Evaluation of ABL90 and ABL800 Radiometer Blood Gas Analyzers: Challenges and Applications in Point-of-Care Cancer Diagnostics in Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Settings
2.3. Study Population
2.4. Measurement Tool
2.5. Inclusion and Exclusion Criteria
2.6. Data Collection
2.7. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dave, V.P.; Ngo, T.A.; Pernestig, A.K.; Tilevik, D.; Kant, K.; Nguyen, T.; Wolff, A.; Bang, D.D. MicroRNA amplification and detection technologies: Opportunities and challenges for point-of-care diagnostics. Lab. Investig. 2019, 99, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Noah, N.M.; Ndangili, P.M. Current trends of nanobiosensors for point-of-care diagnostics. J. Anal. Methods Chem. 2019, 2019, 2179718. [Google Scholar] [CrossRef] [PubMed]
- Haney, K.; Tandon, P.; Divi, R.; Ossandon, M.R.; Baker, H.; Pearlman, P.C. The role of affordable, point-of-care technologies for cancer care in low-and middle-income countries: A review and commentary. IEEE J. Transl. Eng. Health Med. 2017, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, N.R.; Wada, M.; Fang, M.L.; Sixsmith, A. Defining organizational contributions to sustaining an ageing workforce: A bibliometric review. Eur. J. Ageing 2019, 16, 337–361. [Google Scholar] [CrossRef]
- Chandra, P.; Tan, Y.N.; Singh, S.P. (Eds.) Next Generation Point-of-Care Biomedical Sensors Technologies for Cancer Diagnosis; Springer: Singapore, 2017; pp. 10–25. [Google Scholar] [CrossRef]
- Hazra, R.S.; Hasan Khan, M.R.; Kale, N.; Tanha, T.; Khandare, J.; Ganai, S.; Quadir, M. Bioinspired materials for wearable devices and point-of-care testing of cancer. ACS Biomater. Sci. Eng. 2022, 9, 2103–2128. [Google Scholar] [CrossRef]
- Sandbhor Gaikwad, P.; Banerjee, R. Advances in point-of-care diagnostic devices in cancers. Analyst 2018, 143, 1326–1348. [Google Scholar] [CrossRef]
- Khabour, O.F.; Al Ali, K.H.; Mahallawi, W.H. Occupational infection and needle stick injury among clinical laboratory workers in Al-Madinah city, Saudi Arabia. J. Occup. Med. Toxicol. 2018, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Alenazy, A.A.; Al-Shammari, N.H.; Aldhafeeri, M.M.; Alanzi, N.G.; Ibrahim al-Shdaid, A.; Almutairi, R.A. Assessing laboratory technician and nurse knowledge, attitudes, and practices related to effective specimen collection and handling: An opportunity to improve quality in Saudi healthcare settings. J. Saf. Stud. 2022, 5, 1496–1504. [Google Scholar]
- Setiawan, L.; Graef, K.; Schmolze, D.; Alem, A.; Taylor, L. Building pathology capacity in sub-Saharan Africa to improve breast cancer diagnosis and treatment: Training laboratory technicians in high-quality manual immunohistochemistry. BMC Cancer 2024, 24, 32. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Bennett, K.M.; Deignan, J.L.; Hendrix, E.C.; Orton, S.M.; Verma, S.; Schutzbank, T.E. Molecular pathology curriculum for medical laboratory scientists: A report of the association for molecular pathology training and education committee. J. Mol. Diagn. 2014, 16, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Malaysia, K.K.; Kesihatan Wakaf Bharu, W.B. Hyperkalemia measurement between blood gas analyser and main laboratory biochemistry analyser. Med. J. Malaysia 2021, 76, 157–162. [Google Scholar]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Liu, Y. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020, 146, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, S.; Alhadidi, T.; Ben Azouz, M. Breast cancer detection using low-frequency bioimpedance device. Breast Cancer Targets Ther. 2020, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, I.G.; Almadi, M.; Alsohaibani, F.; Mosli, M.; De Vol, E.B.; Abaalkhail, F.; Alqahtani, S.A. Epidemiology of pancreatic cancer in Saudi Arabia: A retrospective analysis of pancreatic cancer diagnosed in Saudi Arabia between 2004 and 2015. Clin. Exp. Gastroenterol. 2021, 14, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Al-Mandeel, H.M.; Sagr, E.; Sait, K.; Latifah, H.M.; Al-Obaid, A.; Al-Badawi, I.A.; Brignardello-Petersen, R. Clinical practice guidelines on the screening and treatment of precancerous lesions for cervical cancer prevention in Saudi Arabia. Ann. Saudi Med. 2016, 36, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Albadr, H. Designing a Decision Support System for Improving Medical Devices Maintenance in Saudi Arabia. Ph.D. Thesis, Brunel University London, London, UK, 2019. [Google Scholar]
- Alshalani, A.J.; Salama, K.F. Assessment of occupational safety practices among medical laboratory staff in Governmental Hospitals in Riyadh, Saudi Arabia. J. Saf. Stud. 2019, 5, 1–23. [Google Scholar] [CrossRef]
- Rasmi, Y.; Abubakar, F.; Suleiman, W.; Wong, K.W.; Biswas, A.; Yusof, N.A.; Gopal, K. Emerging point-of-care biosensors for rapid diagnosis of COVID-19: Current progress, challenges, and future prospects. Anal. Bioanal. Chem. 2021, 413, 4137–4159. [Google Scholar] [CrossRef] [PubMed]
- Rempell, J.S.; Noble, V.E.; Liteplo, A.S.; Woolley, A.M.; Cooke, M.H.; Field, M.; Kim, D.J.; Murray, A.; Saul, T.; Sierzenski, P.R.; et al. Pilot point-of-care ultrasound curriculum at Harvard Medical School: Early experience. West. J. Emerg. Med. 2016, 17, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Briggs, C.; Kimber, S.; Green, L. Guidelines for point-of-care testing: Haematology. Br. J. Haematol. 2008, 142, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.R. Development of point-of-care biosensors for COVID-19. Front. Chem. 2020, 8, 517. [Google Scholar] [CrossRef] [PubMed]
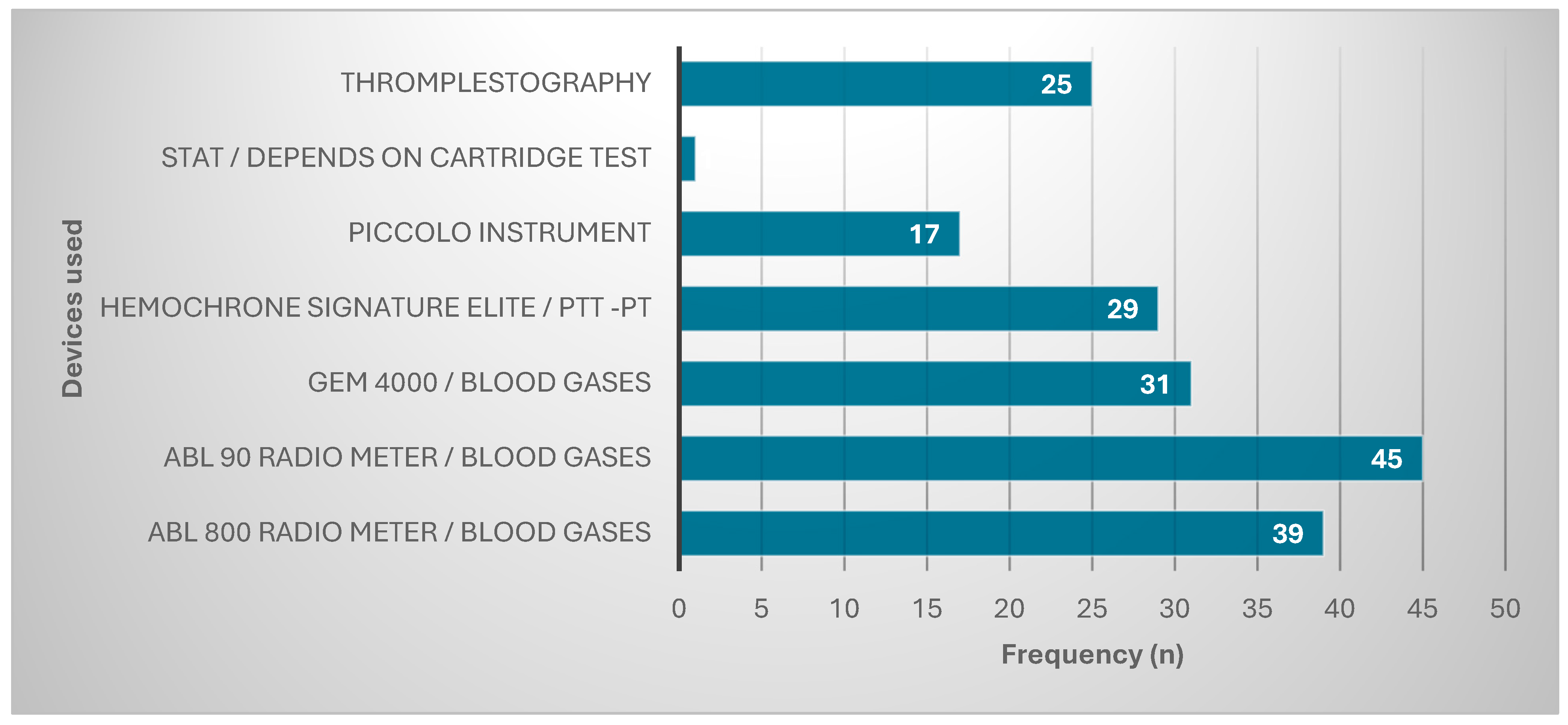
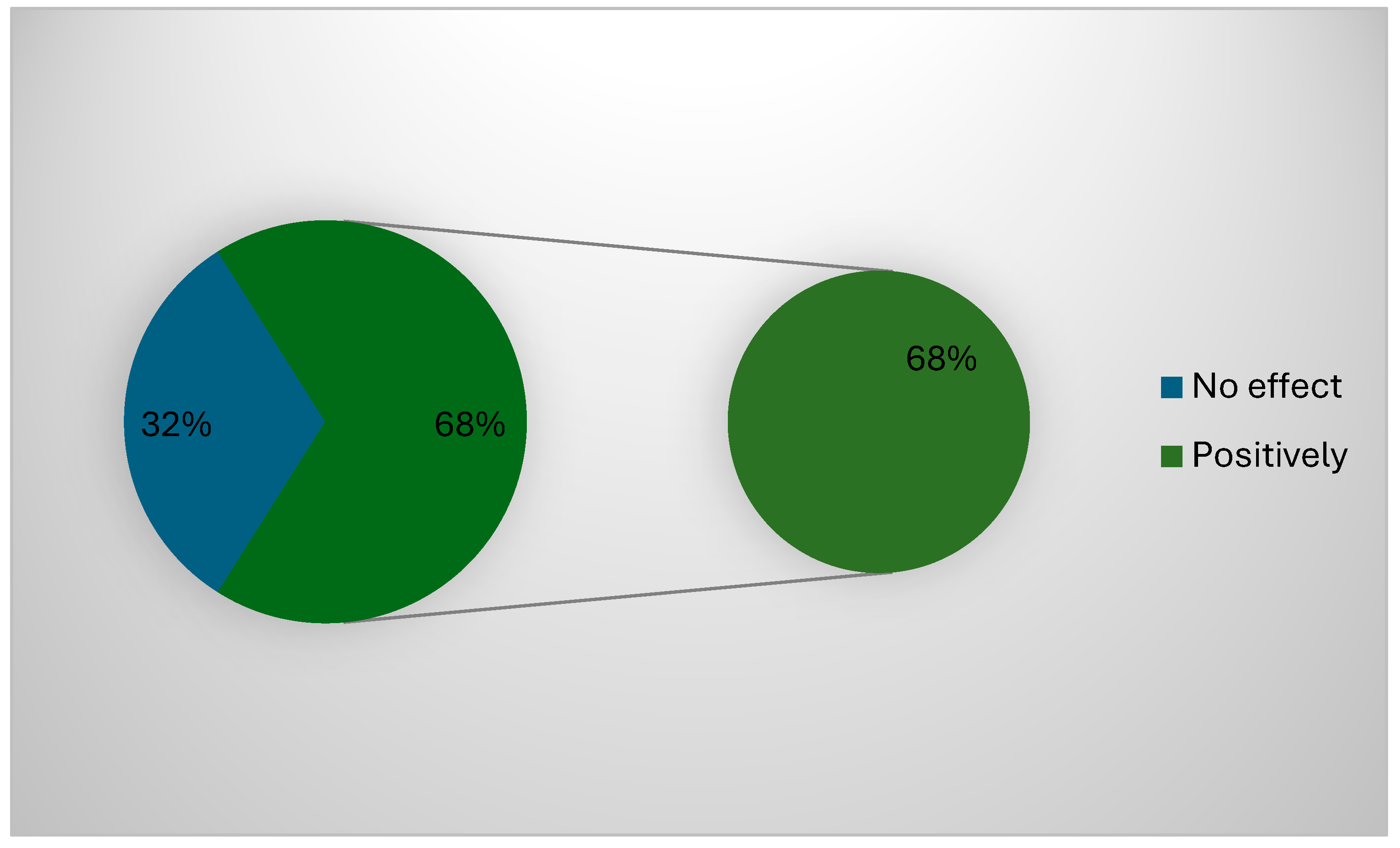
| Demographic Data | Frequency (n) | Percentage (%) |
|---|---|---|
| Years of experience | ||
| <1 year | 2 | 1.1 |
| >10 years | 26 | 13.9 |
| 1–5 years | 159 | 85.0 |
| Area of positioning | ||
| Cytology | 8 | 4.3 |
| Flow cytometry | 26 | 13.9 |
| Hematology | 109 | 58.3 |
| Histology | 8 | 4.3 |
| Serology | 10 | 5.3 |
| Specimen collection and processing | 26 | 13.9 |
| Specialty | ||
| Cytotechnologist | 13 | 7.0 |
| Lab assistants | 70 | 37.4 |
| Medical technologists | 13 | 7.0 |
| Pathologist | 91 | 48.7 |
| Challenges of Using POCT | Mean ± S.D |
|---|---|
| Devices and chemicals used are feasible | 2.59 ± 0.49 |
| SOPs are readable | 1.87 ± 0.53 |
| There is a prior training device before practice | 2.12 ± 0.67 |
| Large number of cancer samples | 2.99 ± 0.1 |
| There is a shortage of staff | 2.99 ± 0.07 |
| Maintenance of devices is poorly performed | 1.86 ± 0.55 |
| Devices are not user-friendly | 2.51 ± 0.5 |
| Specimens are not readily prepared to be added directly | 1.71 ± 0.92 |
| Devices have old technological procedures | 2.76 ± 0.47 |
| It takes time to use devices | 2.99 ± 0.07 |
| Total | 2.44 ± 0.4 |
| Challenges of Using POCT | Agree | Neutral | Disagree |
|---|---|---|---|
| n (%) | |||
| Devices and chemicals used are feasible | 111(59.4) | 76(40.6) | 0 |
| SOPs are readable | 16(8.6) | 40(21.4) | 131(70.1) |
| There is a prior training device before practice | 54(28.9) | 32(17.1) | 101(54) |
| Large number of cancer samples | 185(98.9) | 2(1.2) | 0 |
| There is a shortage of staff | 186(99.5) | 1(0.5) | 0 |
| Maintenance of devices is poorly performed | 17(9.1) | 44(23.5) | 126(67.4) |
| Devices are not user-friendly | 96(51.3) | 0 | 91(48.7) |
| Specimens are not readily prepared to be added directly | 60(32.1) | 13(7) | 114(61) |
| Devices have old technological procedures | 145(77.5) | 39(20.9) | 3(1.6) |
| It takes time to use devices | 186(99.5) | 1(0.5) | 0 |
| Item | Frequency (n) | Percentage (%) |
|---|---|---|
| Inclusion of control testing | ||
| No | 74 | 39.6 |
| Yes | 113 | 60.4 |
| Special precautions to keep the instrument safe | ||
| No | 25 | 13.4 |
| Yes | 162 | 86.6 |
| Results comparison with central laboratory | ||
| Yes | 187 | 100.0 |
| Need For instrument validation before use | ||
| No | 107 | 57.2 |
| Yes | 80 | 42.8 |
| Need for committee to monitor the operation of POCT devices | ||
| No | 63 | 33.7 |
| Yes | 124 | 66.3 |
| You are making good protection from specimen | ||
| Yes | 187 | 100.0 |
| You are making good protection during the transmission of the specimen | ||
| No | 44 | 23.5 |
| Yes | 143 | 76.5 |
| Isolation is optimum for a procedure | ||
| No | 99 | 52.9 |
| Yes | 88 | 47.1 |
| Hand hygiene | ||
| No | 8 | 4.3 |
| Yes | 179 | 95.7 |
| Use of personal protective equipment (PPE) | ||
| No | 93 | 49.7 |
| Yes | 94 | 50.3 |
| Use of medical protective mask | ||
| No | 21 | 11.2 |
| Yes | 166 | 88.8 |
| Challenges of Using POCT | p-Value | |
|---|---|---|
| Devices and chemicals used are feasible | Types of devices used | 0.000 |
| SOPs are readable | ||
| There is a prior training device before practice | ||
| Large number of cancer samples | ||
| There is a shortage of staff | ||
| Maintenance of devices is poorly performed | ||
| Devices are not user-friendly | ||
| Specimens are not readily prepared to be added directly | ||
| Devices have old technological procedures | ||
| It takes time to use devices | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-shahrani, A.Y.; Khan, J. Evaluation of ABL90 and ABL800 Radiometer Blood Gas Analyzers: Challenges and Applications in Point-of-Care Cancer Diagnostics in Saudi Arabia. Healthcare 2025, 13, 331. https://doi.org/10.3390/healthcare13030331
Al-shahrani AY, Khan J. Evaluation of ABL90 and ABL800 Radiometer Blood Gas Analyzers: Challenges and Applications in Point-of-Care Cancer Diagnostics in Saudi Arabia. Healthcare. 2025; 13(3):331. https://doi.org/10.3390/healthcare13030331
Chicago/Turabian StyleAl-shahrani, Abdulaziz Yahya, and Johra Khan. 2025. "Evaluation of ABL90 and ABL800 Radiometer Blood Gas Analyzers: Challenges and Applications in Point-of-Care Cancer Diagnostics in Saudi Arabia" Healthcare 13, no. 3: 331. https://doi.org/10.3390/healthcare13030331
APA StyleAl-shahrani, A. Y., & Khan, J. (2025). Evaluation of ABL90 and ABL800 Radiometer Blood Gas Analyzers: Challenges and Applications in Point-of-Care Cancer Diagnostics in Saudi Arabia. Healthcare, 13(3), 331. https://doi.org/10.3390/healthcare13030331






