Development of an Evidence-Based Cognitive Training Application for Elderly Individuals with Cognitive Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Study Design
2.2.1. Phase of Development: Analysis Phase
2.2.2. Phase of Development: Design Phase
2.2.3. Phase of Development: Development Phase
2.2.4. Phase of Development: Implementation Phase
2.2.5. Phase of Development: Evaluation Phase
2.3. Statistical Analysis
3. Results
3.1. Analysis Phase Findings: Systematic Review and Needs of Spouses of Dementia Patients
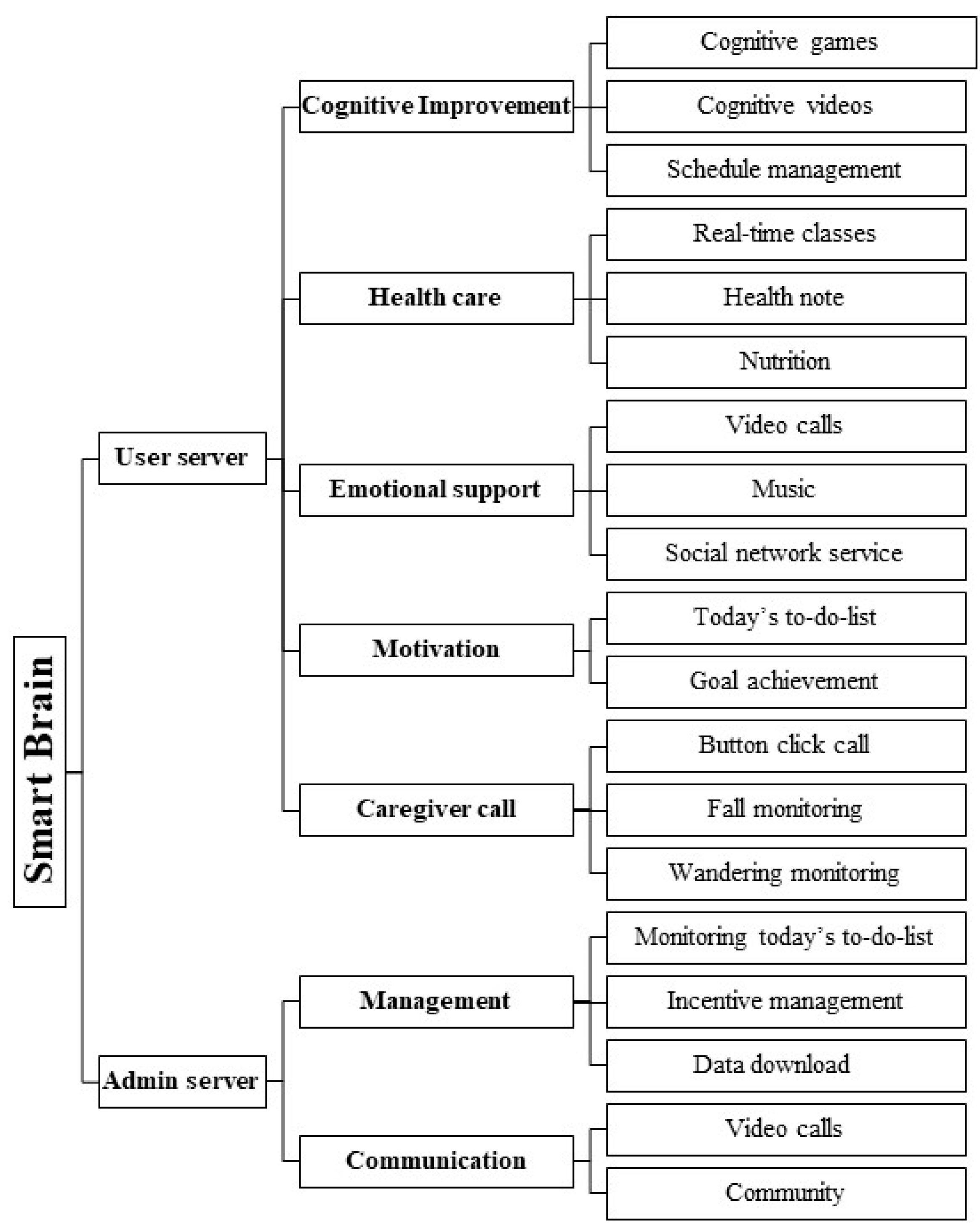
3.2. Design Phase Findings
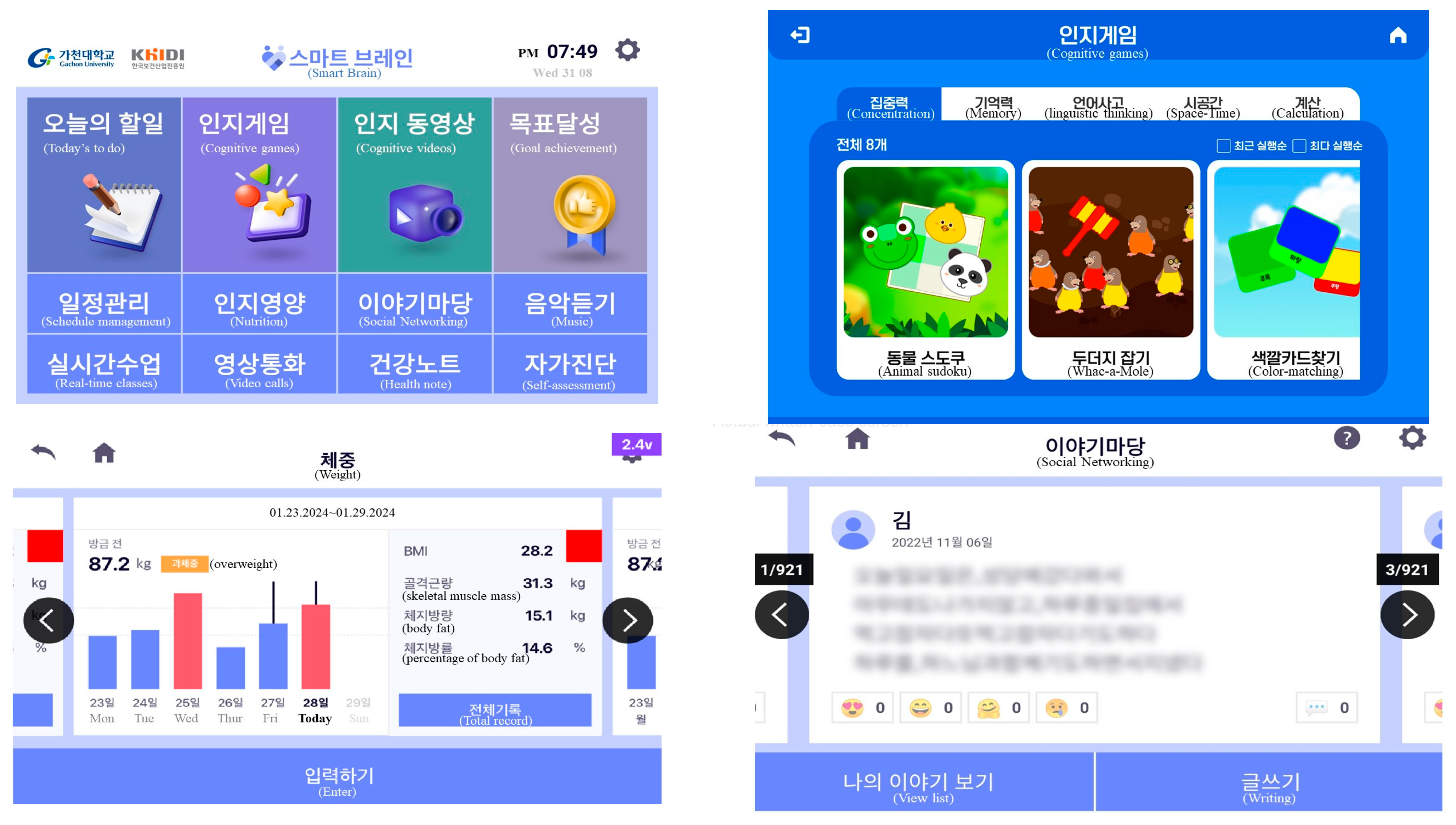
3.3. Development Phase Findings
3.4. Implementation and Evaluation Phase Finding: Usability Test of Uses and Experts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harper, L.C. 2022 Alzheimer’s Association Facts and Figures. 2022. Available online: https://www.cambridge.org/core/books/abs/tattoo-on-my-brain/resources/915A476B938D0AF39A218D34852AF645 (accessed on 8 August 2024).
- World Health Organization. Dementia. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 8 August 2024).
- Willmott, R.; Martin West, I.; Yung, P.; Giri Shankar, V.; Perera, G.; Tsamakis, K.; Stewart, R.; Mueller, C. An investigation of neuropsychiatric symptoms, contextual factors, and antidepressant treatment as risk factors for dementia development in people with mild cognitive impairment. Int. J. Geriatr. Psychiatry 2024, 39, e6097. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Lopez, O.; Armstrong, M.J.; Getchius, T.S.D.; Ganguli, M.; Gloss, D.; Gronseth, G.S.; Marson, D.; Pringsheim, T.; Day, G.S.; et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology 2018, 90, 126–135. [Google Scholar] [CrossRef]
- Rasmussen, J.; Langerman, H. Alzheimer’s Disease—Why We Need Early Diagnosis. Degener. Neurol. Neuromuscul. Dis. 2019, 9, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Abraha, I.; Rimland, J.M.; Trotta, F.M.; Dell, G.; Cruz-Jentoft, A.; Petrovic, M.; Gudmundsson, A.; Soiza, R.; O’Mahony, D.; Guaita, A.; et al. Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series. BMJ Open 2017, 7, e012759. [Google Scholar] [CrossRef] [PubMed]
- Chae, H.J.; Lee, S.H. Effectiveness of online-based cognitive intervention in community-dwelling older adults with cognitive dysfunction: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2023, 38, e5853. [Google Scholar] [CrossRef]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early Alzheimer’s disease. New Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, S.; Wang, J.; Shang, H.; Chen, X. Advancements in Pharmacological Treatment of Alzheimer’s Disease: The Advent of Disease-Modifying Therapies (DMTs). Brain Sci. 2024, 14, 990. [Google Scholar] [CrossRef]
- Ngandu, T.; Lehtisalo, J.; Solomon, A.; Levälahti, E.; Ahtiluoto, S.; Antikainen, R.; Bäckman, L.; Hänninen, T.; Jula, A.; Laatikainen, T. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 2015, 385, 2255–2263. [Google Scholar] [CrossRef]
- Coelho, T. Digital technologies in dementia care. In Digital Therapies in Psychosocial Rehabilitation and Mental Health; IGI Global: Hershey, PA, USA, 2022; pp. 115–140. [Google Scholar]
- Cheung, G.; Peri, K. Challenges to dementia care during COVID-19: Innovations in remote delivery of group Cognitive Stimulation Therapy. Aging Ment. Health 2021, 25, 977–979. [Google Scholar] [CrossRef]
- Delmastro, F.; Di Martino, F.; Dolciotti, C. Cognitive training and stress detection in mci frail older people through wearable sensors and machine learning. IEEE Access 2020, 8, 65573–65590. [Google Scholar] [CrossRef]
- Nakaoku, Y.; Ogata, S.; Murata, S.; Nishimori, M.; Ihara, M.; Iihara, K.; Iihara, K.; Takegami, M.; Nishimura, K. AI-assisted in-house power monitoring for the detection of cognitive impairment in older adults. Sensors 2021, 21, 6249. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, M.; Angilletta, C. Long-Lasting Neuropsychological Effects of a Computerized Cognitive Training in Patients Affected by Early Stage Alzheimer’s Disease: Are They Stable Over Time? J. Appl. Gerontol. 2019, 38, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Manenti, R.; Gobbi, E.; Baglio, F.; Macis, A.; Ferrari, C.; Pagnoni, I.; Rossetto, F.; Di Tella, S.; Alemanno, F.; Cimino, V.; et al. Effectiveness of an innovative cognitive treatment and telerehabilitation on subjects with mild cognitive impairment: A multicenter, randomized, active-controlled study. Front. Aging Neurosci. 2020, 12, 585988. [Google Scholar] [CrossRef]
- Poptsi, E.; Lazarou, I.; Markou, N.; Vassiloglou, M.; Nikolaidou, E.; Diamantidou, A.; Siatra, V.; Karathanassi, E.; Karakostas, A.; Zafeiropoulou, F.K.; et al. A comparative single-blind randomized controlled trial with language training in people with mild cognitive impairment. Am. J. Alzheimer’s Dis. Other Dement. 2019, 34, 176–187. [Google Scholar] [CrossRef]
- Martínez-Alcalá, C.I.; Pliego-Pastrana, P.; Rosales-Lagarde, A.; Lopez-Noguerola, J.S.; Molina-Trinidad, E.M. Information and communication technologies in the care of the elderly: Systematic review of applications aimed at patients with dementia and caregivers. JMIR Rehabil. Assist. Technol. 2016, 3, e5226. [Google Scholar] [CrossRef]
- Hagovská, M.; Dzvoník, O.; Olekszyová, Z. Comparison of Two Cognitive Training Programs With Effects on Functional Activities and Quality of Life. Res. Gerontol. Nurs. 2017, 10, 172–180. [Google Scholar] [CrossRef]
- Kwan, R.Y.; Lee, D.; Lee, P.H.; Tse, M.; Cheung, D.S.; Thiamwong, L.; Choi, K.-S. Effects of an mHealth brisk walking intervention on increasing physical activity in older people with cognitive frailty: Pilot randomized controlled trial. JMIR Mhealth Uhealth 2020, 8, e16596. [Google Scholar] [CrossRef]
- Moon, S.; Park, K. The effect of digital reminiscence therapy on people with dementia: A pilot randomized controlled trial. BMC Geriatr. 2020, 20, 166. [Google Scholar] [CrossRef]
- Soo-Jung, K.; Yun-Jin, C. Predictable Effect and Usability of Smart-Phone Application for Elderly Dementia Prevention. J. Ind. Converg. 2019, 17, 87–94. [Google Scholar]
- Karyotaki, M.; Drigas, A. Online and other ICT Applications for Cognitive Training and Assessment. Int. J. Online Eng. 2015, 11, 36–42. [Google Scholar] [CrossRef]
- Baquero, A.A.D.; Bartolomé, M.V.P.; Toribio-Guzmán, J.M.; Martínez-Abad, F.; Vidales, E.P.; Aguado, Y.B.; van der Roest, H.G.; Franco-Martín, M.A. Determinants of adherence to a “GRADIOR” computer-based cognitive training program in people with mild cognitive impairment (MCI) and mild dementia. J. Clin. Med. 2022, 11, 1714. [Google Scholar] [CrossRef] [PubMed]
- Muruganantham, G. Developing of E-content package by using ADDIE model. Int. J. Appl. Res. 2015, 1, 52–54. [Google Scholar]
- Mexia, S.G.; Ponce, M.A.; Franco-Campos, R. Instructional Design by Employing the ADDIE Model to Enhance the Performance of Undergraduate Students; Boletín Científico INVESTIGIUM de la Escuela Superior de Tizayuca: Hidalgo, TX, USA, 2017; Volume 3. [Google Scholar]
- Dwitiyanti, N.; Kumala, S.A.; Widiyatun, F. Using the ADDIE model in development of physics unit convertion application based on Android as learning media. Form. J. Ilm. Pendidik. MIPA 2020, 10, 125–132. [Google Scholar] [CrossRef]
- Stoyanov, S.R.; Hides, L.; Kavanagh, D.J.; Zelenko, O.; Tjondronegoro, D.; Mani, M. Mobile app rating scale: A new tool for assessing the quality of health mobile apps. JMIR Mhealth Uhealth 2015, 3, e27. [Google Scholar] [CrossRef]
- Ainscough, K.; Kennelly, M.; Lindsay, K.L.; McAuliffe, F.M. Impact of an mHealth supported healthy lifestyle intervention on behavioural stage of change in overweight and obese pregnancy. In Proceedings of the Nutrition Society (OCE3), Dublin, Ireland, 11–14 July 2016; Volume 75. [Google Scholar]
- Beentjes, K.M.; Neal, D.P.; Kerkhof, Y.J.F.; Broeder, C.; Moeridjan, Z.D.J.; Ettema, T.P.; Pelkmans, W.; Muller, M.M.; Graff, M.J.L.; Dröes, R.-M. Impact of the FindMyApps program on people with mild cognitive impairment or dementia and their caregivers; an exploratory pilot randomised controlled trial. Disabil. Rehabil. Assist. Technol. 2020, 18, 253–265. [Google Scholar] [CrossRef]
- de Souto Barreto, P.; Pothier, K.; Soriano, G.; Lussier, M.; Bherer, L.; Guyonnet, S.; Piau, A.; Ousset, P.-J.; Vellas, B. A web-based multidomain lifestyle intervention for older adults: The eMIND randomized controlled trial. J. Prev. Alzheimer’s Dis. 2021, 8, 142–150. [Google Scholar] [CrossRef]
- Forstmeier, S.; Maercker, A. Motivational processes in mild cognitive impairment and Alzheimer’s disease: Results from the Motivational Reserve in Alzheimer’s (MoReA) study. BMC Psychiatry 2015, 15, 293. [Google Scholar] [CrossRef]
- Chen, X.; Pan, W. The treatment strategies for neurodegenerative diseases by integrative medicine. Integr. Med. Int. 2014, 1, 223–225. [Google Scholar] [CrossRef]
- Byrne, L.M.; Wilson, P.M.; Bucks, R.S.; Hughes, A.O.; Wilcock, G.K. The sensitivity to change over time of the Bristol Activities of Daily Living Scale in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2000, 15, 656–661. [Google Scholar] [CrossRef]
- Heßmann, P.; Seeberg, G.; Reese, J.P.; Dams, J.; Baum, E.; Müller, M.J.; Dodel, R.; Balzer-Geldsetzer, M. Health-related quality of life in patients with Alzheimer’s disease in different German health care settings. J. Alzheimer’s Dis. 2016, 51, 545–561. [Google Scholar] [CrossRef]
- Li, B.-Y.; He, N.-Y.; Qiao, Y.; Xu, H.-M.; Lu, Y.-Z.; Cui, P.-J.; Ling, H.-W.; Yan, F.-H.; Tang, H.-D.; Chen, S.-D. Computerized cognitive training for Chinese mild cognitive impairment patients: A neuropsychological and fMRI study. NeuroImage Clin. 2019, 22, 101691. [Google Scholar] [CrossRef] [PubMed]
- Robert, P.; Manera, V.; Derreumaux, A.; Montesino, M.F.Y.; Leone, E.; Fabre, R.; Bourgeois, J. Efficacy of a web app for cognitive training (MeMo) regarding cognitive and behavioral performance in people with neurocognitive disorders: Randomized controlled trial. J. Med. Internet Res. 2020, 22, e17167. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Wu, B.; Tanaka, E.; Watanabe, T.; Watanabe, K.; Chen, W.; Ito, S.; Okumura, R.; Arai, T.; Anme, T. Association between a change in social interaction and dementia among elderly people. Int. J. Gerontol. 2016, 10, 76–80. [Google Scholar] [CrossRef]
- Arai, A.; Khaltar, A.; Ozaki, T.; Katsumata, Y. Influence of social interaction on behavioral and psychological symptoms of dementia over 1 year among long-term care facility residents. Geriatr. Nurs. 2021, 42, 509–516. [Google Scholar] [CrossRef]
- Bahar-Fuchs, A.; Webb, S.; Bartsch, L.; Clare, L.; Rebok, G.; Cherbuin, N.; Anstey, K.J. Tailored and adaptive computerized cognitive training in older adults at risk for dementia: A randomized controlled trial. J. Alzheimer’s Dis. 2017, 60, 889–911. [Google Scholar] [CrossRef]

| Variable | Contents | Mean ± SD 1/N (%) |
|---|---|---|
| Age | 73.2 ± 7.0 | |
| Gender | Male | 33 (33.0%) |
| Female | 67 (67.0%) | |
| Subjective economic status | High | 13 (13.0%) |
| Moderate | 67 (67.0%) | |
| Low | 20 (20.0%) | |
| Education level | No formal education | 7 (7.0%) |
| Elementary/Middle school | 31 (31.0%) | |
| High School/college | 60 (60.0%) | |
| Graduate or Higher | 2 (2.0%) | |
| Primary caregiver | Spouse | 54 (54.0%) |
| Child | 22 (22.0%) | |
| Sibling | 1 (1.0%) | |
| Caregiver | 1 (1.0%) | |
| None | 22 (22.0%) |
| Category | Contents | Mean ± SD 1 |
|---|---|---|
| Cognitive improvement | Cognitive games | 3.68 ± 1.11 |
| Cognitive videos | 3.26 ± 1.27 | |
| Schedule management | 3.05 ± 1.14 | |
| Health care | Online classes | 3.06 ± 1.32 |
| Health note | 3.67 ± 1.17 | |
| Cognitive improvement nutrition | 2.78 ± 1.13 | |
| Emotional support | Video calls | 3.20 ± 1.19 |
| Listening to music | 3.37 ± 1.15 | |
| Social networking service | 3.41 ± 1.25 | |
| Motivation | Today’s to-do list | 3.48 ± 1.21 |
| Goal achievement | 3.49 ± 1.29 | |
| Caregiver call | Button click call | 3.38 ± 1.30 |
| Fall monitoring | 3.09 ± 1.24 | |
| Wandering monitoring | 2.99 ± 1.32 |
| Category | Component | Score (Mean ± SD 1) |
|---|---|---|
| Quality of application | Mean score | 4.00 ± 0.19 |
| Engagement | Mean score | 3.94 ± 0.36 |
| Entertainment | 3.71 ± 0.76 | |
| Interest | 4.00 ± 0.58 | |
| Customization | 4.14 ± 0.90 | |
| Interactivity | 4.00 ± 0.82 | |
| Target group | 3.86 ± 1.07 | |
| Functionality | Mean score | 4.00 ± 0.41 |
| Performance | 4.00 ± 0.81 | |
| Ease of use | 3.71 ± 0.76 | |
| Navigation | 4.14 ± 0.69 | |
| Gestural design | 4.14 ± 0.69 | |
| Esthetics | Mean score | 3.86 ± 0.26 |
| Layout | 3.86 ± 0.90 | |
| Graphics | 3.71 ± 1.11 | |
| Visual appeal | 4.00 ± 0.82 | |
| Information | Mean score | 3.92 ± 0.25 |
| Accuracy of app description (in app store) | 4.14 ± 0.69 | |
| Goals | 4.29 ± 0.76 | |
| Quality of information | 4.00 ± 0.82 | |
| Quantity of information | 4.29 ± 0.76 | |
| Visual information | 4.14 ± 0.90 | |
| Credibility | 3.86 ± 0.90 | |
| Evidence base | 2.71 ± 0.49 | |
| App subjective quality | Mean score | 4.29 ± 0.39 |
| Willingness to recommend app to others | 4.71 ± 0.49 | |
| Estimated number of uses per year | 3.86 ± 0.90 | |
| Willingness to pay for app | 4.43 ± 0.98 | |
| Overall star rating of app | 4.14 ± 1.07 |
| Category | Component | Score (Mean ± SD 1) |
|---|---|---|
| Quality of application | Mean score | 3.80 ± 0.24 |
| Engagement | Mean score | 3.78 ± 0.63 |
| Entertainment | 3.64 ± 1.12 | |
| Interest | 3.73 ± 1.01 | |
| Customization | 4.00 ± 1.00 | |
| Interactivity | 3.82 ± 0.98 | |
| Target group | 3.73 ± 1.01 | |
| Functionality | Mean score | 3.78 ± 0.63 |
| Performance | 3.09 ± 0.83 | |
| Ease of use | 3.55 ± 0.93 | |
| Navigation | 3.27 ± 0.79 | |
| Gestural design | 3.27 ± 0.79 | |
| Esthetics | Mean score | 4.12 ± 0.43 |
| Layout | 3.09 ± 0.83 | |
| Graphics | 4.18 ± 0.87 | |
| Visual appeal | 4.09 ± 1.04 | |
| Information | Mean score | 4.16 ± 0.42 |
| Quality of information | 4.09 ± 0.94 | |
| Quantity of information | 4.09 ± 0.94 | |
| Visual information | 4.18 ± 0.87 | |
| Credibility | 4.27 ± 0.91 | |
| App subjective quality | Mean score | 3.66 ± 0.49 |
| Willingness to recommend app to others | 4.64 ± 0.51 | |
| Estimated number of uses per year | 4.00 ± 0.89 | |
| Willingness to pay for app | 2.27 ± 1.01 | |
| Overall star rating of app | 3.73 ± 1.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chae, H.-J.; Kim, C.-H.; Lee, S.-H. Development of an Evidence-Based Cognitive Training Application for Elderly Individuals with Cognitive Dysfunction. Healthcare 2025, 13, 215. https://doi.org/10.3390/healthcare13030215
Chae H-J, Kim C-H, Lee S-H. Development of an Evidence-Based Cognitive Training Application for Elderly Individuals with Cognitive Dysfunction. Healthcare. 2025; 13(3):215. https://doi.org/10.3390/healthcare13030215
Chicago/Turabian StyleChae, Hee-Jae, Chan-Hee Kim, and Seon-Heui Lee. 2025. "Development of an Evidence-Based Cognitive Training Application for Elderly Individuals with Cognitive Dysfunction" Healthcare 13, no. 3: 215. https://doi.org/10.3390/healthcare13030215
APA StyleChae, H.-J., Kim, C.-H., & Lee, S.-H. (2025). Development of an Evidence-Based Cognitive Training Application for Elderly Individuals with Cognitive Dysfunction. Healthcare, 13(3), 215. https://doi.org/10.3390/healthcare13030215






