Cost-Effectiveness Analysis of Recombinant Tumor Necrosis Factor Receptor: Fc Fusion Protein as First-Line Treatment for Active Rheumatoid Arthritis in China
Highlights
- rhTNFR:Fc provided 0.74 additional QALYs at an incremental cost of CNY 9447.96 versus methotrexate, giving an ICER of CNY 12,783.56 per QALY, well below the 2024 China per capita GDP threshold. Results were robust in probabilistic analysis and most sensitive to utility inputs; adverse events were broadly similar across arms.
- Scenario analyses were consistent with the base case. rhTNFR:Fc plus methotrexate remained cost-effective versus methotrexate alone with an ICER of CNY 12,834.05 per QALY. Under patient and payer perspectives, the ICERs were CNY 8079.04 and CNY 7630.34 per QALY.
- Clinicians can adopt rhTNFR:Fc more proactively, including early in the treatment course, to achieve greater symptom relief and longer-term benefits.
- Policymakers can provide stronger preferential policies and safeguards, such as higher reimbursement, to support wider standardized use and improve population health outcomes.
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Population
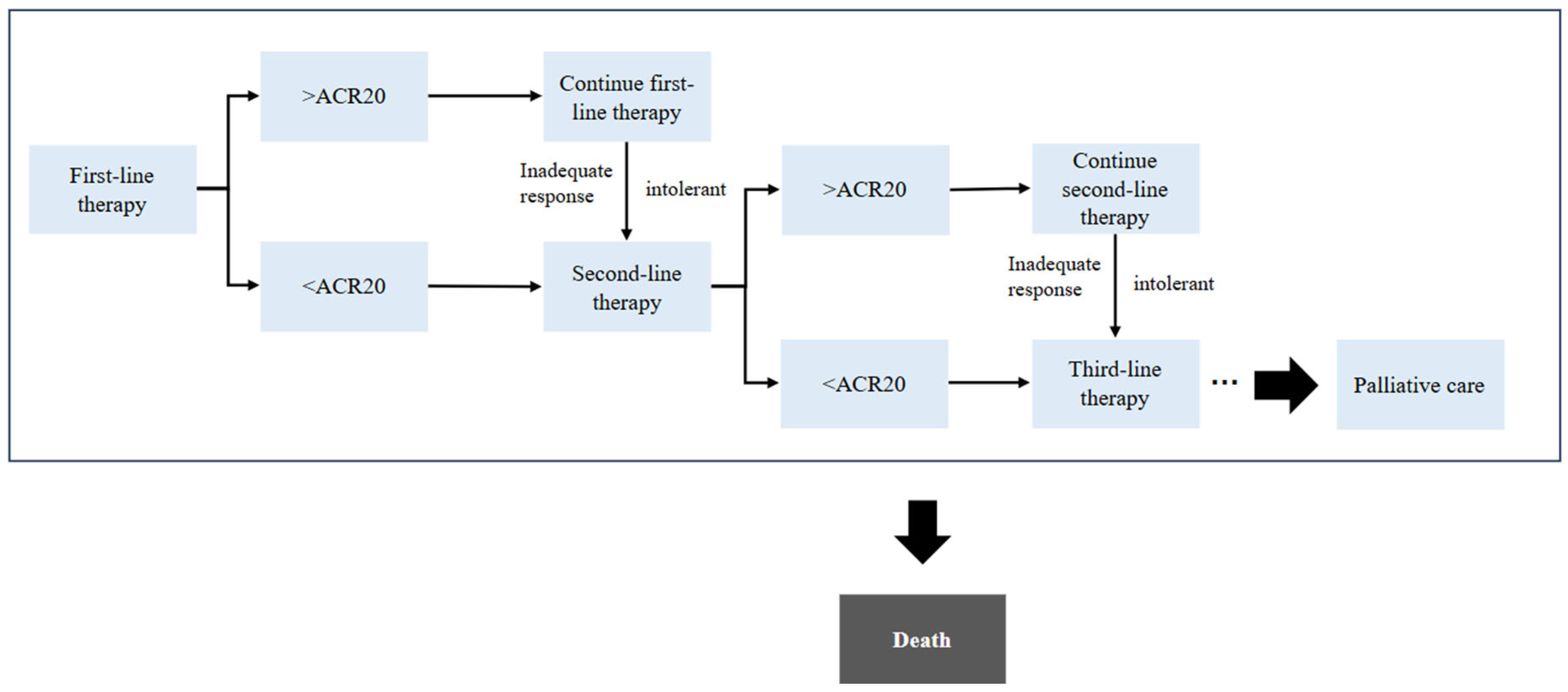
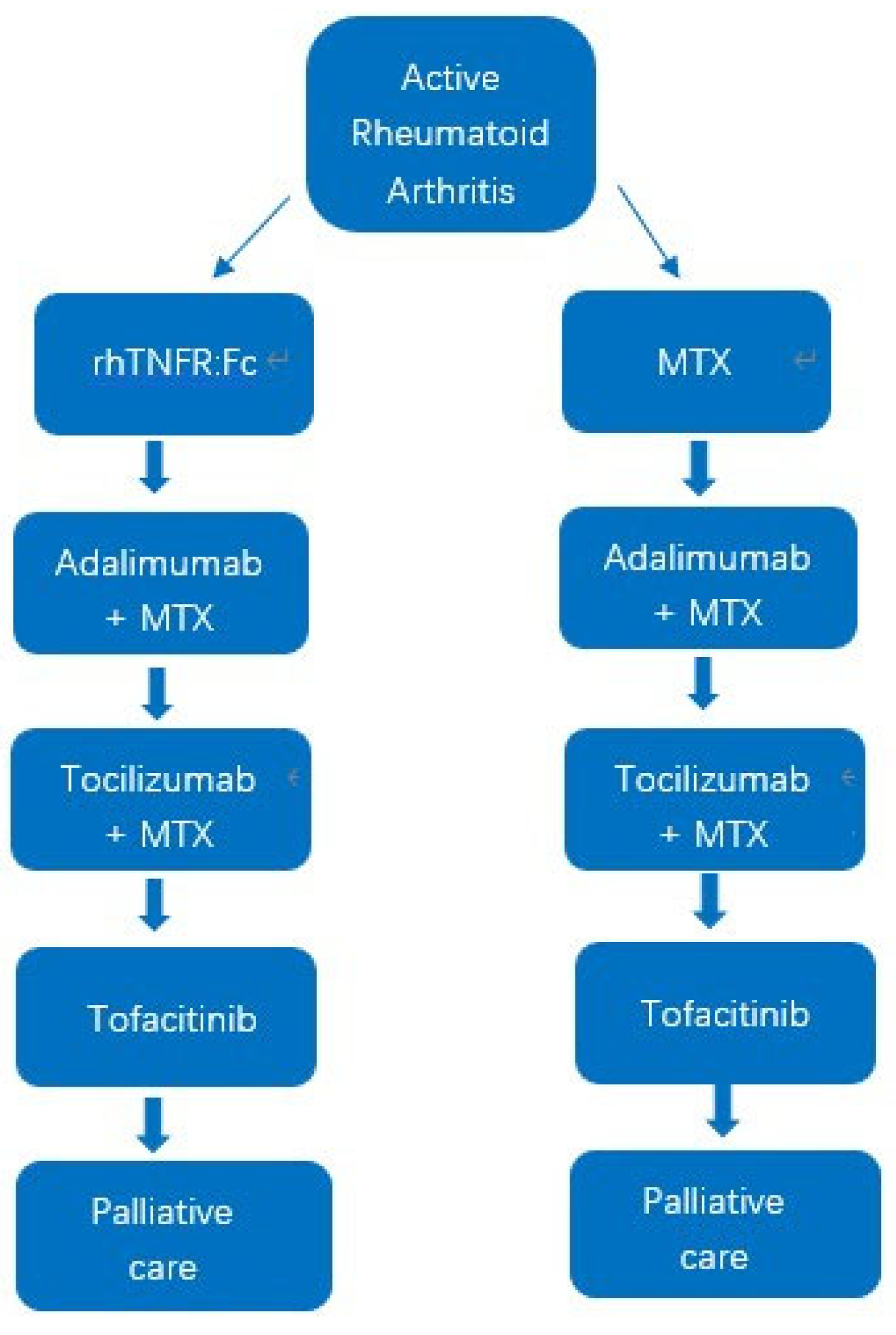
2.2. Model Structure
2.3. Study Perspective and Model Assumptions
2.4. Transition Probabilities
2.5. Cost Parameters
2.6. Utility
2.7. Sensitivity Analyses
3. Results
3.1. Base-Case Analysis
3.2. Sensitivity Analyses
3.2.1. Scenario Analyses
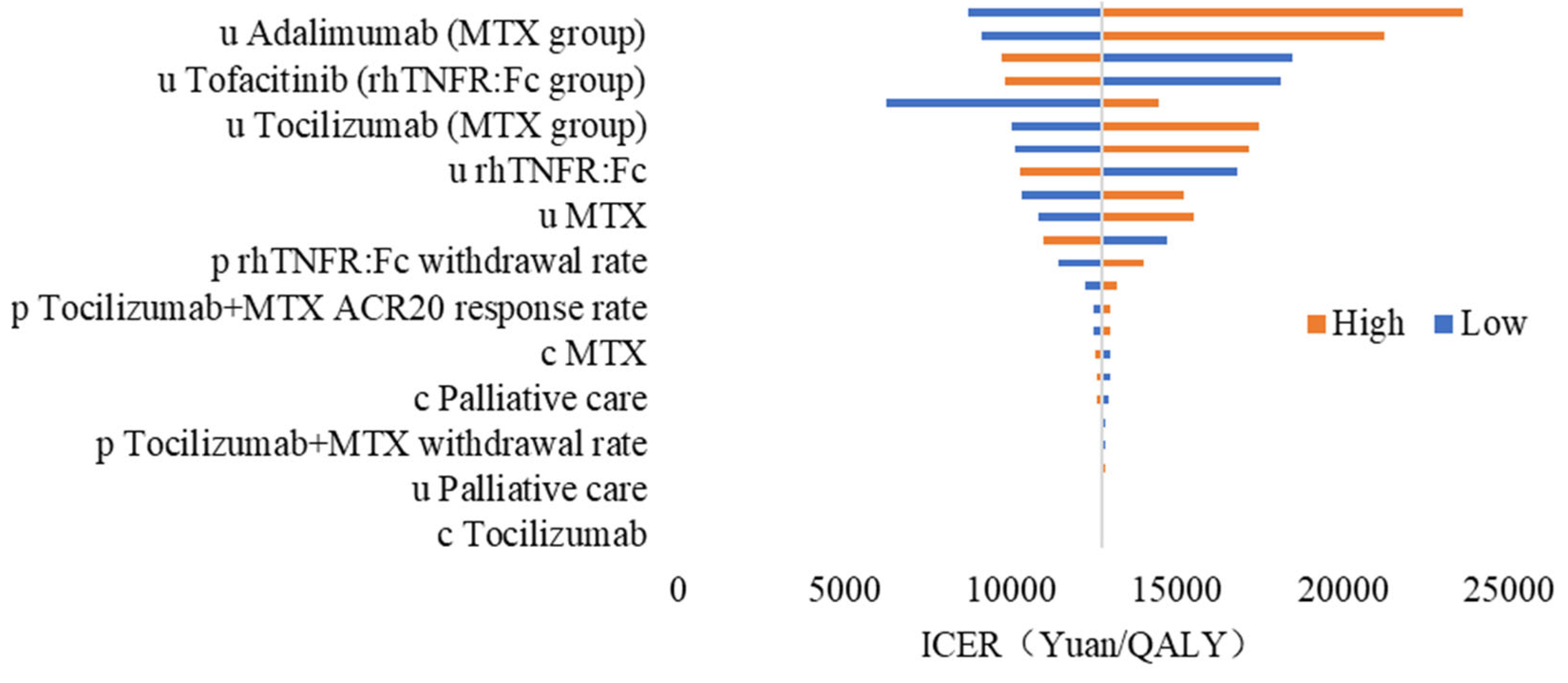
3.2.2. One-Way Sensitivity Analysis
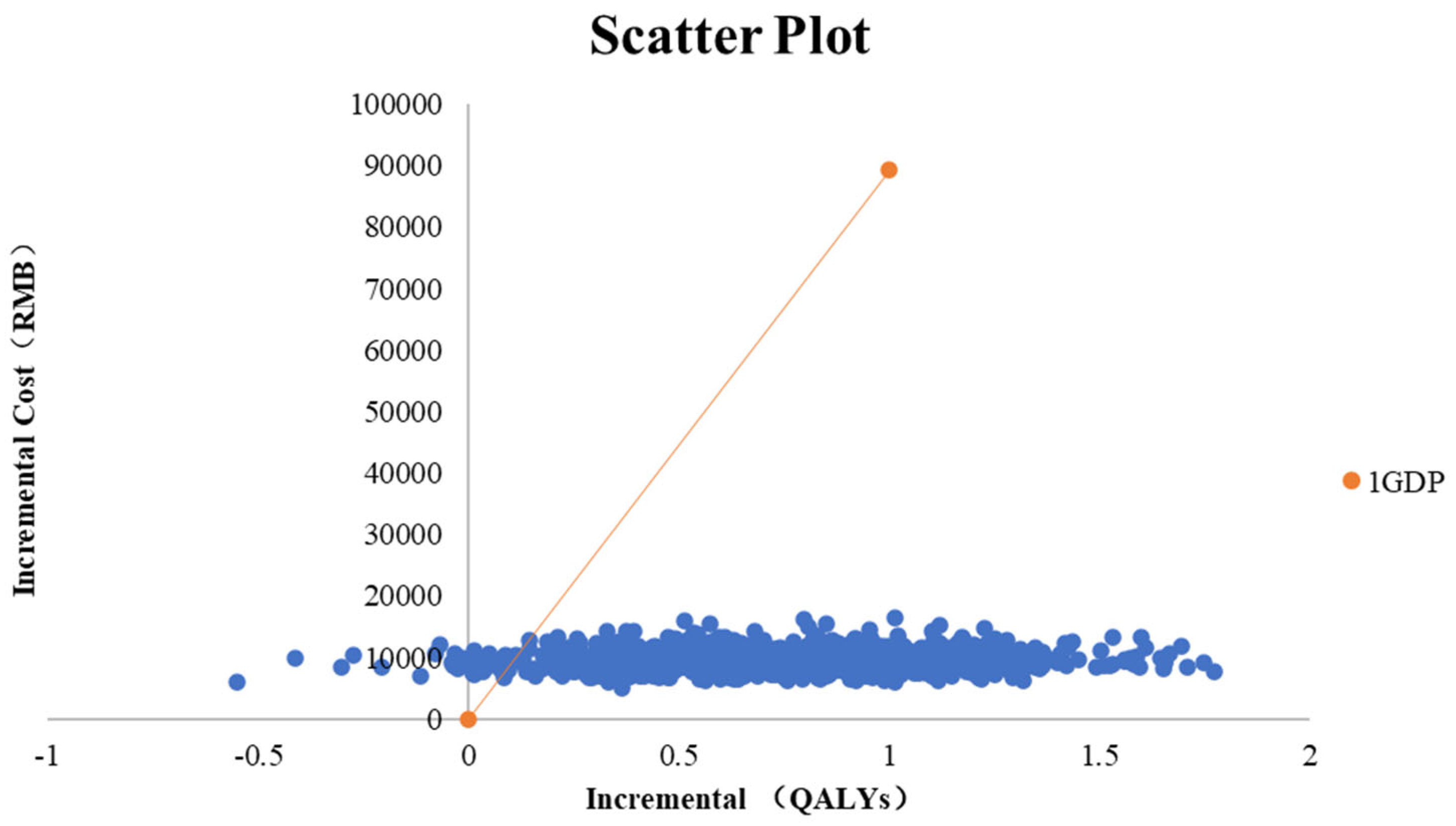
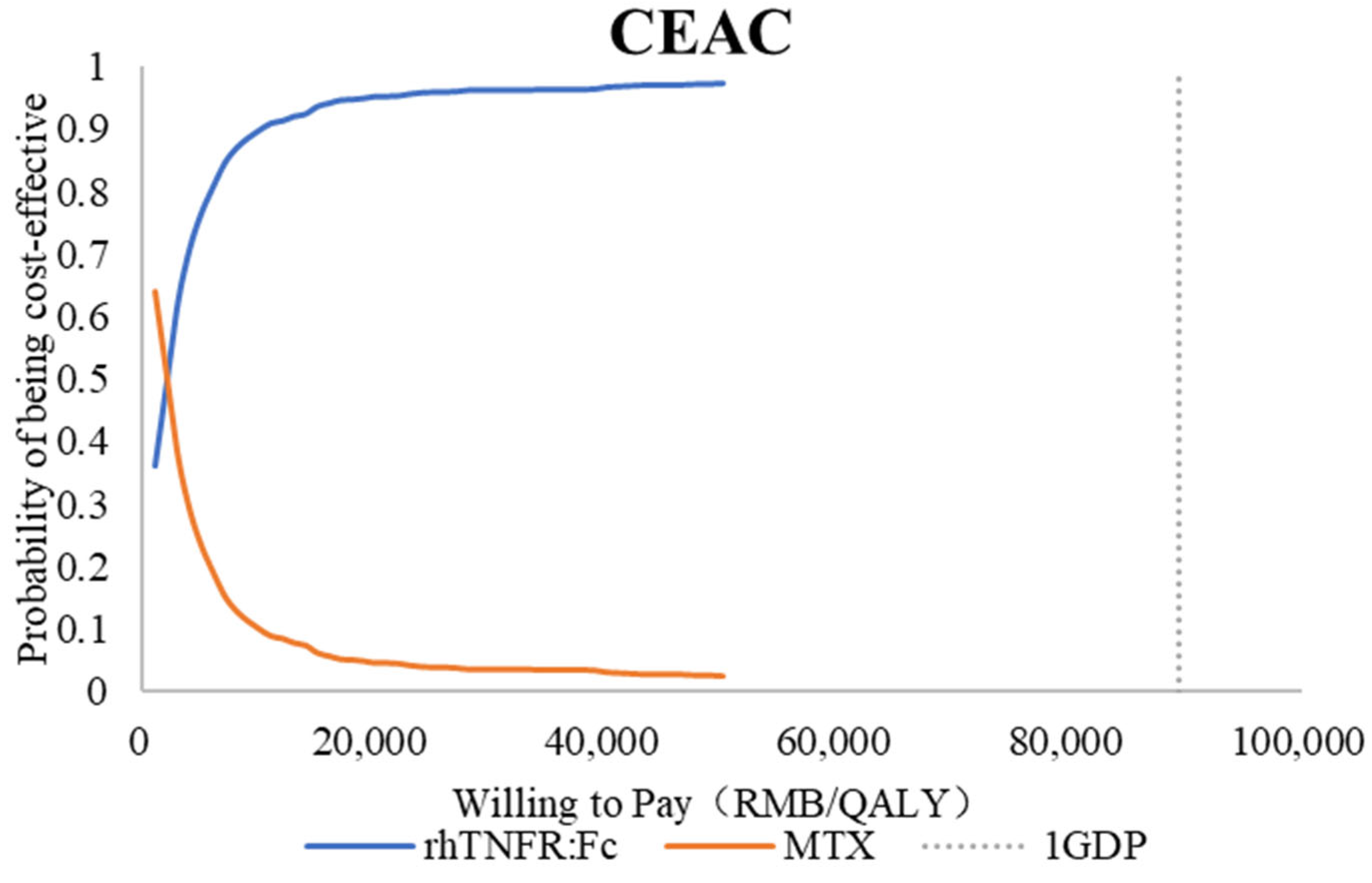
3.2.3. Probabilistic Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scott, D.L.; Wolfe, F.; Huizinga, T.W.J. Rheumatoid Arthritis. Lancet 2010, 376, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, T.; Moe, R.H.; Kvien, T.K. The Burden of Disease in Rheumatoid Arthritis. PharmacoEconomics 2014, 32, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Kiadaliri, A.A.; Felson, D.T.; Neogi, T.; Englund, M. Brief Report: Rheumatoid Arthritis as the Underlying Cause of Death in Thirty-One Countries, 1987-2011: Trend Analysis of World Health Organization Mortality Database. Arthritis Rheumatol. 2017, 69, 1560–1565. [Google Scholar] [CrossRef]
- Wolfe, F. The Burden of Rheumatoid Arthritis. Am. J. Manag. Care 1999, 5 (Suppl. S14), S852–S859; discussion S866–S869. [Google Scholar]
- Cross, M.; Smith, E.; Hoy, D.; Carmona, L.; Wolfe, F.; Vos, T.; Williams, B.; Gabriel, S.; Lassere, M.; Johns, N.; et al. The Global Burden of Rheumatoid Arthritis: Estimates from the global Burden of Disease 2010 Study. Ann. Rheum. Dis. 2014, 73, 1316–1322. [Google Scholar] [CrossRef]
- Hu, H.; Luan, L.; Yang, K.; Li, S. Burden of Rheumatoid Arthritis from a Societal Perspective: A Prevalence-Based Study on Cost of This Illness for Patients in China. Int. J. Rheum. Dis. 2018, 21, 1572–1580. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Mu, R.; Yang, L.; Zhang, Y.; Han, S.; Li, X.; Wang, Y.; Wang, G.; Zhu, P.; et al. Societal Costs of Rheumatoid Arthritis in China: A Hospital-Based Cross-Sectional Study. Arthritis Care Res. 2013, 66, 523–531. [Google Scholar] [CrossRef]
- National Collaborating Centre for Chronic Conditions (UK). Rheumatoid Arthritis: National Clinical Guideline for Management and Treatment in Adults. National Institute for Health and Clinical Excellence: Guidance; Royal College of Physicians: London, UK, 2009. [Google Scholar]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St. Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Calabrò, A.; Caterino, A.L.; Elefante, E.; Valentini, V.; Vitale, A.; Talarico, R.; Cantarini, L.; Frediani, B. One Year in Review 2016: Novelties in the Treatment of Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2016, 34, 357–372. [Google Scholar]
- Emery, P. Optimizing Outcomes in Patients with Rheumatoid Arthritis and an Inadequate Response to Anti-TNF Treatment. Rheumatology 2012, 51 (Suppl. S5), v22–v30. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR Recommendations for the Management of Rheumatoid Arthritis with Synthetic and Biological Disease-Modifying Antirheumatic Drugs: 2022 Update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Fiscella, K.; Franks, P. Cost-Effectiveness of the Transdermal Nicotine Patch as an Adjunct to Physicians’ Smoking Cessation Counseling. JAMA 1996, 275, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Cantor, S.B.; Ganiats, T.G. Incremental Cost-Effectiveness Analysis: The Optimal Strategy Depends on the Strategy Set. J. Clin. Epidemiol. 1999, 52, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.; Pang, L.; Liu, S. The Data Fitting and Optimal Control of a Hand, Foot and Mouth Disease (HFMD) Model with Stage Structure. Appl. Math. Comput. 2016, 276, 61–74. [Google Scholar] [CrossRef]
- Shen, M.; Xiao, Y.; Rong, L.; Meyers, L.A.; Bellan, S.E. The Cost-Effectiveness of Oral HIV Pre-Exposure Prophylaxis and Early Antiretroviral Therapy in the Presence of Drug Resistance among Men Who Have Sex with Men in San Francisco. BMC Med. 2018, 16, 58. [Google Scholar] [CrossRef]
- Hu, D.; Bao, C.; Chen, S.; Gu, J.; Li, Z.; Sun, L.; Han, X.; Ni, L. A Comparison Study of a Recombinant Tumor Necrosis Factor Receptor:Fc Fusion Protein (rhTNFR:Fc) and Methotrexate in Treatment of Patients with Active Rheumatoid Arthritis in China. Rheumatol. Int. 2009, 29, 297–303. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Q.; Jiang, N.; Zhao, Y.; Huang, C.; Liu, Y.; Xu, H.; Chen, Y.; Wu, L.; Xu, J.; et al. Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis: 2024 update. Rheumatol. Immunol. Res. 2025, 5, 189–208. [Google Scholar] [CrossRef]
- Modena, V.; Bianchi, G.; Roccatello, D. Cost-Effectiveness of Biologic Treatment for Rheumatoid Arthritis in Clinical Practice: An Achievable Target? Autoimmun. Rev. 2013, 12, 835–838. [Google Scholar] [CrossRef]
- Klareskog, L.; Gaubitz, M.; Rodriguez-Valverde, V.; Malaise, M.; Dougados, M.; Wajdula, J. Assessment of Long-Term Safety and Efficacy of Etanercept in a 5-Year Extension Study in Patients with Rheumatoid Arthritis. Clin. Exp. Rheumatol. 2011, 29, 238–247. [Google Scholar]
- Strand, V.; Singh, J.A. Improved Health-Related Quality of Life with Effective Disease-Modifying Antirheumatic Drugs: Evidence from Randomized Controlled Trials. Am. J. Manag. Care 2007, 13 Suppl. S9, S237–S251. [Google Scholar]
- Weinblatt, M.E.; Keystone, E.C.; Furst, D.E.; Moreland, L.W.; Weisman, M.H.; Birbara, C.A.; Teoh, L.A.; Fischkoff, S.A.; Chartash, E.K. Adalimumab, a Fully Human Anti-Tumor Necrosis Factor Alpha Monoclonal Antibody, for the Treatment of Rheumatoid Arthritis in Patients Taking Concomitant Methotrexate: The ARMADA Trial. Arthritis Rheum. 2003, 48, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Emery, P.; Keystone, E.; Tony, H.P.; Cantagrel, A.; van Vollenhoven, R.; Sanchez, A.; Alecock, E.; Lee, J.; Kremer, J. IL-6 Receptor Inhibition with Tocilizumab Improves Treatment Outcomes in Patients with Rheumatoid Arthritis Refractory to Anti-Tumour Necrosis Factor Biologicals: Results from a 24-Week Multicentre Randomised Placebo-Controlled Trial. Ann. Rheum. Dis. 2008, 67, 1516–1523. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Fleischmann, R.; Cohen, S.; Lee, E.B.; Meijide, J.A.G.; Wagner, S.; Forejtova, S.; Zwillich, S.H.; Gruben, D.; Koncz, T.; et al. Tofacitinib or Adalimumab versus Placebo in Rheumatoid Arthritis. N. Engl. J. Med. 2012, 367, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Qiao, L.; Li, L.; Zuo, K.; Li, L.; Wang, L.; Jiang, N.; Wang, Q.; Li, M.; Wang, Y.; et al. Average Annual Costs of Rheumatoid Arthritis Estimated by Inverse Probability Weighting and Their Influence Factors: A Cross-Sectional Study Based on Chinese Registry of Rheumatoid Arthritis (CREDIT) Cohort. PLoS ONE 2025, 20, e0330261. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Wang, Q.; Xu, Z.; Huang, Y.; Xi, X. Mapping Health Assessment Questionnaire Disability Index onto EQ-5D-5L in China. Front. Public Health 2023, 11, 1123552. [Google Scholar] [CrossRef]
- Weinblatt, M.E.; Kremer, J.M.; Bankhurst, A.D.; Bulpitt, K.J.; Fleischmann, R.M.; Fox, R.I.; Jackson, C.G.; Lange, M.; Burge, D.J. A Trial of Etanercept, a Recombinant Tumor Necrosis Factor Receptor:Fc Fusion Protein, in Patients with Rheumatoid Arthritis Receiving Methotrexate. N. Engl. J. Med. 1999, 340, 253–259. [Google Scholar] [CrossRef]
- Jansen, J.P.; Incerti, D.; Mutebi, A.; Peneva, D.; MacEwan, J.P.; Stolshek, B.; Kaur, P.; Gharaibeh, M.; Strand, V. Cost-effectiveness of sequenced treatment of rheumatoid arthritis with targeted immune modulators. J. Med Econ. 2017, 20, 703–714. [Google Scholar] [CrossRef]
- Wu, B.; Wilson, A.; Wang, F.-F.; Wang, S.-L.; Wallace, D.J.; Weisman, M.H.; Lu, L.-J. Cost effectiveness of different treatment strategies in the treatment of patients with moderate to severe rheumatoid arthritis in China. PLoS ONE 2012, 7, e47373. [Google Scholar] [CrossRef]
- Wu, B.; Song, Y.; Leng, L.; Bucala, R.; Lu, L.-J. Treatment of moderate rheumatoid arthritis with different strategies in a health resource-limited setting: A cost-effectiveness analysis in the era of biosimilars. Clin. Exp. Rheumatol. 2014, 33, 20–26. [Google Scholar]
- Peng, K.; Chan, S.C.W.; Wang, Y.; Cheng, F.W.T.; Yeung, W.W.Y.; Jiao, Y.; Chan, E.W.Y.; Wong, I.C.K.; Lau, C.-S.; Li, X. Cost-effectiveness of biosimilars vs leflunomide in patients with rheumatoid arthritis. JAMA Netw. Open 2024, 7, e2418800. [Google Scholar] [CrossRef]
| rhTNFR:Fc | MTX | |
|---|---|---|
| Age (years) | 48.74 ± 10.41 | 48.66 ± 10.63 |
| Female sex (%) | 85.59% | 84.17% |
| Duration of disease (months) | 90.78 ± 98.75 | 93.74 ± 94.29 |
| ACR20 (%) | 75.42% | 70.00% |
| ACR50 (%) | 40.68% | 30.83% |
| ACR70 (%) | 20.34% | 10.83% |
| AE incidence (%) | 51.28% | 43.7% |
| Parameters | Base | Upper | Lower | Distribution | Source |
|---|---|---|---|---|---|
| Age (years) | 49.00 | 49.00 | 49.00 | - | |
| Male (%) | 15% | 18% | 12% | - | |
| p rhTNFR:Fc ACR20 response rate | 75.42% | 67.30% | 82.73% | Beta | 13 |
| p rhTNFR:Fc withdrawal rate | 13.56% | 8.02% | 20.26% | Beta | 13 |
| p MTX ACR20 response rate | 70.00% | 48.02% | 63.83% | Beta | 13 |
| p MTX withdrawal rate | 9.17% | 4.71% | 14.91% | Beta | 13 |
| p Adalimumab + MTX ACR20 response rate | 67.00% | 60.35% | 73.33% | Beta | 17 |
| p Adalimumab + MTX withdrawal rate | 8.00% | 4.67% | 12.13% | Beta | 17 |
| p Tocilizumab + MTX ACR20 response rate | 50.00% | 43.10% | 56.90% | Beta | 18 |
| p Tocilizumab + MTX withdrawal rate | 8.00% | 4.67% | 12.13% | Beta | 18 |
| p Tofacitinib ACR20 response rate | 52.00% | 45.08% | 58.88% | Beta | 19 |
| p Tofacitinib withdrawal rate | 11.00% | 7.06% | 15.68% | Beta | 19 |
| c rhTNFR:Fc | 126.60 | 151.92 | 101.28 | Gamma | |
| c MTX | 1.94 | 2.32 | 1.55 | Gamma | |
| c Adalimumab | 998.00 | 1197.60 | 798.40 | Gamma | |
| c Tocilizumab | 1506.38 | 1807.66 | 1205.10 | Gamma | |
| c Tofacitinib | 1.30 | 1.56 | 1.04 | Gamma | |
| c Loratadine | 2.3137 | 2.78 | 1.85 | Gamma | |
| c Buprofen Sustained-Release Capsules | 0.2246 | 0.27 | 0.18 | Gamma | |
| c Amoxicillin | 0.1449 | 0.17 | 0.12 | Gamma | |
| c Omeprazole enteric-coated capsules | 0.4201 | 0.50 | 0.34 | Gamma | |
| c Polyene Phosphatidylcholine | 1.29 | 1.55 | 1.03 | Gamma | |
| c Registration | 14 | 16.80 | 11.20 | Gamma | |
| c Complete blood count | 54 | 64.80 | 43.20 | Gamma | |
| c Lipid profile tests | 36 | 43.20 | 28.80 | Gamma | |
| c Biochemical tests | 64 | 76.80 | 51.20 | Gamma | |
| c Ultrasound scans | 105 | 126.00 | 84.00 | Gamma | |
| c_CT | 265 | 318.00 | 212.00 | Gamma | |
| c Bone density tests | 40 | 48.00 | 32.00 | Gamma | |
| c Injection fee, subcutaneous | 3.5 | 4.20 | 2.80 | Gamma | |
| c Injection fee, intravenous | 5 | 6.00 | 4.00 | Gamma | |
| c_Bospitalization | 34 | 40.80 | 27.20 | Gamma | |
| c_Nursing | 12.5 | 15.00 | 10.00 | Gamma | |
| c Palliative care | 41,971 | 47,046.00 | 37,017.00 | Gamma | 20 |
| p Discount | 5.00% | 8% | 0.00% | - | |
| u base | 0.193193 | 0.23 | 0.15 | Gamma | 13 |
| u rhTNFR:Fc | 0.514056 | 0.62 | 0.41 | Gamma | 22 |
| u MTX | 0.37967 | 0.46 | 0.30 | Gamma | 22 |
| u Adalimumab (rhTNFR:Fc group) | 0.737562 | 0.89 | 0.59 | Gamma | 17 |
| u Adalimumab (MTX group) | 0.64043 | 0.77 | 0.51 | Gamma | 17 |
| u Tocilizumab (rhTNFR:Fc group) | 0.737562 | 0.89 | 0.59 | Gamma | 11 |
| u Tocilizumab (MTX group) | 0.64043 | 0.77 | 0.51 | Gamma | 11 |
| u Tofacitinib (rhTNFR:Fc group) | 0.737562 | 0.89 | 0.59 | Gamma | 19 |
| u Tofacitinib (MTX group) | 0.64043 | 0.77 | 0.51 | Gamma | 19 |
| u Palliative care | 0.193193 | 0.23 | 0.15 | Gamma | 13 |
| Scenario | Incremental Cost (CNY) | Incremental QALYs | ICER |
|---|---|---|---|
| Base case analysis | 9447.96 | 0.74 | 12,783.56 |
| Combination use perspective | 8357.07 | 0.71 | 11,776.31 |
| Patient out-of-pocket expenses perspective | 5970.98 | 0.74 | 8079.04 |
| Medical insurance perspective | 5639.36 | 0.74 | 7630.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Ma, A. Cost-Effectiveness Analysis of Recombinant Tumor Necrosis Factor Receptor: Fc Fusion Protein as First-Line Treatment for Active Rheumatoid Arthritis in China. Healthcare 2025, 13, 3267. https://doi.org/10.3390/healthcare13243267
Zhang R, Ma A. Cost-Effectiveness Analysis of Recombinant Tumor Necrosis Factor Receptor: Fc Fusion Protein as First-Line Treatment for Active Rheumatoid Arthritis in China. Healthcare. 2025; 13(24):3267. https://doi.org/10.3390/healthcare13243267
Chicago/Turabian StyleZhang, Rui, and Aixia Ma. 2025. "Cost-Effectiveness Analysis of Recombinant Tumor Necrosis Factor Receptor: Fc Fusion Protein as First-Line Treatment for Active Rheumatoid Arthritis in China" Healthcare 13, no. 24: 3267. https://doi.org/10.3390/healthcare13243267
APA StyleZhang, R., & Ma, A. (2025). Cost-Effectiveness Analysis of Recombinant Tumor Necrosis Factor Receptor: Fc Fusion Protein as First-Line Treatment for Active Rheumatoid Arthritis in China. Healthcare, 13(24), 3267. https://doi.org/10.3390/healthcare13243267





