The Future Dynamics of Long-Term Care Pressure in China’s Longevity Era: A Prediction Based on the Discrete-Time Markov Model
Highlights
- The number of disabled older adults in China will peak at 160 million in 2070 and remain above 115 million by 2100, with the disability rate rising from 39.75% to 45.28%.
- Person-years with disability (PYD) will increase significantly among the oldest-old and women, highlighting sharp age and gender disparities in long-term care burdens.
- China’s long-term care system will face sustained and structural pressure, demanding urgent adaptation to demographic aging.
- Timely policy interventions are needed to mitigate future health risks and avoid a care crisis in the longevity era.
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Definition of Health States
2.3. Construction of the Health Transition Model
- (1)
- Markov Chain Assumption
- (2)
- Introducing Transition Strength to Construct the Health State Transition Probability Matrix
- (3)
- Sensitivity analysis with scenario-specific time variation
- (4)
- Population Projection Model by Health Status
- (5)
- Estimating Care Time Demand for Elderly People in Different Disability States
- (6)
- Estimating person-years with disability (PYD) Among old adults
3. Results
3.1. Transition Probabilities Across Health States
3.2. Projected Population Size of Older Adults with Different Levels of Disability
3.3. Projected Duration of Disability States in Older Adults
3.4. Projected Scale of Disability Person-Years Across Different Disability States
4. Discussion
4.1. The Continuous Increase in Older Population with Impairment and Dysfunction
4.2. The Impact of Longer Disability Duration and Rising LTC Demand
4.3. The Variations in Health and Care Needs Across Age Groups
4.4. The Health Disparities Across Gender Categories
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PYD | Person-Years with Disability |
| LE | Life Expectancy |
| HLE | Healthy Life Expectancy |
| LTC | Long-Term Care |
| LTCI | Long-Term Care Insurance |
| HICs | High-Income Countries |
| LMICs | Low- and Middle-Income Countries |
Appendix A
| Age Group (Years) and Health State | ||||||||
|---|---|---|---|---|---|---|---|---|
| Total Population | 2030 | 2040 | 2050 | 2060 | 2070 | 2080 | 2090 | 2100 |
| 65~74 | ||||||||
| Mild Disability | 5.4 | 5.66 | 5.88 | 6.1 | 6.3 | 6.5 | 6.71 | 6.91 |
| Severe Disability | 1.19 | 1.25 | 1.3 | 1.35 | 1.39 | 1.44 | 1.48 | 1.53 |
| 75~84 | ||||||||
| Mild Disability | 4.48 | 4.69 | 4.93 | 5.12 | 5.33 | 5.51 | 5.73 | 5.93 |
| Severe Disability | 1.44 | 1.5 | 1.58 | 1.64 | 1.71 | 1.77 | 1.84 | 1.9 |
| 85~94 | ||||||||
| Mild Disability | 2.91 | 3.04 | 3.17 | 3.33 | 3.47 | 3.61 | 3.75 | 3.94 |
| Severe Disability | 2.15 | 2.25 | 2.35 | 2.46 | 2.57 | 2.67 | 2.78 | 2.91 |
| 95+ | ||||||||
| Mild Disability | 1.1 | 1.14 | 1.16 | 1.21 | 1.26 | 1.31 | 1.37 | 1.43 |
| Severe Disability | 2.18 | 2.25 | 2.3 | 2.4 | 2.49 | 2.6 | 2.71 | 2.84 |
| Male population | ||||||||
| 65~74 | ||||||||
| Mild Disability | 4.41 | 4.62 | 4.8 | 4.97 | 5.13 | 5.3 | 5.47 | 5.63 |
| Severe Disability | 1 | 1.04 | 1.09 | 1.12 | 1.16 | 1.2 | 1.24 | 1.27 |
| 75~84 | ||||||||
| Mild Disability | 4.23 | 4.43 | 4.65 | 4.84 | 5.03 | 5.2 | 5.41 | 5.61 |
| Severe Disability | 1.15 | 1.2 | 1.26 | 1.31 | 1.37 | 1.41 | 1.47 | 1.52 |
| 85~94 | ||||||||
| Mild Disability | 2.89 | 3.02 | 3.15 | 3.31 | 3.45 | 3.59 | 3.73 | 3.92 |
| Severe Disability | 1.68 | 1.76 | 1.83 | 1.92 | 2.01 | 2.09 | 2.17 | 2.28 |
| 95+ | ||||||||
| Mild Disability | 0.93 | 0.96 | 0.98 | 1.02 | 1.06 | 1.11 | 1.15 | 1.21 |
| Severe Disability | 2.1 | 2.17 | 2.22 | 2.31 | 2.4 | 2.51 | 2.61 | 2.74 |
| Female population | ||||||||
| 65~74 | ||||||||
| Mild Disability | 5.79 | 6.07 | 6.3 | 6.53 | 6.75 | 6.97 | 7.19 | 7.4 |
| Severe Disability | 1.44 | 1.51 | 1.57 | 1.63 | 1.68 | 1.74 | 1.79 | 1.85 |
| 75~84 | ||||||||
| Mild Disability | 4.26 | 4.46 | 4.68 | 4.86 | 5.06 | 5.23 | 5.44 | 5.63 |
| Severe Disability | 2.59 | 2.72 | 2.85 | 2.96 | 3.08 | 3.19 | 3.31 | 3.43 |
| 85~94 | ||||||||
| Mild Disability | 3.18 | 3.33 | 3.47 | 3.64 | 3.8 | 3.95 | 4.11 | 4.31 |
| Severe Disability | 2.29 | 2.4 | 2.5 | 2.63 | 2.74 | 2.85 | 2.96 | 3.11 |
| 95+ | ||||||||
| Mild Disability | 1.23 | 1.27 | 1.3 | 1.35 | 1.41 | 1.47 | 1.53 | 1.6 |
| Severe Disability | 2.13 | 2.21 | 2.26 | 2.35 | 2.45 | 2.55 | 2.66 | 2.79 |
References
- Jugran, D. Too well to die; too ill to live: An update on the lifespan versus health span debate. J. Glob. Health 2025, 15, 03022. [Google Scholar] [CrossRef] [PubMed]
- United Nations. World Population Prospects 2024. 2024. Available online: https://www.un.org/zh/global-issues/ageing (accessed on 1 August 2025).
- Cassell, A.; Edwards, D.; Harshfield, A.; Rhodes, K.; Brimicombe, J.; Payne, R.; Griffin, S. The epidemiology of multimorbidity in primary care: A retrospective cohort study. Br. J. Gen. Pract. 2018, 68, e245–e251. [Google Scholar] [CrossRef]
- Mokdad, A.H.; Bisignano, C.; Hsu, J.M.; Ababneh, H.S.; Abbasgholizadeh, R.; Abdelkader, A.; PubMed, M.; Abiodun, O.O.; Aboagye, R.G.; Abu-Zaid, A.; et al. The burden of diseases, injuries, and risk factors by state in the USA, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 404, 2314–2340. [Google Scholar] [CrossRef]
- Garmany, A.; Yamada, S.; Terzic, A. Longevity leap: Mind the healthspan gap. npj Regen. Med. 2021, 6, 57. [Google Scholar] [CrossRef]
- Nguyen, H.; Chua, K.C.; Dregan, A.; Vitoratou, S.; Bayes-Marin, I.; Olaya, B.; Prina, A.M. Factors associated with multimorbidity patterns in older adults in England: Findings from the English Longitudinal Study of Aging (ELSA). J. Aging Health 2020, 32, 1120–1132. [Google Scholar] [CrossRef]
- Simpson, G.; Kaluvu, L.M.; Stokes, J.; Roderick, P.; Chapman, A.; Akyea, R.K.; Zaccardi, F.; Santer, M.; Farmer, A.; Dambha-Miller, H. Understanding social care need through primary care big data: A rapid scoping review. BJGP Open 2022, 6, BJGPO.2022.0016. [Google Scholar] [CrossRef]
- Yue, L.; Jia, C.; Hu, B.; Zhang, Z.; Bai, M.; Wang, S.; Yao, N. Caregiving stress among family caregivers of older adults living with disabilities in China. Geriatr. Nurs. 2022, 47, 226–231. [Google Scholar] [CrossRef]
- Zhan, H. Chinese caregiving burden and the future burden of elder care in life-course perspective. Int. J. Aging Hum. Dev. 2002, 54, 267–290. [Google Scholar] [CrossRef]
- De, C.L.; Declercq, A.; Bouckaert, L.; Vermandere, M.; Graff, M.; Aertgeert, B. Perspectives of older adults with a chronic condition on functioning, social participation and health: A qualitative study. BMC Geriatr. 2021, 21, 418. [Google Scholar] [CrossRef] [PubMed]
- Cherry, K.; Marks, L.; Benedetto, T.; Sullivan, M.; Barker, A. Perceptions of longevity and successful aging in very old adults. J. Relig. Spiritual. Aging 2013, 25, 288–310. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Wu, B.; Ling, L. Undermet needs for assistance in personal activities of daily living among community-dwelling oldest old in China from 2005 to 2008. Res. Aging 2015, 37, 148–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhao, J.; Hu, J.; Liang, D.; Luo, Y. Predicting unmet activities of daily living needs among the oldest old with disabilities in China: A machine learning approach. Front. Public Health. 2023, 11, 1257818. [Google Scholar] [CrossRef] [PubMed]
- Haß, L.; Knippschild, S.; Tönnies, T.; Hoyer, A.; Palm, R.; Voss, S.; Brinks, R. Projected number of people in need for long-term care in Germany until 2050. Front. Public Health 2024, 12, 1456320. [Google Scholar] [CrossRef]
- Hu, B.; Brimblecombe, N.; Cartagena-Farias, J.; Silva-Ribeiro, W. Projected costs of long-term care for older people in England: The impacts of housing quality improvements. Health Policy 2025, 152, 105246. [Google Scholar] [CrossRef]
- Comas-Herrera, A.; Wittenberg, R.; Costa-Font, J.; Gori, C.; Di Maio, A.; Patxot, C.; Pickard, L.; Pozzi, A.; Rothgang, H. Future long-term care expenditure in Germany, Spain, Italy and the United Kingdom. Ageing Soc. 2006, 26, 285–302. [Google Scholar] [CrossRef]
- World Health Organization. Ageing and Health. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 14 February 2025).
- Hou, C.; Ma, Y.; Yang, X.; Tao, L.; Zheng, D.; Liu, X.; Wang, X.; Li, X.; Wang, W.; Fang, X.; et al. Disability transitions and health expectancies among elderly people aged 65 years and over in China: A nationwide longitudinal study. Aging Dis. 2019, 10, 1246–1257. [Google Scholar] [CrossRef]
- The World Bank. Public Data from the World Bank. Available online: https://databank.worldbank.org/ (accessed on 29 October 2025).
- Huang, G.; Guo, F.; Cheng, Z.; Tani, M.; Chen, G. Projections of future demand and costs of aged care services in China. Res. Policy Rev. 2023, 42, 56. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Y.; Roshanmehr, F.; Bai, X.; Taghizadeh-Hesary, F.; Taghizadeh-Hesary, F. The health status transition and medical expenditure evaluation of elderly population in China. Int. J. Environ. Res. Public Health 2021, 18, 6907. [Google Scholar] [CrossRef]
- Hu, B.; Shin, P.; Han, E.; Rhee, Y. Projecting Informal Care demand among older Koreans between 2020 and 2067. Int. J. Environ. Res. Public Health 2022, 19, 6391. [Google Scholar] [CrossRef]
- Hu, B.; Cartagena-Farias, J.; Brimblecombe, N.; Jadoolal, S.; Wittenberg, R. Projected costs of informal care for older people in England. Eur. J. Health Econ. 2024, 25, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Du, P. Changing face of the extent to which needs were met among disabled older persons in China and its determinants. Popul. Dev. 2021, 27, 96–104. [Google Scholar]
- Zhai, Z.W.; Chen, J.J.; Li, L. Future trends of China’s population and ageing: 2015–2100. Popul. Res. 2017, 4, 60–71. [Google Scholar]
- Cui, X.D. Forecasting demand for long-term care: Based on multistate piecewise constant markov process. Chin. J. Popul. Sci. 2017, 6, 82–93+128. [Google Scholar]
- Zhu, Y.; Zhang, Z.X. Health status transferring of the elderly and related labor demand for caring. Chin. J. Popul. Sci. 2019, 2, 63–74+127. [Google Scholar]
- Shahid, A.; Wilkinson, K.; Marcu, S.; Shapiro, C.M. Mini-mental State Examination (MMSE). In STOP, THAT and One Hundred Other Sleep Scales; Shahid, A., Wilkinson, K., Marcs, S., Eds.; Springer: New York, NY, USA, 2012. [Google Scholar]
- Huang, F.; Wu, C.J. Simulation-based comparative analysis of financing polices for long-term care in China. J. Appl. Stat. Manag. 2018, 4, 587–602. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, H.H.; Wang, Z.L. Analysis on trends of future home-based care needs and costs for elderly in China. Econ. Res. J. 2012, 10, 134–149. [Google Scholar]
- Lin, B. An analysis on the status and trend of old population without self-care ability in China. Popul. Econ. 2015, 4, 77–84. [Google Scholar]
- Reither, E.; Olshansky, S.; Yang, Y. New forecasting methodology indicates more disease and earlier mortality ahead for today’s younger Americans. Health Aff. 2011, 30, 1562–1568. [Google Scholar] [CrossRef]
- Beltrán-Sánchez, H.; Soneji, S.; Crimmins, E. Past, Present, and future of healthy life expectancy. Cold Spring Harb. Perspect. Med. 2015, 5, a025957. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.; Kim, J.; Vasunilashorn, S. Biodemography: New approaches to understanding trends and differences in population health and mortality. Demography 2010, 47, S41–S64. [Google Scholar] [CrossRef]
- Zeng, Y.; Gu, D.; Purser, J.; Hoenig, H.; Christakis, N. Associations of environmental factors with Elderly health and mortality in China. Am. J. Public Health 2010, 100, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, L.; Jette, A. The disablement process. Soc. Sci. Med. 1994, 38, 1–14. [Google Scholar] [CrossRef]
- Hardy, S.; Gill, T. Recovery from disability among community-dwelling older persons. JAMA 2004, 291, 1596–1602. [Google Scholar] [CrossRef]
- Robine, J.M.; Michel, J.P. Looking forward to a general theory on population aging. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, M590–M597. [Google Scholar] [CrossRef]
- Case, A.; Fertig, A.; Paxson, C. The lasting impact of childhood health and circumstance. J. Health Econ. 2005, 24, 365–389. [Google Scholar] [CrossRef]
- Oksuzyan, A.; Juel, K.; Vaupel, J.; Christensen, K. Men: Good health and high mortality. Sex differences in health and aging. Aging Clin. Exp. Res. 2008, 20, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, E.M.; Kim, J.K.; Solé-Auró, A. Gender differences in health: Results from SHARE, ELSA and HRS. Eur. J. Public Health 2011, 21, 81–91. [Google Scholar] [CrossRef]
- Crimmins, E.; Beltrán-Sánchez, H. Mortality and morbidity trends: Is there compression of morbidity? J. Gerontol. B Psychol. Sci. Soc. Sci. 2011, 66B, 75–86. [Google Scholar] [CrossRef]
- Zimmer, Z.; Martin, L.G.; Nagin, D.S.; Jones, B.L. Modeling disability trajectories and mortality of the oldest-old in China. Demography 2012, 49, 291–314. [Google Scholar] [CrossRef]
- Freedman, V.A.; Spillman, B.C.; Andreski, P.M.; Cornman, J.C.; Crimmins, E.M.; Kramarow, E.; Lubitz, J.; Martin, L.G.; Merkin, S.S.; Schoeni, R.F.; et al. Trends in late-life activity limitations in the United States: An update from five national surveys. Demography 2013, 50, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, Y.; Ding, J.; Hu, Q.; Xu, J.; Luo, J.; Wu, Z.; Chu, T. Construction of disability risk prediction model for the elderly based on machine learning. Sci. Rep. 2025, 15, 16247. [Google Scholar] [CrossRef]
- Xi, J.Y.; Lin, X.; Hao, Y.T. Measurement and projection of the burden of disease attributable to population aging in 188 countries, 1990–2050: A population-based study. J. Glob. Health 2022, 12, 04093. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, T.R. The age mode of elderly disability in China and the disabled population projection. Popul. J. 2020, 42, 57–72. [Google Scholar] [CrossRef]
- He, S.; Lai, S.L. Determinants of functional disability trajectories: An assessment of the disablement model and life-course perspective. Geriatr. Gerontol. Int. 2023, 23, 817–829. [Google Scholar] [CrossRef]
- Bao, J.; Zhou, L.; Liu, G.; Tang, J.; Lu, X.; Cheng, C.; Jin, Y.; Bai, J. Current state of care for the elderly in China in the context of an aging population. Biosci. Trends 2022, 16, 107–118. [Google Scholar] [CrossRef] [PubMed]
- National Health Commission of the People’s Republic of China. Surveillance of Residents’ Health Literacy in China (2024). 2025. Available online: https://www.gov.cn/lianbo/bumen/202501/P020250110406390443744.pdf (accessed on 16 September 2025).
- National Health Commission of the People’s Republic of China. Progress Report on the Strengthening and Promotion of Older Affairs. 2022. Available online: https://www.nhc.gov.cn/wjw/mtbd/202209/00c3a74bf4c742aba8a991ac594d18fb.shtml (accessed on 16 September 2025).
- Zeng, Y.; Feng, Q.S.; Therese, H.; Kaare, C.; James, W.V. Trends of disability and mortality among the oldest-old in China. Popul. Res. 2017, 41, 22–32. [Google Scholar]
- Jiao, K.S. Inequality of healthy life expectancy for the Chinese elderly and its future trend. Sociol. Stud. 2018, 33, 116–141+244–245. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Y.; Wang, W.J.; Zhang, X.Y. The prediction of health status and family care needs of the elderly people in China. Popul. J. 2024, 46, 73–89. [Google Scholar] [CrossRef]
- Zhong, Y.; Qin, G.; Xi, H.; Cai, D.; Wang, Y.; Wang, T.; Gao, Y. Prevalence, patterns of multimorbidity and associations with health care utilization among middle-aged and older people in China. BMC Public Health 2023, 23, 537. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Ma, H.; Zhang, H.; Zhang, Y.; Tang, S.; Xiong, J. Dynamic cross-lagged effects between healthy lifestyles and multimorbidity among middle-aged and older adults in China. BMC Public Health 2025, 25, 2132. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ai, C.; Liu, Z.; Wang, G. Neuroimaging studies of resting-state functional magnetic resonance imaging in eating disorders. BMC Med. Imaging 2024, 24, 265. [Google Scholar] [CrossRef]
- Stephan, A.J.; Strobl, R.; Schwettmann, L.; Meisinger, C.; Ladwig, K.H.; Linkohr, B.; Thorand, B.; Peters, A.; Grill, E. The times we are born into and our lifestyle choices determine our health trajectories in older age-Results from the KORA-Age study. Prev. Med. 2020, 133, 106025. [Google Scholar] [CrossRef]
- Scott, A.J. The longevity society. Lancet Healthy Longev. 2021, 2, e820–e827. [Google Scholar] [CrossRef]
- Rosa, W.; Bhadelia, A.; Knaul, F.; Travers, J.; Metheny, N.; Fulmer, T. A longevity society requires integrated palliative care models for historically excluded older people. Lancet Healthy Longev. 2022, 3, e227–e228. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P. Investing in health to create a third demographic dividend. Gerontologist 2016, 56, S167–S177. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.; Xin, M.; Lu, E.; Cheung, W.; Tsang, H. Double disadvantage of carers with a disability: A cross-sectional study of care duration and perceived importance for service improvement in Hong Kong, China. Int. J. Environ. Res. Public Health 2022, 20, 20. [Google Scholar] [CrossRef]
- Dann, T. Global elderly care in crisis. Lancet 2014, 383, 927. [Google Scholar] [CrossRef]
- Pearlin, L.I. The life course and the stress process: Some conceptual comparisons. J. Gerontol. B Psychol. Sci. Soc. Sci. 2010, 65, 207–215. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Hu, H. Multidimensional poverty and disability of older adults in China: Will long-term care insurance make a difference? Appl. Res. Qual. Life 2024, 19, 3439–3462. [Google Scholar] [CrossRef]
- Yang, E.; Lee, K.H. The moderating effects of disability on mobile internet use among older adults: Population-based cross-sectional study. J. Med. Internet Res. 2022, 24, e37127. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, L. Pension reform in China. J. Aging Soc. Policy 2016, 28, 15–28. [Google Scholar] [CrossRef]
- Kawachi, M. Is the Benefit from Public Pension Adequate for Retired Workers in Japan? Available online: https://general.jscpa.or.jp/aniv/pbss/pdf/program/KAWACHI_MunekazuP.pdf (accessed on 28 October 2025).
- Lee, K.; Shim, Y. Fiscal and Welfare Effects of Balanced-budget Reforms of Korea’s National Pension System. Glob. Econ. Rev. 2018, 47, 367–394. [Google Scholar] [CrossRef]
- Castro, R. Country-level, mandatory, self-financeable pension replacement rates in OECD countries. J. Pension. Econ. Finance 2024, 23, 321–334. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, X.; Yang, S. The effect of delayed retirement on social welfare in China from the perspective of the pension replacement rate. Singap. Econ. Rev. 2024, 69, 1907–1932. [Google Scholar] [CrossRef]
- Zhou, J.; Lv, Y.; Mao, C.; Duan, J.; Gao, X.; Wang, J.; Yin, Z.; Shi, W.; Luo, J.; Kang, Q.; et al. Development and validation of a nomogram for predicting the 6-year risk of cognitive impairment among Chinese older adults. J. Am. Med. Dir. Assoc. 2020, 21, 864–871. e6. [Google Scholar] [CrossRef]
- National Bureau of Statistics. The Data of the Seventh National Population Census. 2021. Available online: https://www.stats.gov.cn/sj/pcsj/ (accessed on 28 October 2025).
- Chen, S.; Zheng, J.; Chen, C.; Xing, Y.; Cui, Y.; Ding, Y.; Li, X. Unmet needs of activities of daily living among a community-based sample of disabled elderly people in Eastern China: A cross-sectional study. BMC Geriatr. 2018, 18, 160. [Google Scholar] [CrossRef] [PubMed]
- Fougère, B.; Gammack, J.K. Overview of Two Different Nursing Home Health Systems: Examples from France and the United States. J. Am. Med. Dir. Assoc. 2017, 18, 998–999. [Google Scholar] [CrossRef]
- Fei, X.T. The Issue of older Support Amidst Changing Family Structures: Revisiting the Evolution of Family Patterns in China. J. Peking Univ. (Philos. Soc. Sci.) 1983, 7–16. [Google Scholar]
- The Central People’s Government of the People’s Republic of China. China’s Long-Term Care Insurance Program Currently Covers 2.6 Million Beneficiaries and Has Alleviated Financial Burdens for the Public by More Than 80 Billion Yuan. 2024. Available online: https://www.gov.cn/lianbo/bumen/202411/content_6988388.htm. (accessed on 16 September 2025).
- Xu, X.; Chen, L. Projection of long-term care costs in China, 2020–2050: Based on the Bayesian quantile regression method. Sustainability 2019, 11, 3530. [Google Scholar] [CrossRef]
- Hu, H.W.; Hu, X.Y. Care support for the disabled elderly in the context of negative population growth: Logical mechanisms and governance paths. J. Huazhong Univ. Sci. Technol. (Soc. Sci. Ed.) 2023, 37, 28–40. [Google Scholar] [CrossRef]
- Bai, X.; Yu, R.W.L.; Liu, C.; Sörensen, S. Digital Literacy, Intergenerational Relationships, and Future Care Preparation in Aging Chinese Adults in Hong Kong: Does the Gender of Adult Children Make a Difference? Health Soc. Care Community 2025, 2025, 6198111. [Google Scholar] [CrossRef]
- Jaul, E.; Barron, J. Age-related diseases and clinical and public health implications for the 85 years old and over population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef]
- Collerton, J.; Davies, K.; Jagger, C.; Kingston, A.; Bond, J.; Eccles, M.P.; Robinson, L.A.; Martin-Ruiz, C.; von Zglinicki, T.; James, O.F.; et al. Health and disease in 85 year olds: Baseline findings from the Newcastle 85+ cohort study. BMJ 2009, 339, e4462. [Google Scholar] [CrossRef] [PubMed]
- Ostchega, Y.; Harris, T.; Hirsch, R.; Parsons, V.; Kington, R. The prevalence of functional limitations and disability in older persons in the US: Data from the National Health and Nutrition Examination Survey III. J. Am. Geriatr. Soc. 2000, 48, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Pengpid, S.; Peltzer, K.; Hajek, A.; Gyasi, R. Determinants of limitations in activities of daily living among community-dwelling persons aged 80 years and older: Longitudinal national evidence from the Health, Aging and Retirement in Thailand study, 2015–2022. Geriatr. Gerontol. Int. 2025, 25, 403–410. [Google Scholar] [CrossRef]
- Ye, X. Deconstruction of health gradients and optimization paths of health policies among older adults in China. Acad. Forum 2025, 48, 66–80. [Google Scholar] [CrossRef]
- Phillips, S.P. Defining and measuring gender: A social determinant of health whose time has come. Int. J. Equity Health 2005, 4, 11. [Google Scholar] [CrossRef]
- Ipsen, C.; Hall, J.P.; Lui, J. Rural disability and community participation. Front. Rehabil. Sci. 2022, 3, 1049578. [Google Scholar] [CrossRef] [PubMed]
- Barria, P.; Andrade, A.; Albayay, B.C.; Covarrubias-Escudero, F.; Cifuentes, C.; Moreno, J.C.; Appelgren-González, J.P. Functional Profile Differences Across Diagnostic Categories Using WHODAS 2.0 in Adults with Neurological, Musculoskeletal, and Chronic Pain Conditions. J. Funct. Morphol. Kinesiol. 2025, 10, 312. [Google Scholar] [CrossRef]
- Rieker, P.P.; Bird, C.E. Rethinking gender differences in health: Why we need to integrate social and biological perspectives. J. Gerontol. B Psychol. Sci. Soc. Sci. 2005, 60, S40–S47. [Google Scholar] [CrossRef]
- Song, Y.; Bian, Y. Gender differences in the use of health care in China: Cross-sectional analysis. Int. J. Equity Health 2014, 13, 8. [Google Scholar] [CrossRef]
- Warraich, H.J.; Califf, R.M. Differences in health outcomes between men and women: Biological, behavioral, and societal factors. Clin. Chem. 2019, 65, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Tareque, M.; Tiedt, A.; Islam, T.; Begum, S.; Saito, Y. Gender differences in functional disability and self-care among seniors in Bangladesh. BMC Geriatr. 2017, 17, 177. [Google Scholar] [CrossRef]
- Da Silva, T.H.R. Advancing gender equity in nursing care for older adults: A pathway to achieving the United Nations Sustainable Development Goals. J. Community Med. Public Health Rep. 2024, 5, 7. [Google Scholar] [CrossRef]
- Chica-Pérez, A.; Correa-Casado, M.; Fernández-Sola, C.; Granero-Molina, J.; Cortés-Rodríguez, A.; Hernández-Padilla, J. The Family Caregiver Role from the Perspective of Older Women Experiencing Poverty in a High-Income Country: A Qualitative Study. J. Adv. Nurs. 2025, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.; d’Uva, T.B.; Van Doorslaer, E. The gender health gap in China: A decomposition analysis. Econ. Hum. Biol. 2015, 18, 13–26. [Google Scholar] [CrossRef]
- Liu, Z.W.; Liu, J. Intergenerational reverse knowledge transfer and artificial compensation in health information dissemination: A case study of “Taking Mom to Remove the IUD”. News Writ. 2025, 96–109. [Google Scholar]
- Li, Q.; Gu, J.T.; Huang, J.Y.; Luo, C.L. “They see me as mentally ill”: The stigmatization experiences of Chinese menopausal women in the family. BMC Women’s Health 2023, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P. Gender gap of pension benefits for the elderly in China. J. Beijing Technol. Bus. Univ. (Soc. Sci.) 2020, 35, 90–104. [Google Scholar]
- Zhou, M.; Zhao, S.; Zhao, Z. Gender differences in health insurance coverage in China. Int. J. Equity Health 2021, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Yuying, Z.; Jing, C. Gender differences in the score and influencing factors of social participation among Chinese elderly. J. Women Aging 2022, 34, 537–550. [Google Scholar] [CrossRef]
- Sigurlaugardottir, S.; Sigurdardottir, S.; Aspelund, T.; Bjornsdottir, K.; Jegermalm, M.; Olafsson, K.; Hjaltadottir, I. Elements of burden among informal caregivers of community-dwelling older adults receiving home care nursing: A cross-sectional study on health status, well-being, and gender differences. BMC Geriatr. 2025, 25, 623. [Google Scholar] [CrossRef] [PubMed]
- Brewster, G.; Wang, D.; McPhillips, M.; Epps, F.; Yang, I. Correlates of sleep disturbance experienced by informal caregivers of persons living with dementia: A systematic review. Clin. Gerontol. 2024, 47, 380–407. [Google Scholar] [CrossRef] [PubMed]
- Mattos, M.; Bernacchi, V.; Shaffer, K.; Gallagher, V.; Seo, S.; Jepson, L.; Manning, C. Sleep and caregiver burden among caregivers of persons living with dementia: A scoping review. Innov. Aging 2024, 8, igae005. [Google Scholar] [CrossRef]
- Chakraborty, R.; Jana, A.; Vibhute, V.M. Caregiving: A risk factor of poor health and depression among informal caregivers in India-A comparative analysis. BMC Public Health 2023, 23, 42. [Google Scholar] [CrossRef]
- Wu, F. Characteristics of caregivers and their different input for the elderly in Chinese families based on the third national survey on the status of women in China. J. Chin. Women’s Stud. 2017, 5–13. [Google Scholar]
- Yuan, D.; Chen, T. Informal care and women’s health: A life course approach. Popul. Dev. 2021, 27, 59–73. [Google Scholar]
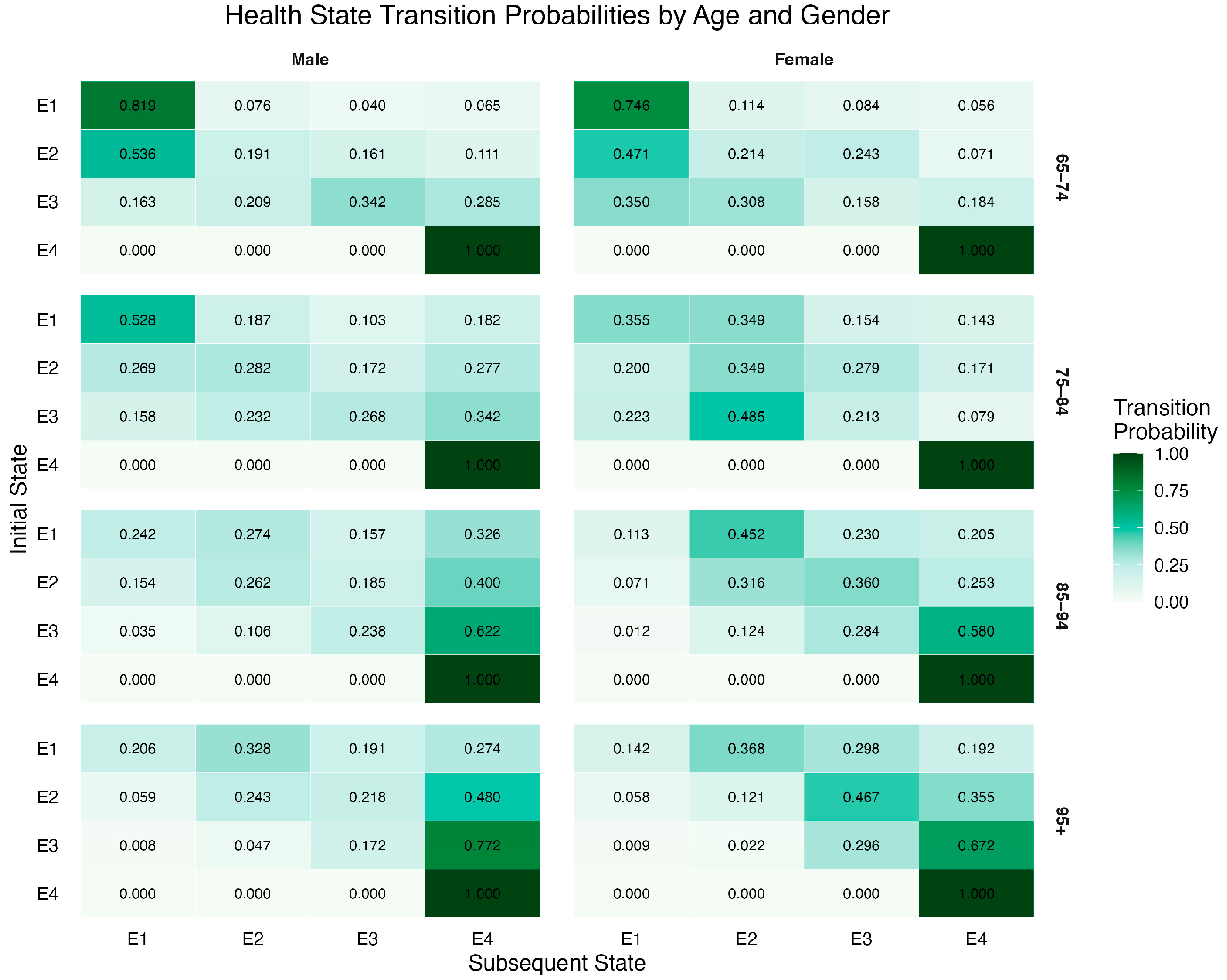
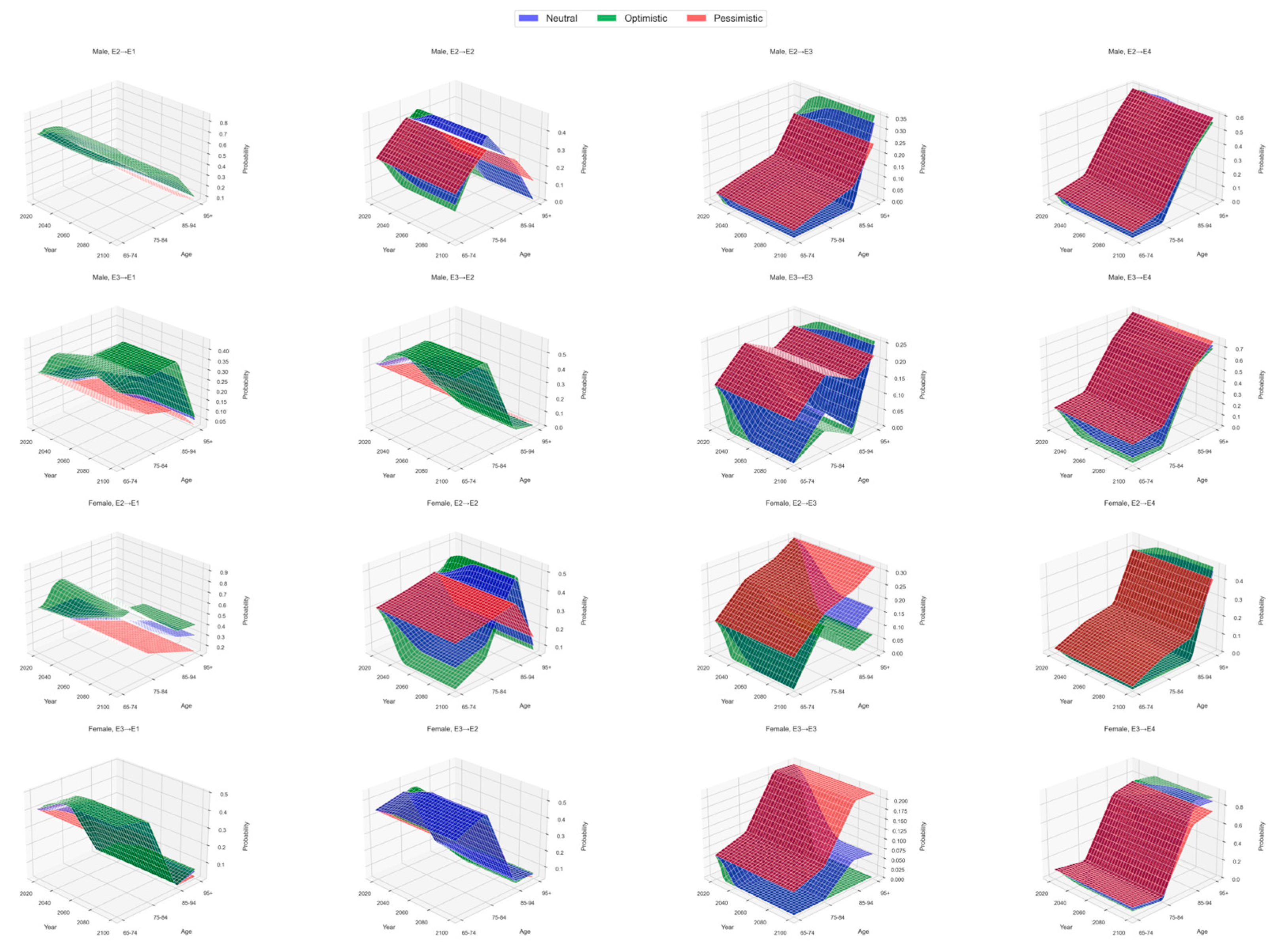
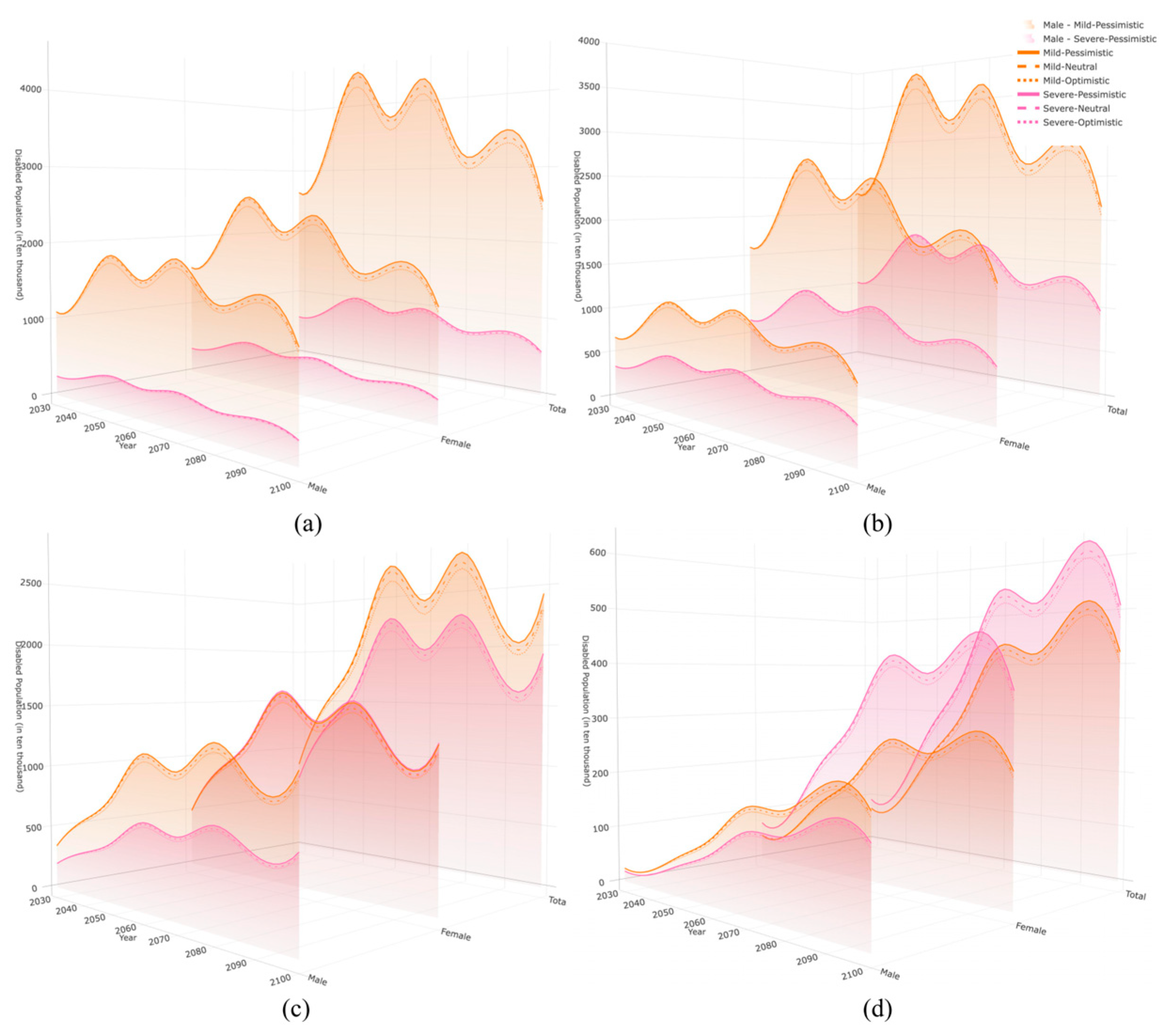

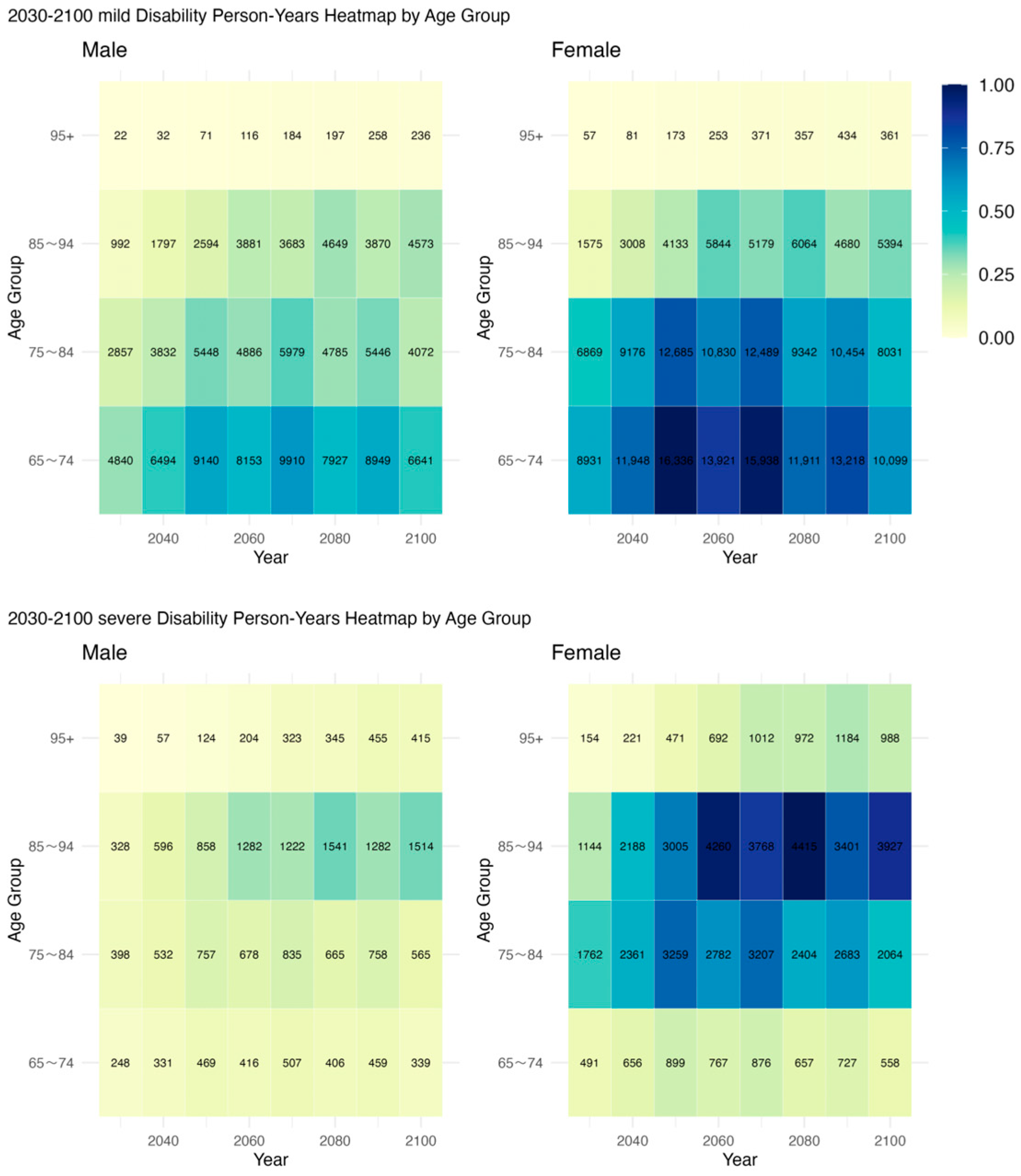
| 2030 | 2040 | 2050 | 2060 | 2070 | 2080 | 2090 | 2100 | |
|---|---|---|---|---|---|---|---|---|
| Total Population-pes | 20,727.29 | 27,674.90 | 37,166.34 | 34,532.08 | 37,610.81 | 31,158.40 | 31,486.32 | 25,422.34 |
| Disabled Older Population-Pes | 8238.17 | 11,332.61 | 15,281.48 | 15,059.27 | 15,956.64 | 13,907.89 | 13,549.29 | 11,510.57 |
| Disabled Older Population-Neu | 7949.83 | 11,015.30 | 14,960.57 | 14,848.44 | 15,844.94 | 13,859.21 | 13,501.87 | 11,470.28 |
| Disabled Older Population-Opt | 7661.50 | 10,697.98 | 14,639.66 | 14,637.61 | 15,621.55 | 13,615.82 | 13,264.75 | 11,268.85 |
| Proportion of Disabled Older Adults (%)-Pes | 39.75 | 40.95 | 41.12 | 43.61 | 42.43 | 44.64 | 43.03 | 45.28 |
| Proportion of Disabled Older Adults (%)-Neu | 38.35 | 39.80 | 40.25 | 43.00 | 42.13 | 44.48 | 42.88 | 45.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, R.; Tan, Y.; Huang, J. The Future Dynamics of Long-Term Care Pressure in China’s Longevity Era: A Prediction Based on the Discrete-Time Markov Model. Healthcare 2025, 13, 3024. https://doi.org/10.3390/healthcare13233024
Feng R, Tan Y, Huang J. The Future Dynamics of Long-Term Care Pressure in China’s Longevity Era: A Prediction Based on the Discrete-Time Markov Model. Healthcare. 2025; 13(23):3024. https://doi.org/10.3390/healthcare13233024
Chicago/Turabian StyleFeng, Ran, Yiting Tan, and Jianyuan Huang. 2025. "The Future Dynamics of Long-Term Care Pressure in China’s Longevity Era: A Prediction Based on the Discrete-Time Markov Model" Healthcare 13, no. 23: 3024. https://doi.org/10.3390/healthcare13233024
APA StyleFeng, R., Tan, Y., & Huang, J. (2025). The Future Dynamics of Long-Term Care Pressure in China’s Longevity Era: A Prediction Based on the Discrete-Time Markov Model. Healthcare, 13(23), 3024. https://doi.org/10.3390/healthcare13233024






