Unsupervised Clustering of 41,728 Emergency Department Visits: Insights into Patient Profiles and KTAS Reliability
Abstract
1. Introduction
2. Methods
2.1. Korean Triage and Acuity Scale (KTAS)
2.2. Research Design and Study Population
2.3. Analysis Procedures
3. Results
3.1. Characteristics of the Study Population
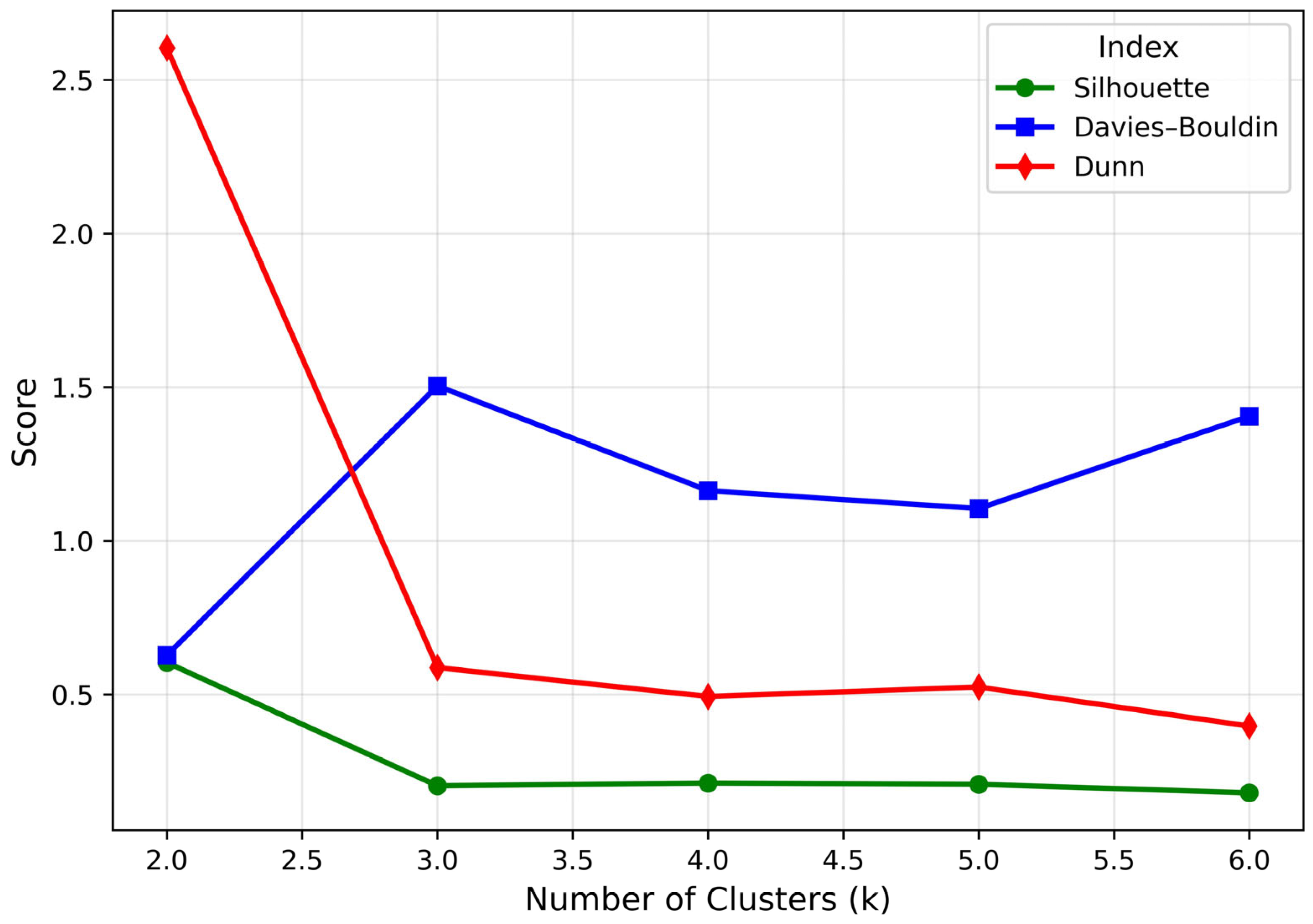
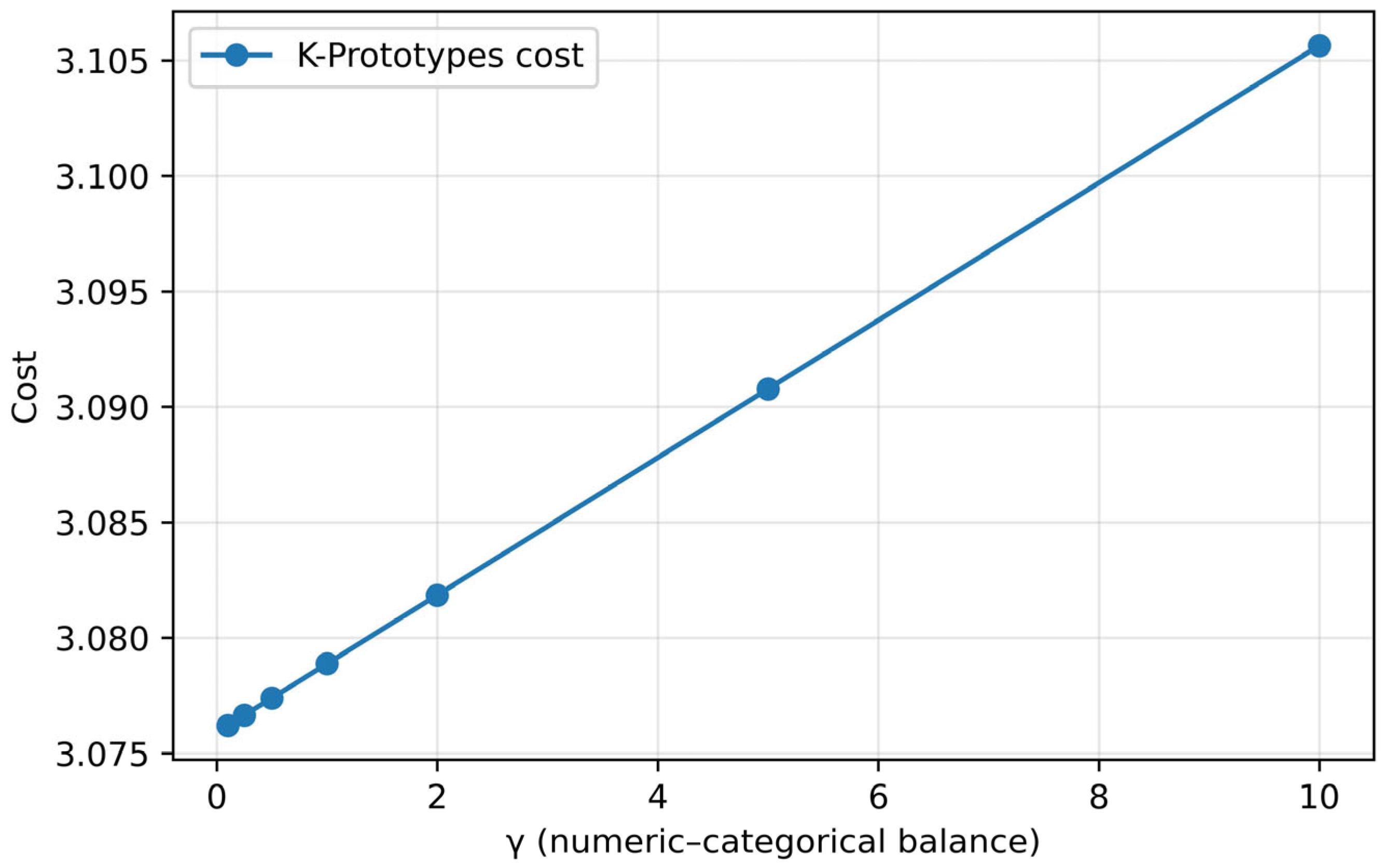
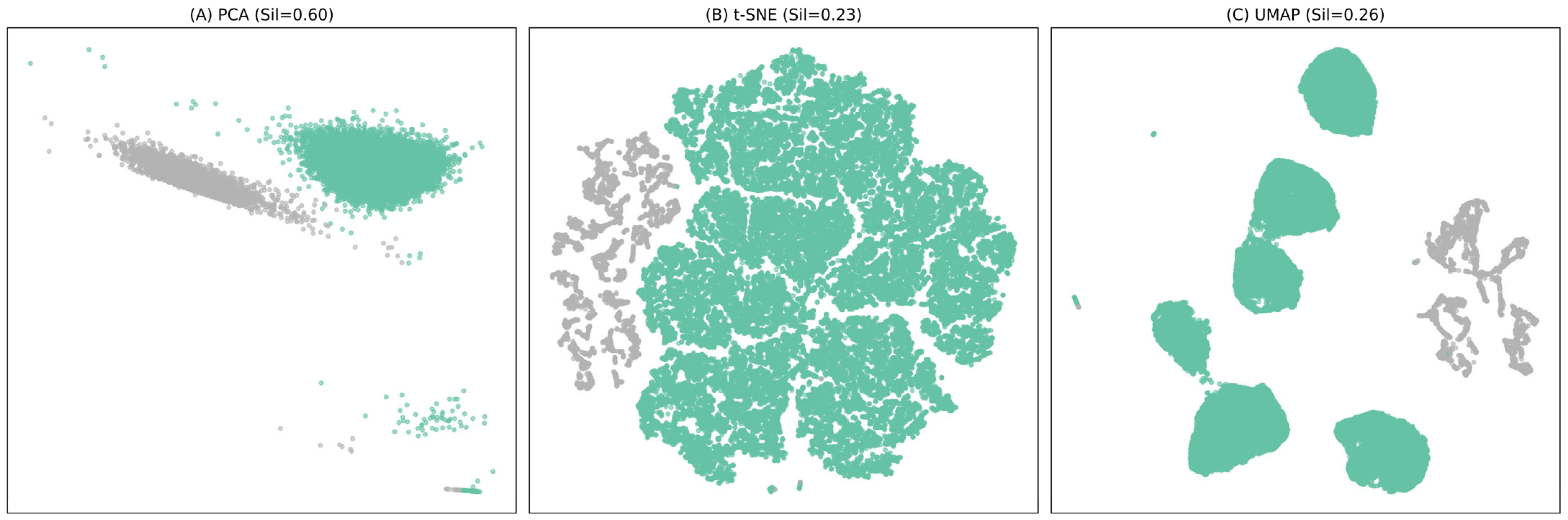
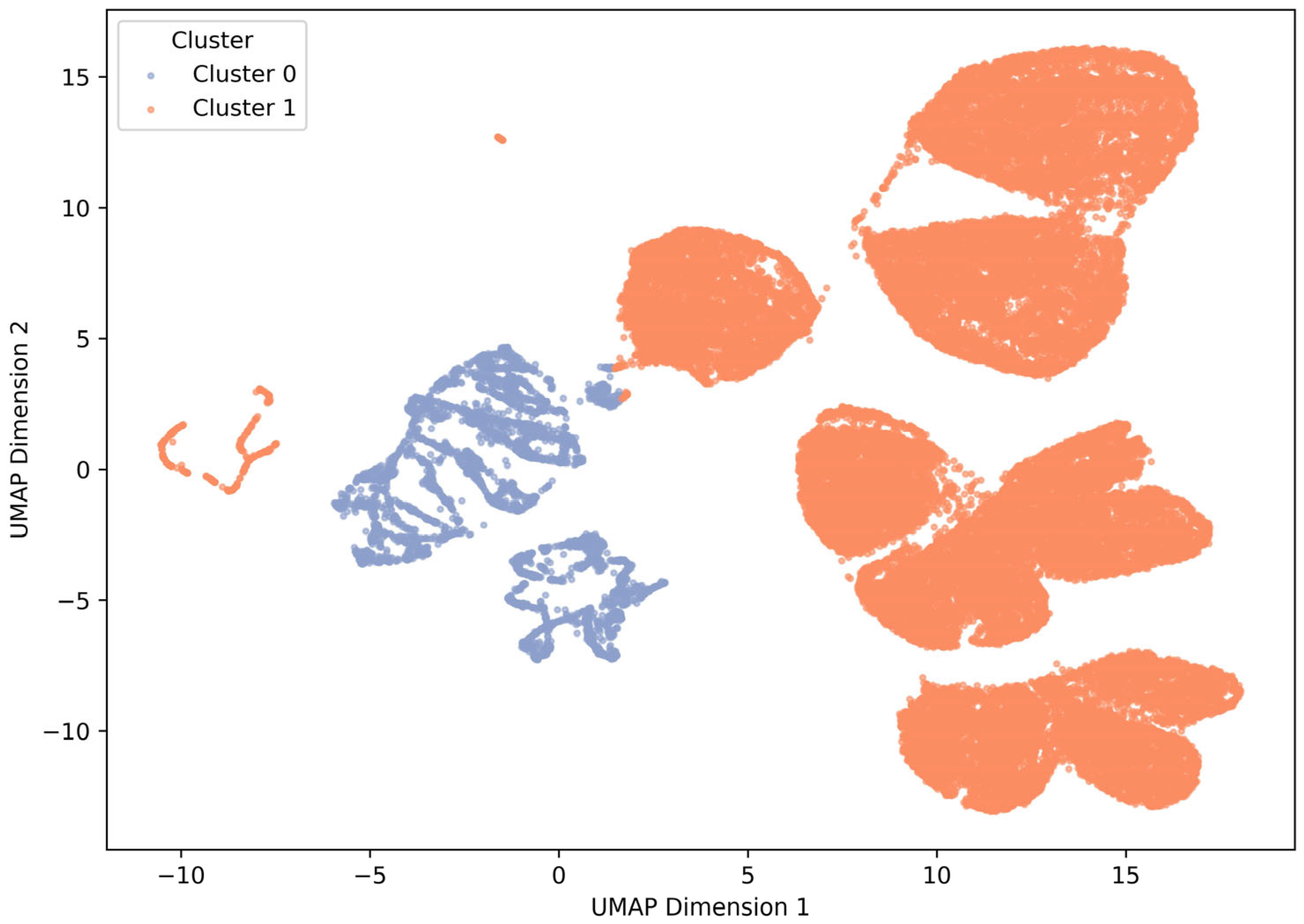
3.2. Cluster Analysis
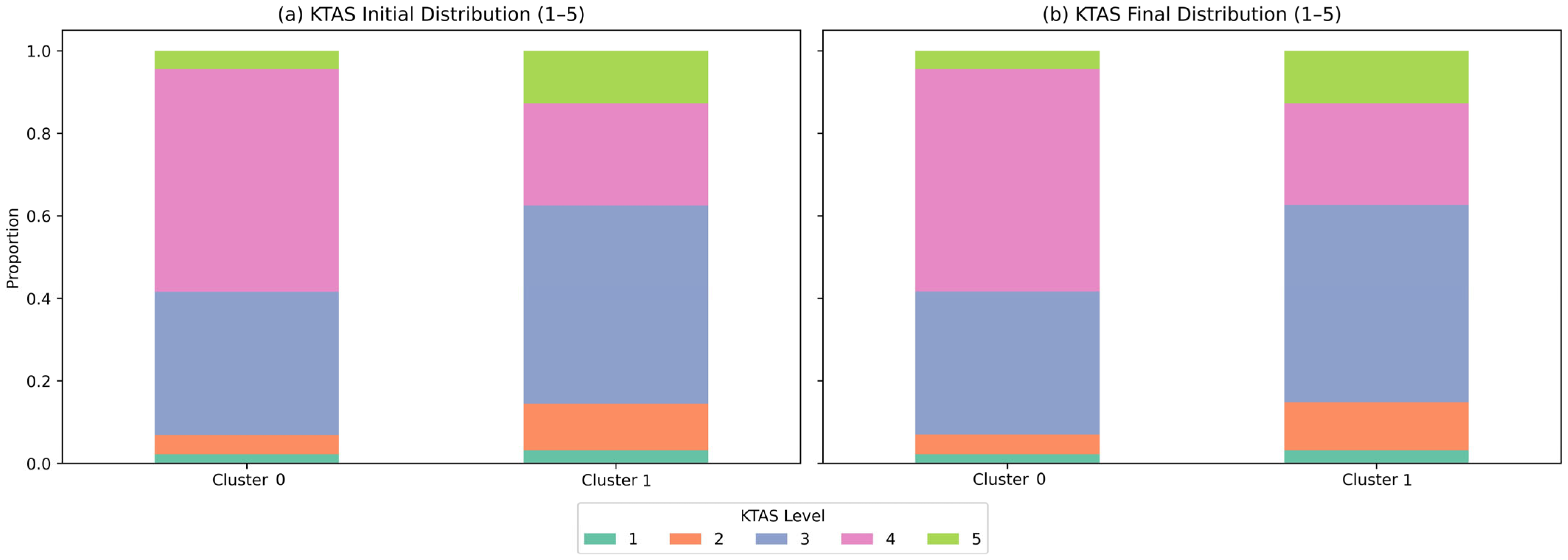
3.3. KTAS Distribution and Severity Patterns
3.4. Sequential Logistic Regression
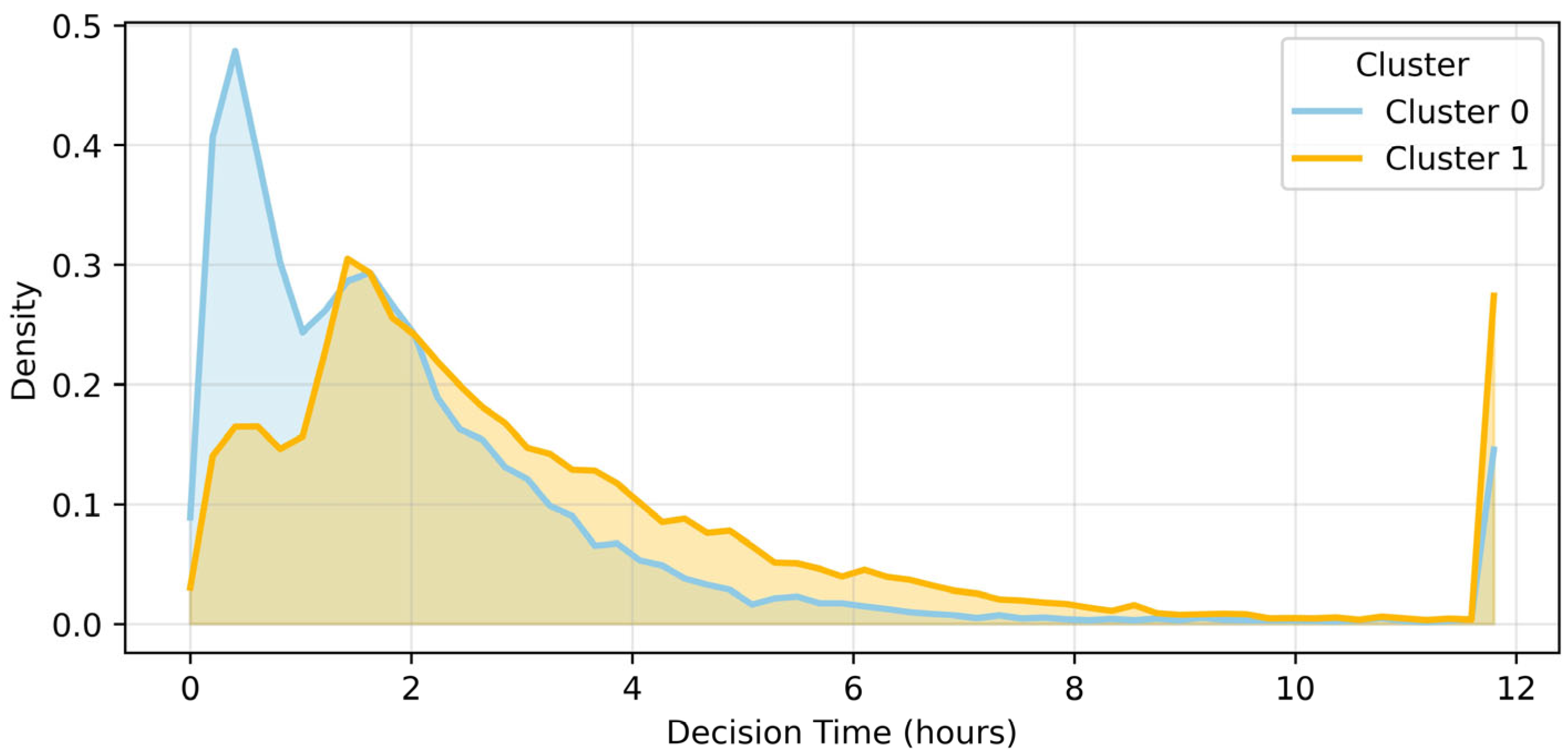
3.5. Decision Time Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schinkel, M.; Bergsma, L.; Veldhuis, L.I.; Ridderikhof, M.L.; Holleman, F. Comparing complaint-based triage scales and early warn-ing scores for emergency department triage. Emerg. Med. J. 2022, 39, 691–696. [Google Scholar] [CrossRef]
- Cameron, A.; Rodgers, K.; Ireland, A.J.; Jamdar, R.; McKay, G.A. A simple tool to predict admission at the time of triage. Emerg. Med. J. 2015, 32, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, J.; Kim, Y.J.; Lee, J.H.; Lim, T.H. Reliability of Korean Triage and Acuity Scale: Interrater Agreement between Two Experienced Nurses by Real-Time Triage and Analysis of Influencing Factors to Disagreement of Triage Levels. J. Korean Med. Sci. 2019, 34, e189. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Wang, C.-L.; Li, C.-J.; Huang, C.-H.; Ng, C.-J.; Lin, G.-Y.; Chiou, M.-J.; Lo, H.-Y.; Chen, S.-Y. A machine learning model for predicting unscheduled 72 h return visits to the emergency department by patients with abdominal pain. Diagnostics 2022, 12, 82. [Google Scholar] [CrossRef]
- Savioli, G.; Ceresa, I.F.; Bressan, M.A.; Bavestrello Piccini, G.; Novelli, V.; Cutti, S.; Ricevuti, G.; Esposito, C.; Longhitano, Y.; Piccioni, A.; et al. Geriatric population triage: The risk of real-life over- and under-triage in an overcrowded ED: 4- and 5-level triage systems compared: The CREONTE (Crowding and R E Organization National TriagE) study. J. Pers. Med. 2024, 14, 195. [Google Scholar] [CrossRef]
- Zachariasse, J.M.; van der Hagen, V.; Seiger, N.; Mackway-Jones, K.; van Veen, M.; A Moll, H. Validity of the Manchester Triage System in emergency care: A prospective observational study. PLoS ONE 2017, 12, e0170811. [Google Scholar] [CrossRef]
- Sezik, S.; Cingiz, M.Ö.; İbiş, E. Machine Learning-Based Model for Emergency Department Disposition at a Public Hospital. Appl. Sci. 2025, 15, 1628. [Google Scholar] [CrossRef]
- Aityan, S.K.; Shoustikov, D.; Arandjelović, O. Integrated AI medical emergency diagnostics advising system. Electronics 2024, 13, 4389. [Google Scholar] [CrossRef]
- Ocak, M.; Tascanov, M.B.; Yurt, N.Ş.; Yurt, Y.C. Predictive efficacy of frontal QRS-T angle in COVID-19 patients. Am. J. Emerg. Med. 2022, 57, 210. [Google Scholar] [CrossRef] [PubMed]
- Wartelle, A.; Mourad-Chehade, F.; Yalaoui, F.; Questiaux, H.; Monneret, T.; Soliveau, G.; Chrusciel, J.; Duclos, A.; Laplanche, D.; Sanchez, S. Multimorbidity Clustering of the Emergency Department Patient Flow: Impact Analysis of New Unscheduled Care Clinics. PLoS ONE 2022, 17, e0262914. [Google Scholar] [CrossRef]
- Orso, D.; Peric, D.; Di Gioia, C.C.; Comisso, I.; Bove, T.; Ban, A.; Fonda, F.; Federici, N. Renal and genitourinary ultrasound evaluation in emergency and critical care: An overview. Healthcare 2024, 12, 1356. [Google Scholar] [CrossRef]
- Narayanan, N.; Gross, A.K.; Pintens, M.; Fee, C.; MacDougall, C. Effect of an Electronic Medical Record Alert for Severe Sepsis among ED Patients. Am. J. Emerg. Med. 2016, 34, 185–188. [Google Scholar] [CrossRef]
- Huang, Z. Extensions to the k-means algorithm for clustering large data sets with categorical values. Data Min. Knowl. Discov. 1998, 2, 283–304. [Google Scholar] [CrossRef]
- Sisto, U.G.; Orso, D.; Maione, D.; Venturelli, F.; De Luca, A. Tissue Doppler imaging in acute and critical care: Enhancing diagnostic precision. Medicina 2025, 61, 1051. [Google Scholar] [CrossRef]
- Hastings, S.N.; Whitson, H.E.; Sloane, R.; Landerman, L.R.; Horney, C.; Johnson, K.S. Using the past to predict the future: Latent class analysis of patterns of health service use of older adults in the emergency department. J. Am. Geriatr. Soc. 2014, 62, 711–715. [Google Scholar] [CrossRef] [PubMed]
- White, N.J.; Contaifer, D., Jr.; Martin, E.J.; Newton, J.C.; Mohammed, B.M.; Bostic, J.L.; Brophy, G.M.; Spiess, B.D.; Pusateri, A.E.; Ward, K.R.; et al. Early hemostatic responses to trauma identified with hierarchical clustering analysis. J. Thromb. Haemost. 2015, 13, 978–988. [Google Scholar] [CrossRef]
- Grant, R.W.; McCloskey, J.; Hatfield, M.; Uratsu, C.; Ralston, J.D.; Bayliss, E.; Kennedy, C.J. Use of latent class analysis and k-means clustering to identify complex patient profiles. JAMA Netw. Open 2020, 3, e2029068. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.M.; Dufour, I.; Courteau, J.; Vanasse, A.; Chouinard, M.C.; Dubois, M.F.; Dubuc, N.; Elazhary, N.; Hudon, C. Profiles of frequent emergency department users with chronic conditions: A latent class analysis. BMJ Open 2022, 12, e055297. [Google Scholar] [CrossRef]
- Orso, D.; Furlanis, G.; Romanelli, A.; Gheller, F.; Tecchiolli, M.; Cominotto, F. Risk factors analysis for 90-day mortality of adult patients with mild traumatic brain injury in an Italian emergency department. Geriatrics 2024, 9, 23. [Google Scholar] [CrossRef]
- Abar, B.; Holub, A.; Hong, S.; Aaserude, E.; DeRienzo, V. Latent class analysis of barriers to care among emergency department patients. West. J. Emerg. Med. 2019, 20, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Rathlev, N.K.; Visintainer, P.; Schmidt, J.; Hettler, J.; Albert, V.; Li, H. Patient characteristics and clinical process predictors of patients leaving without being seen from the emergency department. West. J. Emerg. Med. 2020, 21, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Wang, A.; Wang, D.; McCarty, T.; Herzig, S.J.; Samuels-Kalow, M.E. Patient-centered outcomes of an emergency department social and medical resource intervention. West. J. Emerg. Med. 2022, 24, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Riazi, K.; Pan, J.; Thavorn, K.; Ziegler, J.; Rochwerg, B.; Quan, H.; Prescott, H.C.; Dodek, P.M.; Li, B.; et al. Unsupervised clustering for sepsis identification in large-scale patient data: A model development and validation study. Intensive Care Med. Exp. 2025, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-J.; Lin, C.-H.; Chu, C.-C.J.; Lo, H.Y.; Ng, C.J.; Hsu, C.C.; Chen, S.Y. Machine learning models for predicting mortality in patients with cirrhosis and acute upper GI bleeding at an emergency department: A retrospective cohort study. Diagnostics 2024, 14, 1919. [Google Scholar] [CrossRef]
- Ahmad, A.; Dey, L. A k-mean clustering algorithm for mixed numeric and categorical data. Data Knowl. Eng. 2007, 63, 503–527. [Google Scholar] [CrossRef]
- Sánchez-Salmerón, R.; Gómez-Urquiza, J.L.; Albendín-García, L.; Correa-Rodríguez, M.; Martos-Cabrera, M.B.; Velando-Soriano, A.; Suleiman-Martos, N. Machine learning methods applied to triage in emergency services: A systematic review. Int. Emerg. Nurs. 2022, 60, 101109. [Google Scholar] [CrossRef]
| Variable | Mean (SD) | 25% | Median | 75% | Max |
|---|---|---|---|---|---|
| Age (years) | 49.7 (21.4) | 32 | 51 | 65 | 107 |
| Heart rate (beats/min) | 85.6 (18.5) | 73 | 83 | 97 | 234 |
| Respiratory rate (/min) | 17.9 (2.2) | 16 | 18 | 18 | 99 |
| Body temperature (°C) | 36.8 (0.7) | 36.4 | 36.7 | 37.0 | 42.0 |
| Mean arterial pressure (mmHg) | 95.8 (16.3) | 85.7 | 95.0 | 105.3 | 212.7 |
| Variable | Cluster 0 (n = 4465) | Cluster 1 (n = 37.263) | p-Value * |
|---|---|---|---|
| Age (years) | 58.2 ± 14.5 | 45.6 ± 16.3 | <0.001 |
| HR (/min) | 78.2 ± 12.4 | 90.3 ± 15.7 | <0.001 |
| RR (/min) | 17.3 ± 2.8 | 19.2 ± 3.1 | <0.001 |
| BT (°C) | 36.7 ± 0.5 | 37.0 ± 0.6 | <0.001 |
| NRS | 2.1 ± 1.9 | 2.8 ± 2.2 | <0.001 |
| MAP (mmHg) | 104.3 ± 15.7 | 89.7 ± 13.1 | <0.001 |
| Variable | Coefficient (β) | Std. Error | z-Value | p-Value | 95% CI [Lower, Upper] |
|---|---|---|---|---|---|
| Cluster (1 vs. 0) | 0.415 | 0.032 | 13.055 | <0.001 | [0.353, 0.477] |
| Age (years) | −0.022 | 0.000 | −51.905 | <0.001 | [−0.023, −0.021] |
| Sex (male = 1) | −0.266 | 0.017 | −15.418 | <0.001 | [−0.300, −0.232] |
| Cutpoint 1/2 | −4.333 | 0.038 | −113.736 | <0.001 | [−4.408, −4.258] |
| Cutpoint 2/3 | 0.523 | 0.014 | 36.194 | <0.001 | [0.495, 0.551] |
| Cutpoint 3/4 | 0.866 | 0.006 | 141.805 | <0.001 | [0.854, 0.878] |
| Cutpoint 4/5 | 0.489 | 0.008 | 59.752 | <0.001 | [0.473, 0.505] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jang, E.; Kwon, S.; Song, M. Unsupervised Clustering of 41,728 Emergency Department Visits: Insights into Patient Profiles and KTAS Reliability. Healthcare 2025, 13, 3073. https://doi.org/10.3390/healthcare13233073
Kim J, Jang E, Kwon S, Song M. Unsupervised Clustering of 41,728 Emergency Department Visits: Insights into Patient Profiles and KTAS Reliability. Healthcare. 2025; 13(23):3073. https://doi.org/10.3390/healthcare13233073
Chicago/Turabian StyleKim, Jongsun, EunChul Jang, SoonChan Kwon, and MyoungJe Song. 2025. "Unsupervised Clustering of 41,728 Emergency Department Visits: Insights into Patient Profiles and KTAS Reliability" Healthcare 13, no. 23: 3073. https://doi.org/10.3390/healthcare13233073
APA StyleKim, J., Jang, E., Kwon, S., & Song, M. (2025). Unsupervised Clustering of 41,728 Emergency Department Visits: Insights into Patient Profiles and KTAS Reliability. Healthcare, 13(23), 3073. https://doi.org/10.3390/healthcare13233073






