.9 (5.2) | −0.64 (p = 0.002) | −1.12 (p < 0.001) |
| Quality of Life | |||||
| SF-12v2—PCS | 31.9 (4.9) | 34.9 (6.5) | 35.2 (6.01) | 1.047 (p = 0.019) | 1.58 (p = 0.012) |
| SF-12v2—MCS | 41.9 (5.7) | 41.9 (6.12) | 44.4 (6.7) | 0.96 (p = 0.096) | 1.49 (p = 0.018) |
| Total | Subscales | |||||
|---|---|---|---|---|---|---|
| Activity | Self-Management | |||||
| Pearson | p-Value | Pearson | p-Value | Pearson | p-Value | |
| SSEQ Total | ||||||
| Difference at 6 weeks | 0.481 ** | 0.010 | 0.618 ** | <0.001 | 0.084 | 0.671 |
| Difference 6–12 weeks | 0.845 ** | <0.001 | 0.875 ** | <0.001 | 0.35 | 0.068 |
| Difference at 12 weeks | 0.592 ** | <0.001 | 0.697 ** | <0.001 | 0.282 | 0.146 |
| SIS16 | ||||||
| Difference at 6 weeks | 0.677 ** | <0.001 | ||||
| Difference 6–12 weeks | 0.898 ** | <0.001 | ||||
| Difference at 12 weeks | 0.627 ** | <0.001 | ||||
| HADS | HADS-A | HADS-D | ||||
| Difference at 6 weeks | 0.674 ** | <0.001 | 0.481 ** | 0.01 | ||
| Difference 6–12 weeks | 0.725 ** | <0.001 | 0.635 ** | <0.001 | ||
| Difference at 12 weeks | 0.615 ** | <0.001 | 0.414 * | 0.029 | ||
| SF-12v2 | PCS | MCS | ||||
| Difference at 6 weeks | 0.619 ** | <0.001 | 0.625 ** | <0.001 | ||
| Difference 6–12 weeks | 0.587 * | 0.001 | 0.616 ** | <0.001 | ||
| Difference at 12 weeks | 0.446 * | 0.017 | 0.468 * | 0.012 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, C.M.; Branco, D.; Salvador, D.; Dias, T.L.; Carvalho, D.; Matos, M.; Rodrigues, S.; Calheiros, J.M.; Marques, A.M.; Jones, F. Effects of a Self-Management Program on Adults with Stroke: A Quasi-Experimental Study. Healthcare 2025, 13, 495. https://doi.org/10.3390/healthcare13050495
Pereira CM, Branco D, Salvador D, Dias TL, Carvalho D, Matos M, Rodrigues S, Calheiros JM, Marques AM, Jones F. Effects of a Self-Management Program on Adults with Stroke: A Quasi-Experimental Study. Healthcare. 2025; 13(5):495. https://doi.org/10.3390/healthcare13050495
Chicago/Turabian StylePereira, Carla M., Daniela Branco, Dina Salvador, Teresa L. Dias, Daniel Carvalho, Mara Matos, Sandra Rodrigues, José M. Calheiros, António Manuel Marques, and Fiona Jones. 2025. "Effects of a Self-Management Program on Adults with Stroke: A Quasi-Experimental Study" Healthcare 13, no. 5: 495. https://doi.org/10.3390/healthcare13050495
APA StylePereira, C. M., Branco, D., Salvador, D., Dias, T. L., Carvalho, D., Matos, M., Rodrigues, S., Calheiros, J. M., Marques, A. M., & Jones, F. (2025). Effects of a Self-Management Program on Adults with Stroke: A Quasi-Experimental Study. Healthcare, 13(5), 495. https://doi.org/10.3390/healthcare13050495





