Comparative Outcomes Between Classic and Silent ICU Models During COVID-19 Surge in Taiwan: A Real-World Cohort Analysis from a Dual-Campus Medical Center
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Setting
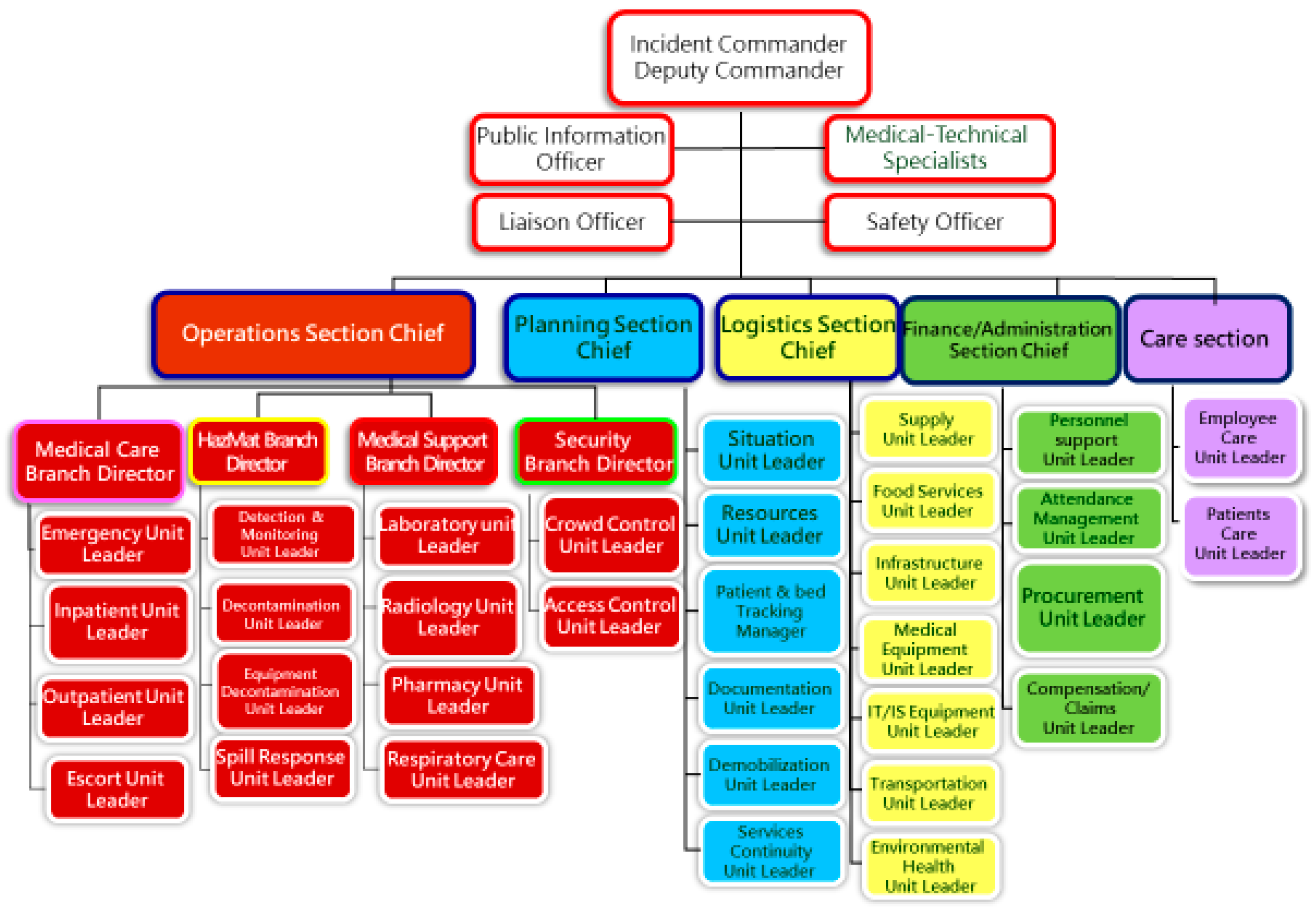
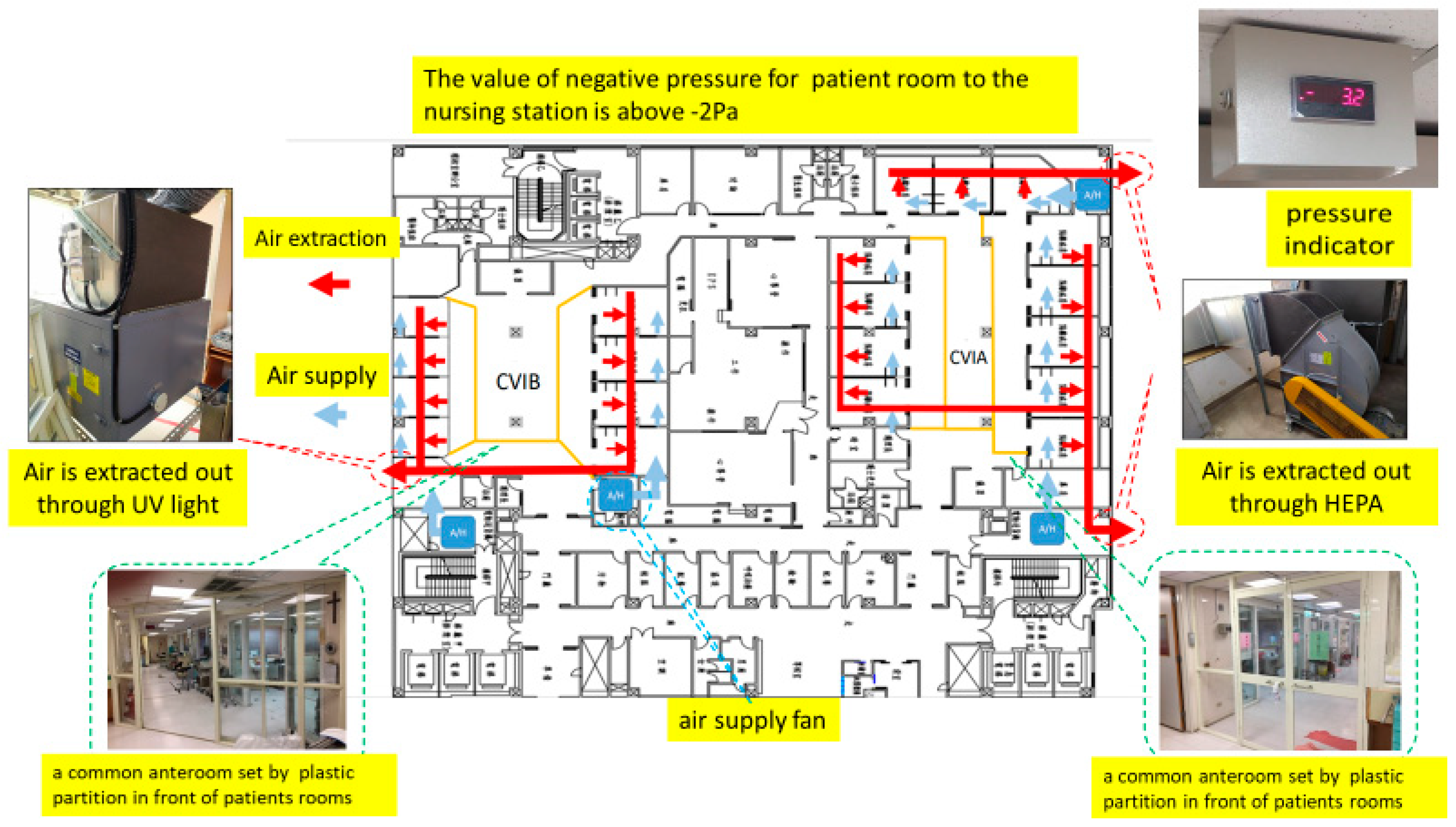
2.2. ICU Models and Expansion Strategy
2.3. Technical Specifications
2.4. Data Collection
2.5. Statistical Analysis
2.6. Ethical Considerations
3. Results
3.1. ICU Expansion and Patient Distribution
3.2. Treatments and Interventions
3.3. Patient Outcomes
3.4. Complications
3.5. Healthcare Worker Safety and Infection Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological Update on COVID-19–22 February 2022; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---22-february-2022 (accessed on 5 November 2025).
- Wang, H.-W.; Chen, G.-W.; Lee, W.-L.; You, S.-H.; Li, C.-W.; Jang, J.-H.; Shieh, C.-L. Learning from each other in the management of natural disaster and COVID-19 pandemic: A case study in Taiwan. Front. Public Heal. 2021, 9, 777255. [Google Scholar] [CrossRef]
- Huang, J.-H.; Chang, H.-T.; Liao, C.-H.; Chiu, K.-M. Rapid response of a medical center upon the surge of COVID-19 epidemic in Taiwan. J. Microbiol. Immunol. Infect. 2022, 55, 1–5. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Murthy, S.; Webb, S. COVID-19: A novel coronavirus and a novel challenge for critical care. Intensiv. Care Med. 2020, 46, 833–836. [Google Scholar] [CrossRef]
- Grasselli, G.; Pesenti, A.; Cecconi, M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: Early experience and forecast during an emergency response. JAMA 2020, 323, 1545–1546. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D.; et al. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Aziz, S.; Arabi, Y.M.; Alhazzani, W.; Evans, L.; Citerio, G.; Fischkoff, K.; Salluh, J.; Meyfroidt, G.; Alshamsi, F.; Oczkowski, S.; et al. Managing ICU surge during the COVID-19 crisis: Rapid guidelines. Intensive Care Med. 2020, 46, 1303–1325. [Google Scholar] [CrossRef]
- Arabi, Y.M.; Azoulay, E.; Al-Dorzi, H.M.; Phua, J.; Salluh, J.; Binnie, A.; Hodgson, C.; Angus, D.C.; Cecconi, M.; Du, B.; et al. How the COVID-19 pandemic will change the future of critical care. Intensiv. Care Med. 2021, 47, 282–291. [Google Scholar] [CrossRef]
- Carenzo, L.; Costantini, E.; Greco, M.; Barra, F.L.; Rendiniello, V.; Mainetti, M.; Bui, R.; Zanella, A.; Grasselli, G.; Lagioia, M.; et al. Hospital surge capacity in a tertiary emergency referral centre during the COVID-19 outbreak in Italy. Anaesthesia 2020, 75, 928–934. [Google Scholar] [CrossRef]
- Peters, A.W.; Chawla, K.S.; Turnbull, Z.A. Transforming ORs into ICUs. N. Engl. J. Med. 2020, 382, e52. [Google Scholar] [CrossRef]
- Bahrani, M.J.; Arabi, Y.M.; Lim, C.M.; Fang, W.F.; Azhari, N.N.; Nishimura, M.; Faris, O.G.; Fong, K.Y.; Pham, T.; Adhikari, N.K.; et al. Critical care bed capacity in Asian countries and regions: 2020 survey. Crit. Care Med. 2020, 48, 654–662. [Google Scholar] [CrossRef]
- Yen, M.-Y.; Lin, Y.-E.; Lee, C.-H.; Ho, M.-S.; Huang, F.-Y.; Chang, S.-C.; Liu, Y.-C. Taiwan’s traffic control bundle and the elimination of nosocomial SARS among healthcare workers. J. Hosp. Infect. 2011, 77, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.Y.S.; Wan, W.T.P.; Chan, A.K.M.; Tong, S.K.; Poon, S.T.; Joynt, G.M. Preparedness for COVID-19: In situ simulation to enhance infection-control systems in the ICU. Br. J. Anaesth. 2020, 125, e236–e239. [Google Scholar] [CrossRef]
- Xie, J.; Tong, Z.; Guan, X.; Du, B.; Qiu, H.; Slutsky, A.S. Critical care crisis and some recommendations during the COVID-19 epidemic in China. Intensiv. Care Med. 2020, 46, 837–840. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Germagnoli, V.; Lorini, F.L.; et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs in Lombardy, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Ghassemieh, B.J.; Nichols, M.; Kim, R.; Jerome, K.R.; Nalla, A.K.; Greninger, A.L.; Meakins, G.D.; Pham, T.; Englund, J.A.; et al. COVID-19 in critically ill patients in the Seattle region—Case series. N. Engl. J. Med. 2020, 382, 2012–2022. [Google Scholar] [CrossRef]
- Fagiuoli, S.; Lorini, F.L.; Remuzzi, G. COVID-19 Bergamo Hospital Crisis Unit. Adaptations and lessons in the province of Bergamo. N. Engl. J. Med. 2020, 382, e71. [Google Scholar] [CrossRef]
- Ng, K.P.; Puar, T.H.K.; Singh, S.R.; Chong, Y.M.; Wong, Y.X.; Tan, T.Y.; Koh, M.S.; Rye, J.; Choo, J.; Lee, J.; et al. COVID-19 and the risk to health care workers: A case report. Ann. Intern. Med. 2020, 172, 766–767. [Google Scholar] [CrossRef]
- Murthy, S.; Gomersall, C.D.; Fowler, R.A. Care for critically ill patients with COVID-19. JAMA 2020, 323, 1499–1500. [Google Scholar] [CrossRef] [PubMed]
- Azoulay, E.; Cariou, A.; Bruneel, F.; Demoule, A.; Kouatchet, A.; Reuter, D.; Souppart, V.; Combes, A.; Klouche, K.; Argaud, L.; et al. Symptoms of anxiety, depression, and peritraumatic dissociation in critical care clinicians managing COVID-19 patients. Am. J. Respir. Crit. Care Med. 2020, 202, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Pappa, S.; Ntella, V.; Giannakas, T.; Giannakoulis, V.G.; Papoutsi, E.; Katsaounou, P. Prevalence of depression, anxiety, and insomnia among healthcare workers during COVID-19: A systematic review and meta-analysis. Brain Behav. Immun. 2020, 88, 901–907. [Google Scholar] [CrossRef]
- Papoutsi, E.; Giannakoulis, V.G.; Ntella, V.; Pappa, S.; Katsaounou, P. Global burden of the COVID-19 pandemic on healthcare workers. ERJ Open Res. 2020, 6, 00195–2020. [Google Scholar] [CrossRef]
- Moss, M.; Good, V.S.; Gozal, D.; Kleinpell, R.; Sessler, C.N. A critical care societies collaborative statement: Burnout syndrome in critical-care professionals—A call for action. Am. J. Respir. Crit. Care Med. 2016, 194, 106–113. [Google Scholar] [CrossRef]
- Azoulay, E.; De Waele, J.; Ferrer, R.; Staudinger, T.; Borkowska, M.; Póvoa, P.; Iliopoulou, K.; Artigas, A.; Schaller, S.J.; Hari, M.S.; et al. Symptoms of burnout in ICU specialists facing the COVID-19 outbreak. Ann. Intensiv. Care 2020, 10, 110. [Google Scholar] [CrossRef]
- Kok, N.; van Gurp, J.; Teerenstra, S.; van der Hoeven, H.; Fuchs, M.; Hoedemaekers, C.; Zegers, M. Recognizing and supporting morally injured ICU professionals during the COVID-19 pandemic. Intensiv. Care Med. 2020, 46, 1653–1654. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, C.; Nafde, C.; Khodatars, S.; Jeanes, A.L.; Habib, S.; Donaldson, E.; Besi, C.; Kooner, G.K. COVID-19: Lessons for junior doctors redeployed to critical care. Postgrad. Med J. 2021, 97, 368–372. [Google Scholar] [CrossRef]
- Bowden, K.R.; Burnham, E.L.; Keniston, A.; Levin, D.; Limes, J.; Persoff, J.; Thurman, L.; Burden, M. Harnessing the power of hospitalists in operational disaster planning: COVID-19. J. Gen. Intern. Med. 2020, 35, 2732–2737. [Google Scholar] [CrossRef] [PubMed]
- Lefrant, J.-Y.; Fischer, M.O.; Potier, H.; Degryse, C.; Jaber, S.; Muller, L.; Pottecher, J.; Charbonneau, H.; Meaudre, E.; Lanot, P.; et al. A national healthcare response to intensive care bed requirements during the COVID-19 outbreak in France. Anaesth. Crit. Care Pain Med. 2020, 39, 709–715. [Google Scholar] [CrossRef]
- Lilly, C.M.; McLaughlin, J.M.; Zhao, H.; Baker, S.P.; Cody, S.; Irwin, R.S.; UMass Memorial Critical Care Operations Group. A multicenter study of ICU telemedicine reengineering of adult critical care. Chest J. 2014, 145, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Halpern, N.A.; Pastores, S.M.; Oropello, J.M.; Kvetan, V. Critical care medicine in the United States: Addressing the intensivist shortage. Crit. Care Med. 2014, 42, 463–474. [Google Scholar] [CrossRef]



| Characteristic | Classic ICU (Units A + B, n = 28) | Silent ICU (Unit C, n = 36) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 67.0 ± 13.1 | 68.5 ± 15.2 | 0.68 |
| Male sex (%) | 22 (78.6%) | 30 (83.3%) | 0.62 |
| APACHE II score (median [IQR]) | 21 [17–27] | 20 [15–24] | 0.75 |
| Any comorbidity (%) | 24 (85.7%) | 30 (83.3%) | 0.78 |
| Chronic hypertension (%) | 18 (64.3%) | 20 (55.6%) | 0.47 |
| Diabetes mellitus (%) | 10 (35.7%) | 12 (33.3%) | 0.84 |
| Coronary artery disease (%) | 3 (10.7%) | 2 (5.6%) | 0.65 |
| Heart failure (%) | 5 (17.9%) | 4 (11.1%) | 0.49 |
| Chronic lung disease (COPD) (%) | 4 (14.3%) | 5 (13.9%) | 0.96 |
| Chronic kidney disease (%) | 2 (7.1%) | 3 (8.3%) | 1 |
| Malignancy (%) | 1 (3.6%) | 1 (2.8%) | 1 |
| Severity of illness upon ICU admission | |||
| Moderate ARDS (%) | 8 (28.6%) | 13 (36.1%) | 0.51 |
| Severe ARDS (%) | 20 (71.4%) | 23 (63.9%) | 0.51 |
| Referred from outside hospital (%) | 10 (35.7%) | 2 (5.6%) | 0.003 |
| Treatment/Intervention | Classic ICU (A + B) (n = 28) | Silent ICU (C) (n = 36) | p-Value |
|---|---|---|---|
| Corticosteroid therapy (dexamethasone) | 28 (100%) | 36 (100%) | – |
| Remdesivir antiviral therapy | 21 (75.0%) | 27 (75.0%) | 1 |
| IL-6 inhibitor (tocilizumab) | 6 (21.4%) | 9 (25.0%) | 0.77 |
| Therapeutic anticoagulation instituted | 27 (96.4%) | 34 (94.4%) | 1 |
| Prone positioning applied | 22 (78.6%) | 28 (77.8%) | 0.93 |
| Non-invasive ventilation pre-intubation | 5 (17.9%) | 8 (22.2%) | 0.76 |
| Mean duration of MV (days) for survivors | 16.5 ± 9.8 | 17.4 ± 10.5 | 0.78 |
| Renal replacement therapy (CVVH/IHD) | 8 (28.6%) | 7 (19.4%) | 0.39 |
| Tracheostomy performed | 2 (7.1%) | 4 (11.1%) | 0.68 |
| Outcome | Classic ICU (A + B) | Silent ICU (C) | p-Value |
|---|---|---|---|
| ICU mortality rate | 8/28 (28.6%) | 13/36 (36.1%) | 0.53 |
| Ventilator-weaning success rate | 14/28 (50.0%) | 23/36 (63.9%) | 0.23 |
| Ventilator-dependent at transfer (%) | 2/28 (7.1%) | 4/36 (11.1%) | 0.68 |
| ICU length of stay—median (IQR), days | 19 (12–27) | 16 (9–31) | 0.97 |
| Hospital length of stay—median, days | 28 (18–46) | 25 (17–45) | 0.88 |
| 28-day mortality (%) | 7 (25.0%) | 10 (27.8%) | 0.8 |
| In-hospital mortality (%) | 9 (32.1%) | 14 (38.9%) | 0.57 |
| Complication | Classic ICU (A + B) (n = 28) | Silent ICU (C) (n = 36) | p-Value |
|---|---|---|---|
| Acute kidney injury (AKI) | 23 (82.1%) | 27 (75.0%) | 0.54 |
| AKI requiring dialysis | 8 (28.6%) | 7 (19.4%) | 0.39 |
| Septic shock (vasopressors required) | 13 (46.4%) | 18 (50.0%) | 0.8 |
| Ventilator-associated pneumonia (VAP) | 7 (25.0%) | 9 (25.0%) | 1 |
| Pneumothorax (barotrauma) | 5 (17.9%) | 7 (19.4%) | 0.87 |
| Bacteremia (non-pulmonary source) | 3 (10.7%) | 2 (5.6%) | 0.65 |
| Deep vein thrombosis | 1 (3.6%) | 0 (0%) | 0.45 |
| Myocarditis/Pericarditis | 0 | 0 | – |
| Any complication above | 26 (92.9%) | 33 (91.7%) | 1 |
| Indicator | Unit A (AIIR ICU) | Unit B (Converted ICU) | Unit C (Silent ICU) |
|---|---|---|---|
| HCW COVID-19 infections, n | 0 | 0 | 0 |
| HCWs quarantined due to exposure, n | 0 | 0 | 0 |
| Breaches in PPE protocol, n | 0 (minor breaches promptly corrected) | 0 | 0 |
| Environmental contamination events, n | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-H.; Hu, T.-Y.; Kuo, L.-K. Comparative Outcomes Between Classic and Silent ICU Models During COVID-19 Surge in Taiwan: A Real-World Cohort Analysis from a Dual-Campus Medical Center. Healthcare 2025, 13, 3092. https://doi.org/10.3390/healthcare13233092
Chang W-H, Hu T-Y, Kuo L-K. Comparative Outcomes Between Classic and Silent ICU Models During COVID-19 Surge in Taiwan: A Real-World Cohort Analysis from a Dual-Campus Medical Center. Healthcare. 2025; 13(23):3092. https://doi.org/10.3390/healthcare13233092
Chicago/Turabian StyleChang, Wei-Hung, Ting-Yu Hu, and Li-Kuo Kuo. 2025. "Comparative Outcomes Between Classic and Silent ICU Models During COVID-19 Surge in Taiwan: A Real-World Cohort Analysis from a Dual-Campus Medical Center" Healthcare 13, no. 23: 3092. https://doi.org/10.3390/healthcare13233092
APA StyleChang, W.-H., Hu, T.-Y., & Kuo, L.-K. (2025). Comparative Outcomes Between Classic and Silent ICU Models During COVID-19 Surge in Taiwan: A Real-World Cohort Analysis from a Dual-Campus Medical Center. Healthcare, 13(23), 3092. https://doi.org/10.3390/healthcare13233092





