Transforming Care Models in Cystic Fibrosis: A Review
Abstract
1. Introduction
2. Growth of the Cystic Fibrosis Care Team
2.1. Medications and the CF Pharmacist
2.2. Anxiety and Depression
2.3. Palliative Care
2.4. Further Initiatives
3. The Changing Cystic Fibrosis Population and Unique Groups Within Cystic Fibrosis Care
3.1. Effects of Highly Effective CFTR Modulator Therapy
3.2. Advanced CF Lung Disease
3.3. CFSPID/CRMS and CFTR-Related Disorders
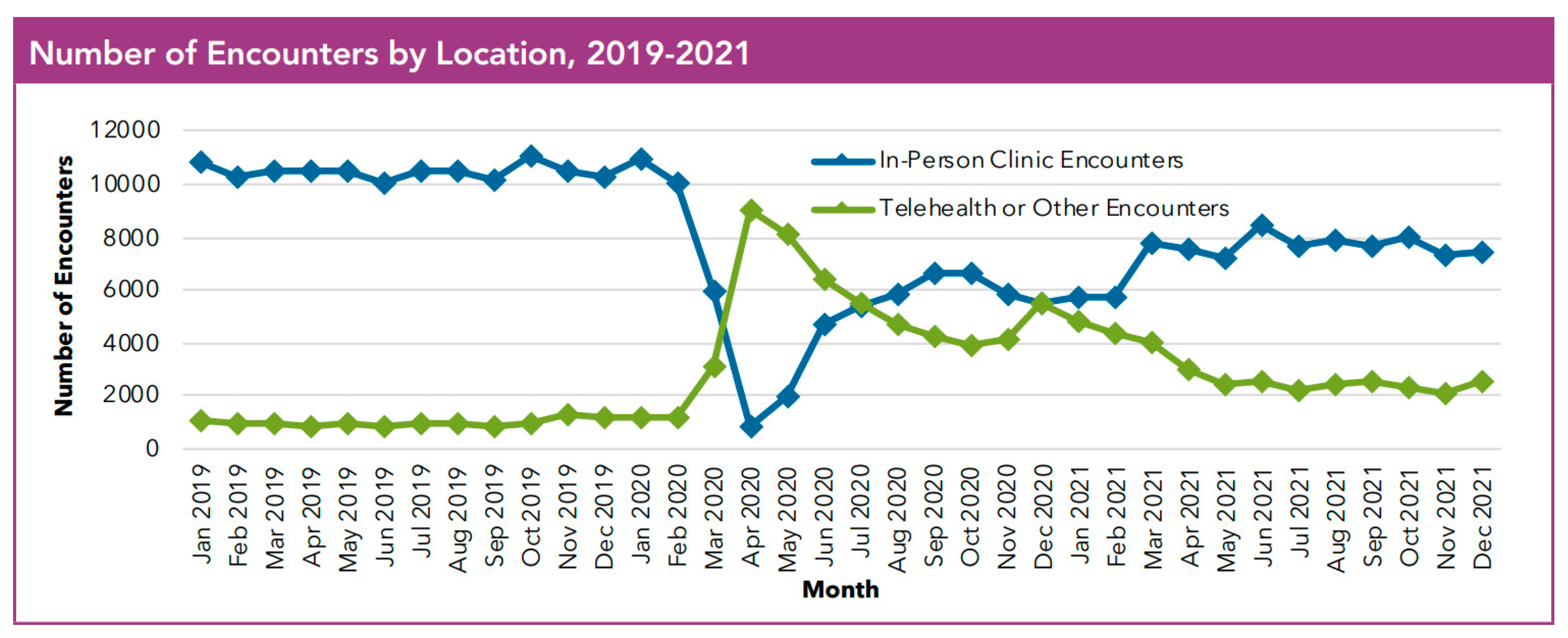
4. Changing CF Care Through the COVID-19 Pandemic
Telemedicine and Remote Monitoring
5. Communication and Partnership
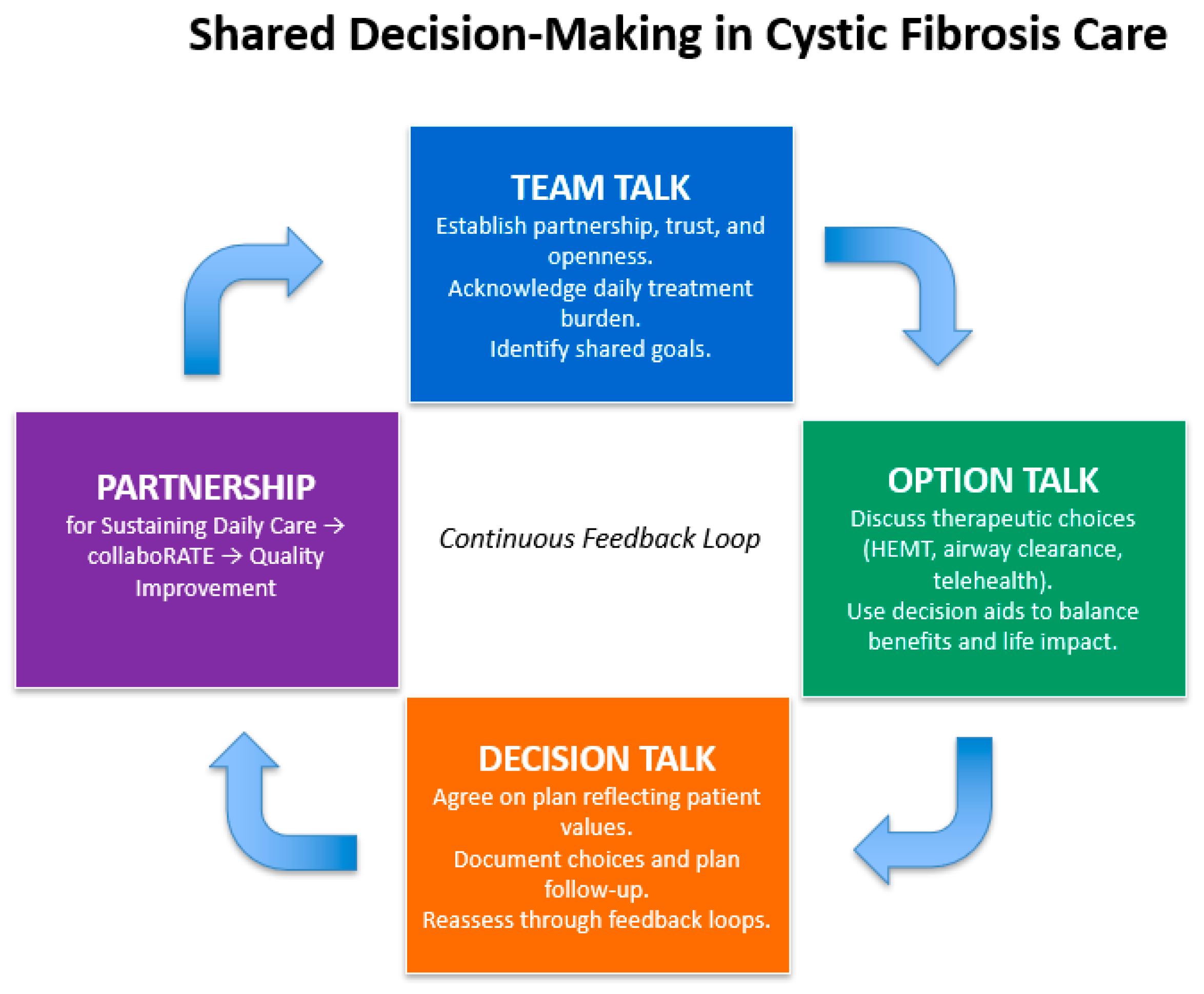
5.1. Shared Decision-Making
5.2. Coproduction
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CF | Cystic fibrosis |
| PwCF | People with CF |
| CFTR | cystic fibrosis transmembrane conductance regulator |
| HEMT | highly effective CFTR modulator therapy |
References
- O’Sullivan, B.P.; Freedman, S.D. Cystic fibrosis. Lancet 2009, 373, 1891–1904. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.V.-N.; Ribeiro, J.D.; Ribeiro, A.F.; Bertuzzo, C.S.; Marson, F.A.L. Novel, rare and common pathogenic variants in the CFTR gene screened by high-throughput sequencing technology and predicted by in silico tools. Sci. Rep. 2019, 9, 6234. [Google Scholar] [CrossRef]
- Goetz, D.; Brown, R.; Filigno, S.; Bichl, S.; Nelson, A.; Merlo, C.; Juel, R.; Lomas, P.; Hempstead, S.; Tran, Q.; et al. Cystic fibrosis foundation position paper: Redefining the CF care model. J. Cyst. Fibros. 2024, 23, 1055–1065. [Google Scholar] [CrossRef]
- Stephenson, A.L.; Stanojevic, S.; Sykes, J.; Burgel, P.-R. The changing epidemiology and demography of cystic fibrosis. Presse Med. 2017, 46 Pt 2, e87–e95. [Google Scholar] [CrossRef] [PubMed]
- De Boeck, K. Cystic fibrosis in the year 2020: A disease with a new face. Acta Paediatr. 2020, 109, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Savant, A.P. Cystic fibrosis year in review 2021. Pediatr. Pulmonol. 2022, 57, 1590–1599. [Google Scholar] [CrossRef]
- Anderson, D.H. Cystic fibrosis of the pancreas and its relation to celiac disease. Am. J. Dis. Child. 1938, 56, 56. [Google Scholar] [CrossRef]
- Burgel, P.R.; Burnet, E.; Regard, L.; Martin, C. The Changing Epidemiology of Cystic Fibrosis: The Implications for Adult Care. Chest 2023, 163, 89–99. [Google Scholar] [CrossRef]
- Koonin, L.M.; Hoots, B.; Tsang, C.A.; Leroy, Z.; Farris, K.; Jolly, B.; Antall, P.; McCabe, B.; Zelis, C.B.; Tong, I.; et al. Trends in the Use of Telehealth During the Emergence of the COVID-19 Pandemic—United States, January–March 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1595–1599. [Google Scholar] [CrossRef]
- Foundation, C.F. Cystic Fibrosis Foundation Patient Registry: 2021 Annual Data Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2022. [Google Scholar]
- Ong, T.; Van Citters, A.D.; Dowd, C.; Fullmer, J.; List, R.; Pai, S.-A.; Ren, C.L.; Scalia, P.; Solomon, G.M.; Sawicki, G.S. Remote monitoring in telehealth care delivery across the U.S. cystic fibrosis care network. J. Cyst. Fibros. 2021, 20 (Suppl. S3), 57–63. [Google Scholar] [CrossRef]
- Stacey, D.; Légaré, F.; Lewis, K.; Barry, M.J.; Bennett, C.L.; Eden, K.B.; Holmes-Rovner, M.; Llewellyn-Thomas, H.; Lyddiatt, A.; Thomson, R.; et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst. Rev. 2017, 4, CD001431. [Google Scholar] [CrossRef]
- Davies, G.; Rowbotham, N.J.; Smith, S.; Elliot, Z.C.; Gathercole, K.; Rayner, O.; Leighton, P.A.; Herbert, S.; Duff, A.J.; Chandran, S.; et al. Characterising burden of treatment in cystic fibrosis to identify priority areas for clinical trials. J. Cyst. Fibros. 2020, 19, 499–502. [Google Scholar] [CrossRef]
- Goetz, D.M.; Savant, A.P. Review of CFTR modulators 2020. Pediatr. Pulmonol. 2021, 56, 3595–3606. [Google Scholar] [CrossRef]
- Davis, P.B. Cystic fibrosis since 1938. Am. J. Respir. Crit. Care Med. 2006, 173, 475–482. [Google Scholar] [CrossRef]
- Foundation, C.F. Our History. 2023. Available online: https://www.cff.org/about-us/our-history#:~:text=When%20the%20Cystic%20Fibrosis%20Foundation,form%20the%20Cystic%20Fibrosis%20Foundation (accessed on 27 January 2023).
- Mogayzel, P.J.; Dunitz, J.; Marrow, L.C.; Hazle, L.A. Improving chronic care delivery and outcomes: The impact of the cystic fibrosis Care Center Network. BMJ Qual. Saf. 2014, 23 (Suppl. S1), i3–i8. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.C.; Penland, C.M.; Hazle, L.; Ashlock, M.; Wetmore, D.; Campbell, P.W.; Beall, R.J. Cystic fibrosis foundation: Achieving the mission. Respir. Care 2009, 54, 788–795; discussion 795. [Google Scholar] [CrossRef]
- Brown, R.F.; Close, C.T.; Mailes, M.G.; Gonzalez, L.J.; Goetz, D.M.; Filigno, S.S.; Preslar, R.; Tran, Q.T.; Hempstead, S.E.; Lomas, P.; et al. Cystic fibrosis foundation position paper: Redefining the cystic fibrosis care team. J. Cyst. Fibros. 2024, 23, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Quittner, A.L.; Abbott, J.; Georgiopoulos, A.M.; Goldbeck, L.; Smith, B.; Hempstead, S.E.; Marshall, B.; Sabadosa, K.A.; Elborn, S. International Committee on Mental Health in Cystic Fibrosis: Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus statements for screening and treating depression and anxiety. Thorax 2016, 71, 26–34. [Google Scholar] [CrossRef]
- Sawicki, G.S.; Sellers, D.E.; Robinson, W.M. High treatment burden in adults with cystic fibrosis: Challenges to disease self-management. J. Cyst. Fibros. 2009, 8, 91–96. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, R.; Touw, D.J.; Heijerman, H.G. Prevention of drug-related complications in cystic fibrosis. Curr. Opin. Pulm. Med. 2019, 25, 666–673. [Google Scholar] [CrossRef]
- Redfern, J.; Webb, A.K. Benefits of a dedicated cystic fibrosis pharmacist. J. R. Soc. Med. 2004, 97 (Suppl. S44), 2–7. [Google Scholar]
- Quittner, A.L.; Zhang, J.; Marynchenko, M.; Chopra, P.A.; Signorovitch, J.; Yushkina, Y.; Riekert, K.A. Pulmonary medication adherence and health-care use in cystic fibrosis. Chest 2014, 146, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wright, B.A.; Singh, S.B.; Schultz, J.L.; Ramsey, L.J.; Spading, K.A.; Mascardo, L.A.; Starner, T.D. Impact of pharmacy services on cystic fibrosis transmembrane conductance regulator modulator prescribing at a pediatric cystic fibrosis center. Pediatr. Pulmonol. 2019, 54, 1591–1595. [Google Scholar] [CrossRef]
- Young, D.C.; Autry, E.; Zobell, J.T.; Kormelink, L.; Homa, K.; Sabadosa, K.A.; Kanga, J.; Anstead, M.; Kuhn, R. Patients and families experience with pharmacist care at cystic fibrosis foundation accredited clinics. Pediatr. Pulmonol. 2019, 54, 1216–1224. [Google Scholar] [CrossRef]
- Guta, M.T.; Tekalign, T.; Awoke, N.; Fite, R.O.; Dendir, G.; Lenjebo, T.L. Global Burden of Anxiety and Depression among Cystic Fibrosis Patient: Systematic Review and Meta-Analysis. Int. J. Chronic Dis. 2021, 2021, 6708865. [Google Scholar] [CrossRef] [PubMed]
- Quittner, A.L.; Goldbeck, L.; Abbott, J.; Duff, A.; Lambrecht, P.; Solé, A.; Tibosch, M.M.; Brucefors, A.B.; Yüksel, H.; Catastini, P.; et al. Prevalence of depression and anxiety in patients with cystic fibrosis and parent caregivers: Results of The International Depression Epidemiological Study across nine countries. Thorax 2014, 69, 1090–1097. [Google Scholar] [CrossRef]
- Lord, L.; McKernon, D.; Grzeskowiak, L.; Kirsa, S.; Ilomaki, J. Depression and anxiety prevalence in people with cystic fibrosis and their caregivers: A systematic review and meta-analysis. Soc. Psychiatry Psychiatr. Epidemiol. 2022, 58, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Riekert, K.A.; Bartlett, S.J.; Boyle, M.P.; Krishnan, J.A.; Rand, C.S. The association between depression, lung function, and health-related quality of life among adults with cystic fibrosis. Chest 2007, 132, 231–237. [Google Scholar] [CrossRef]
- Havermans, T.; Colpaert, K.; Dupont, L.J. Quality of life in patients with Cystic Fibrosis: Association with anxiety and depression. J. Cyst. Fibros. 2008, 7, 581–584. [Google Scholar] [CrossRef]
- Foundation, C.F. Mental Health Coordinator Award. Available online: https://www.cff.org/medical-professionals/mental-health-coordinator-award (accessed on 28 January 2023).
- Ibrahim, H.; Danish, H.; Morrissey, D.; Deasy, K.F.; McCarthy, M.; Dorgan, J.; Fleming, C.; Howlett, C.; Twohig, S.; Vagg, T.; et al. Individualized approach to elexacaftor/tezacaftor/ivacaftor dosing in cystic fibrosis, in response to self-reported anxiety and neurocognitive adverse events: A case series. Front. Pharmacol. 2023, 14, 1156621. [Google Scholar] [CrossRef]
- Piehler, L.; Thalemann, R.; Lehmann, C.; Thee, S.; Röhmel, J.; Syunyaeva, Z.; Stahl, M.; Mall, M.A.; Graeber, S.Y. Effects of elexacaftor/tezacaftor/ivacaftor therapy on mental health of patients with cystic fibrosis. Front. Pharmacol. 2023, 14, 1179208. [Google Scholar] [CrossRef]
- Zhang, L.; Albon, D.; Jones, M.; Bruschwein, H. Impact of elexacaftor/tezacaftor/ivacaftor on depression and anxiety in cystic fibrosis. Ther. Adv. Respir. Dis. 2022, 16, 17534666221144211. [Google Scholar] [CrossRef]
- Pudukodu, H.; Powell, M.Z.; Ceppe, A.; Donaldson, S.H.; Goralski, J.L.; Sowa, N.A. Analysis of Depression and Anxiety Scores Following Initiation of Elexacaftor/Tezacaftor/Ivacaftor in Adults with Cystic Fibrosis. Clin. Respir. J. 2024, 18, e70007. [Google Scholar] [CrossRef]
- Dellon, E.P.; Basile, M.; Hobler, M.R.; Georgiopoulos, A.M.; Chen, E.; Goggin, J.; Goss, C.H.; Hempstead, S.E.; Faro, A.; Kavalieratos, D. Palliative Care Needs of Individuals with Cystic Fibrosis: Perspectives of Multiple Stakeholders. J. Palliat. Med. 2020, 23, 957–963. [Google Scholar] [CrossRef]
- Trandel, E.T.; Pilewski, J.M.; Dellon, E.P.; Jeong, K.; Yabes, J.G.; Moreines, L.T.; Arnold, R.M.; Hoydich, Z.P.; Kavalieratos, D. Prevalence of unmet palliative care needs in adults with cystic fibrosis. J. Cyst. Fibros. 2020, 19, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Dubin, E.; Lowers, J.; Dellon, E.P.; Hempstead, S.; Faro, A.; Tallarico, E.; Fitzpatrick, A.; Hunt, W.R.; Kavalieratos, D. Prevalence of unmet pain and symptom management needs in adults with cystic fibrosis. J. Cyst. Fibros. 2022, 22, 352–355. [Google Scholar] [CrossRef]
- DiFiglia, S.; Georgiopoulos, A.M.; Portenoy, R.; Seng, E.; Berdella, M.; Friedman, D.; Kier, C.; Linnemann, R.W.; Middour-Oxler, B.; Walker, P.; et al. Palliative care needs among outpatient adults with cystic fibrosis: Baseline data from the Improving Life with CF trial. J. Cyst. Fibros. 2024, 23, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Kavalieratos, D.; Georgiopoulos, A.M.; Dhingra, L.; Basile, M.J.; Rabinowitz, E.; Hempstead, S.E.; Faro, A.; Dellon, E.P. Models of Palliative Care Delivery for Individuals with Cystic Fibrosis: Cystic Fibrosis Foundation Evidence-Informed Consensus Guidelines. J. Palliat. Med. 2021, 24, 18–30. [Google Scholar] [CrossRef]
- Kavalieratos, D.; Lowers, J.; Moreines, L.T.; Hoydich, Z.P.; Arnold, R.M.; Yabes, J.G.; Richless, C.; Ikejiani, D.Z.; Teuteberg, W.; Pilewski, J.M. Embedded Specialist Palliative Care in Cystic Fibrosis: Results of a Randomized Feasibility Clinical Trial. J. Palliat. Med. 2023, 26, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Foundation, C.F. Program for Adult Care Excellence (PACE). 2023. Available online: https://www.cff.org/medical-professionals/program-adult-care-excellence-pace (accessed on 30 January 2023).
- Foundation, C.F. Developing Innovative Gastroenterology Specialty Training (DIGEST) Program. Available online: https://www.cff.org/medical-professionals/developing-innovative-gastroenterology-specialty-training-digest-program (accessed on 30 January 2023).
- Brennan, A.L.; Blackman, S.M. EnVisioning the future: Endocrinology in cystic fibrosis. J. Cyst. Fibros. 2019, 18, 743–745. [Google Scholar] [CrossRef]
- Foundation, C.F. Award for a Physical Therapist. 2023. Available online: https://www.cff.org/researchers/award-physical-therapist (accessed on 30 January 2023).
- Foundation, C.F. LEAPP CF: Leadership and Education Advanced Practice Provider Fellowship Program. 2023. Available online: https://www.cff.org/researchers/leapp-cf-leadership-and-education-advanced-practice-provider-fellowship-program (accessed on 30 January 2023).
- Goetz, D.; Ren, C.L. Review of Cystic Fibrosis. Pediatr. Ann. 2019, 48, e154–e161. [Google Scholar] [CrossRef]
- Castellani, C.; Linnane, B.; Pranke, I.; Cresta, F.; Sermet-Gaudelus, I.; Peckham, D. Cystic Fibrosis Diagnosis in Newborns, Children, and Adults. Semin. Respir. Crit. Care Med. 2019, 40, 701–714. [Google Scholar] [CrossRef]
- Cystic Fibrosis, F.; Borowitz, D.; Robinson, K.A.; Rosenfeld, M.; Davis, S.D.; Sabadosa, K.A.; Spear, S.L.; Michel, S.H.; Parad, R.B.; White, T.B.; et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009, 155 (Suppl. S6), S73–S93. [Google Scholar]
- Lahiri, T.; Hempstead, S.E.; Brady, C.; Cannon, C.L.; Clark, K.; Condren, M.E.; Guill, M.F.; Guillerman, R.P.; Leone, C.G.; Maguiness, K.; et al. Clinical Practice Guidelines from the Cystic Fibrosis Foundation for Preschoolers with Cystic Fibrosis. Pediatrics 2016, 137, e20151784. [Google Scholar] [CrossRef] [PubMed]
- Thabut, G.; Christie, J.D.; Mal, H.; Fournier, M.; Brugière, O.; Leseche, G.; Castier, Y.; Rizopoulos, D. Survival benefit of lung transplant for cystic fibrosis since lung allocation score implementation. Am. J. Respir. Crit. Care Med. 2013, 187, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Elborn, J.S.; Bell, S.C.; Madge, S.L.; Burgel, P.-R.; Castellani, C.; Conway, S.; De Rijcke, K.; Dembski, B.; Drevinek, P.; Heijerman, H.G.; et al. Report of the European Respiratory Society/European Cystic Fibrosis Society task force on the care of adults with cystic fibrosis. Eur. Respir. J. 2016, 47, 420–428. [Google Scholar] [CrossRef]
- Flume, P.A. Pneumothorax in cystic fibrosis. Chest 2003, 123, 217–221. [Google Scholar] [CrossRef]
- Flume, P.A.; Yankaskas, J.R.; Ebeling, M.; Hulsey, T.; Clark, L.L. Massive hemoptysis in cystic fibrosis. Chest 2005, 128, 729–738. [Google Scholar] [CrossRef]
- Kapnadak, S.G.; Dimango, E.; Hadjiliadis, D.; Hempstead, S.E.; Tallarico, E.; Pilewski, J.M.; Faro, A.; Albright, J.; Benden, C.; Blair, S.; et al. Cystic Fibrosis Foundation consensus guidelines for the care of individuals with advanced cystic fibrosis lung disease. J. Cyst. Fibros. 2020, 19, 344–354. [Google Scholar] [CrossRef]
- Prayle, A.; Watson, A.; Fortnum, H.; Smyth, A. Side effects of aminoglycosides on the kidney, ear and balance in cystic fibrosis. Thorax 2010, 65, 654–658. [Google Scholar] [CrossRef]
- Poore, T.S.; Taylor-Cousar, J.L.; Zemanick, E.T. Cardiovascular complications in cystic fibrosis: A review of the literature. J. Cyst. Fibros. 2022, 21, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Lowenfels, A.B. Cancer in Cystic Fibrosis: A Narrative Review of Prevalence, Risk Factors, Screening, and Treatment Challenges: Adult Cystic Fibrosis Series. Chest 2022, 161, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.-R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef]
- Prickett, M.H.; Flume, P.A.; Sabadosa, K.A.; Tran, Q.T.; Marshall, B.C. Telehealth and CFTR modulators: Accelerating innovative models of cystic fibrosis care. J. Cyst. Fibros. 2022, 22, 9–16. [Google Scholar] [CrossRef]
- Solomon, G.M.; Marshall, S.G.; Ramsey, B.W.; Rowe, S.M. Breakthrough therapies: Cystic fibrosis (CF) potentiators and correctors. Pediatr. Pulmonol. 2015, 50 (Suppl. S40), S3–S13. [Google Scholar] [CrossRef]
- Ramsey, B.W.; Davies, J.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Dřevínek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef]
- Wainwright, C.E.; Elborn, J.S.; Ramsey, B.W.; Marigowda, G.; Huang, X.; Cipolli, M.; Colombo, C.; Davies, J.C.; De Boeck, K.; Flume, P.A.; et al. Lumacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015, 373, 220–231. [Google Scholar] [CrossRef]
- Taylor-Cousar, J.L.; Munck, A.; McKone, E.F.; Van Der Ent, C.K.; Moeller, A.; Simard, C.; Wang, L.T.; Ingenito, E.P.; McKee, C.; Lu, Y.; et al. Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del. N. Engl. J. Med. 2017, 377, 2013–2023. [Google Scholar] [CrossRef]
- Rowe, S.M.; Daines, C.; Ringshausen, F.C.; Kerem, E.; Wilson, J.; Tullis, E.; Nair, N.; Simard, C.; Han, L.; Ingenito, E.P.; et al. Tezacaftor–Ivacaftor in Residual-Function Heterozygotes with Cystic Fibrosis. N. Engl. J. Med. 2017, 377, 2024–2035. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Vertex Pharmaceuticals Incorporated. Kaylydeco (Ivacaftor) [Package Insert]; Vertex Pharmaceuticals Incorporated: Boston, MA, USA, 2023. [Google Scholar]
- Vertex Pharmaceuticals Incorporated. Orkambi (Tezacaftor/Ivacaftor) [Package Insert]; Vertex Pharmaceuticals Incorporated: Boston, MA, USA, 2023. [Google Scholar]
- Vertex Pharmaceuticals Incorporated. Trikafta (Elexacaftor/Tezacaftor/Ivacaftor) [Package Insert]; Vertex Pharmaceuticals Incorporated: Boston, MA, USA, 2024. [Google Scholar]
- McGarry, M.E.; Gibb, E.R.; Oates, G.R.; Schechter, M.S. Left behind: The potential impact of CFTR modulators on racial and ethnic disparities in cystic fibrosis. Paediatr. Respir. Rev. 2022, 42, 35–42. [Google Scholar] [CrossRef]
- Schütz, K.; Pallenberg, S.T.; Kontsendorn, J.; DeLuca, D.; Sukdolak, C.; Minso, R.; Büttner, T.; Wetzke, M.; Dopfer, C.; Sauer-Heilborn, A.; et al. Spirometric and anthropometric improvements in response to elexacaftor/tezacaftor/ivacaftor depending on age and lung disease severity. Front. Pharmacol. 2023, 14, 1171544. [Google Scholar] [CrossRef]
- Zemanick, E.T.; Taylor-Cousar, J.L.; Davies, J.; Gibson, R.L.; Mall, M.A.; McKone, E.F.; McNally, P.; Ramsey, B.W.; Rayment, J.H.; Rowe, S.M.; et al. A Phase 3 Open-Label Study of Elexacaftor/Tezacaftor/Ivacaftor in Children 6 through 11 Years of Age with Cystic Fibrosis and at Least One F508del Allele. Am. J. Respir. Crit. Care Med. 2021, 203, 1522–1532. [Google Scholar] [CrossRef]
- Hoppe, J.E.; Kasi, A.S.; Pittman, J.E.; Jensen, R.; Thia, L.P.; Robinson, P.; Tirakitsoontorn, P.; Ramsey, B.; Mall, M.A.; Taylor-Cousar, J.L.; et al. Vanzacaftor–tezacaftor–deutivacaftor for children aged 6–11 years with cystic fibrosis (RIDGELINE Trial VX21-121-105): An analysis from a single-arm, phase 3 trial. Lancet Respir. Med. 2025, 13, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Keating, C.; Yonker, L.M.; Vermeulen, F.; Prais, D.; Linnemann, R.W.; Trimble, A.; Kotsimbos, T.; Mermis, J.; Braun, A.T.; O’CArroll, M.; et al. Vanzacaftor–tezacaftor–deutivacaftor versus elexacaftor–tezacaftor–ivacaftor in individuals with cystic fibrosis aged 12 years and older (SKYLINE Trials VX20-121-102 and VX20-121-103): Results from two randomised, active-controlled, phase 3 trials. Lancet Respir. Med. 2025, 13, 256–271. [Google Scholar] [CrossRef]
- Wood, M.; Babowicz, F.; Kennedy, A.G.; Antell, M.; Gilhooly, E.; Tompkins, B.J.; Reddy, S.S. Incidence of transaminitis in adults with cystic fibrosis taking elexacaftor/tezacaftor/ivacaftor. J. Am. Pharm. Assoc. 2023, 63, 920–924. [Google Scholar] [CrossRef]
- Gould, M.J.; Smith, H.; Rayment, J.H.; Machida, H.; Gonska, T.; Galante, G.J. CFTR modulators increase risk of acute pancreatitis in pancreatic insufficient patients with cystic fibrosis. J. Cyst. Fibros. 2022, 21, 600–602. [Google Scholar] [CrossRef]
- Dagenais, R.V.E.; Su, V.C.; Quon, B.S. Real-World Safety of CFTR Modulators in the Treatment of Cystic Fibrosis: A Systematic Review. J. Clin. Med. 2020, 10, 23. [Google Scholar] [CrossRef]
- Heo, S.; Young, D.C.; Safirstein, J.; Bourque, B.; Antell, M.H.; Diloreto, S.; Rotolo, S.M. Mental status changes during elexacaftor/tezacaftor / ivacaftor therapy. J. Cyst. Fibros. 2022, 21, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Daines, C.L.; Tullis, E.; Costa, S.; Linnemann, R.W.; Mall, M.A.; McKone, E.F.; Polineni, D.; Quon, B.S.; Ringshausen, F.C.; Rowe, S.M.; et al. Long-term safety and efficacy of elexacaftor/tezacaftor/ivacaftor in people with cystic fibrosis and at least oneF508delallele: 144-week interim results from a 192-week open-label extension study. Eur. Respir. J. 2023, 62, 2202029. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.L.; O’cAllaghan, L.; Pelligra, C.; Konstan, M.W.; Ward, A.; Ishak, J.K.; Chandler, C.; Liou, T.G. Modeling long-term health outcomes of patients with cystic fibrosis homozygous for F508del-CFTR treated with lumacaftor/ivacaftor. Ther. Adv. Respir. Dis. 2019, 13, 1753466618820186. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.B.; Flume, P.A.; Elborn, J.S.; Cooke, J.; Rowe, S.M.; McColley, S.A.; Rubenstein, R.C.; Higgins, M.; VX11-770-110 (KONDUCT) Study Group. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: A double-blind, randomised controlled trial. Lancet Respir. Med. 2015, 3, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Nichols, D.P.; Paynter, A.C.; Heltshe, S.L.; Donaldson, S.H.; Frederick, C.A.; Freedman, S.D.; Gelfond, D.; Hoffman, L.R.; Kelly, A.; Narkewicz, M.R.; et al. Clinical Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor in People with Cystic Fibrosis: A Clinical Trial. Am. J. Respir. Crit. Care Med. 2022, 205, 529–539. [Google Scholar] [CrossRef]
- Kapnadak, S.G.; Ramos, K.J.; Dellon, E.P. Enhancing care for individuals with advanced cystic fibrosis lung disease. Pediatr. Pulmonol. 2021, 56 (Suppl. S1), S69–S78. [Google Scholar] [CrossRef] [PubMed]
- Burgel, P.-R.; Durieu, I.; Chiron, R.; Ramel, S.; Danner-Boucher, I.; Prevotat, A.; Grenet, D.; Marguet, C.; Reynaud-Gaubert, M.; Macey, J.; et al. Rapid Improvement after Starting Elexacaftor–Tezacaftor–Ivacaftor in Patients with Cystic Fibrosis and Advanced Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2021, 204, 64–73. [Google Scholar] [CrossRef]
- Martin, C.; Hamard, C.; Kanaan, R.; Boussaud, V.; Grenet, D.; Abély, M.; Hubert, D.; Munck, A.; Lemonnier, L.; Burgel, P.-R. Causes of death in French cystic fibrosis patients: The need for improvement in transplantation referral strategies! J. Cyst. Fibros. 2016, 15, 204–212. [Google Scholar] [CrossRef]
- Ramos, K.J.; Quon, B.S.; Heltshe, S.L.; Mayer-Hamblett, N.; Lease, E.D.; Aitken, M.L.; Weiss, N.S.; Goss, C.H. Heterogeneity in Survival in Adult Patients with Cystic Fibrosis with FEV(1) < 30% of Predicted in the United States. Chest 2017, 151, 1320–1328. [Google Scholar]
- Barben, J.; Castellani, C.; Munck, A.; Davies, J.C.; Groot, K.M.d.W.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.J.; McColley, S.; et al. Updated guidance on the management of children with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome/cystic fibrosis screen positive, inconclusive diagnosis (CRMS/CFSPID). J. Cyst. Fibros. 2021, 20, 810–819. [Google Scholar] [CrossRef]
- McGarry, M.E.; Raraigh, K.S.; Farrell, P.; Shropshire, F.; Padding, K.; White, C.; Dorley, M.C.; Hicks, S.; Ren, C.L.; Tullis, K.; et al. Cystic Fibrosis Newborn Screening: A Systematic Review-Driven Consensus Guideline from the United States Cystic Fibrosis Foundation. Int. J. Neonatal Screen. 2025, 11, 24. [Google Scholar] [CrossRef]
- Gonska, T.; Keenan, K.; Au, J.; Dupuis, A.; Chilvers, M.A.; Burgess, C.; Bjornson, C.; Fairservice, L.; Brusky, J.; Kherani, T.; et al. Outcomes of Cystic Fibrosis Screening–Positive Infants with Inconclusive Diagnosis at School Age. Pediatrics 2021, 148, e2021051740. [Google Scholar] [CrossRef]
- Munck, A.; Bourmaud, A.; Bellon, G.; Picq, P.; Farrell, P.M.; on behalf of the DPAM Study Group. Phenotype of children with inconclusive cystic fibrosis diagnosis after newborn screening. Pediatr. Pulmonol. 2021, 55, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.L.; Desai, H.; Platt, M.; Dixon, M. Clinical outcomes in infants with cystic fibrosis transmembrane conductance regulator (CFTR) related metabolic syndrome. Pediatr. Pulmonol. 2011, 46, 1079–1084. [Google Scholar] [CrossRef]
- Terlizzi, V.; Claut, L.; Tosco, A.; Colombo, C.; Raia, V.; Fabrizzi, B.; Lucarelli, M.; Angeloni, A.; Cimino, G.; Castaldo, A.; et al. A survey of the prevalence, management and outcome of infants with an inconclusive diagnosis following newborn bloodspot screening for cystic fibrosis (CRMS/CFSPID) in six Italian centres. J. Cyst. Fibros. 2021, 20, 828–834. [Google Scholar] [CrossRef]
- Hayeems, R.Z.; Miller, F.A.; Barg, C.J.; Bombard, Y.; Carroll, J.C.; Tam, K.; Kerr, E.; Chakraborty, P.; Potter, B.K.; Patton, S.; et al. Psychosocial Response to Uncertain Newborn Screening Results for Cystic Fibrosis. J. Pediatr. 2017, 184, 165–171.e1. [Google Scholar] [CrossRef]
- Johnson, F.; Southern, K.W.; Ulph, F. Psychological Impact on Parents of an Inconclusive Diagnosis Following Newborn Bloodspot Screening for Cystic Fibrosis: A Qualitative Study. Int. J. Neonatal Screen. 2019, 5, 23. [Google Scholar] [CrossRef]
- Green, D.M.; Lahiri, T.; Raraigh, K.S.; Ruiz, F.; Spano, J.; Antos, N.; Bonitz, L.; Christon, L.; Gregoire-Bottex, M.; Hale, J.E.; et al. Cystic Fibrosis Foundation Evidence-Based Guideline for the Management of CRMS/CFSPID. Pediatrics 2024, 153, e2023064657. [Google Scholar] [CrossRef]
- Bieth, E.; Hamdi, S.M.; Mieusset, R. Genetics of the congenital absence of the vas deferens. Hum. Genet. 2020, 140, 59–76. [Google Scholar] [CrossRef]
- Bombieri, C.; Claustres, M.; De Boeck, K.; Derichs, N.; Dodge, J.; Girodon, E.; Sermet, I.; Schwarz, M.; Tzetis, M.; Bareil, C.; et al. Recommendations for the classification of diseases as CFTR-related disorders. J. Cyst. Fibros. 2011, 10 (Suppl. S2), S86–S102. [Google Scholar] [CrossRef] [PubMed]
- Langfelder-Schwind, E.; Karczeski, B.; Strecker, M.N.; Redman, J.; Sugarman, E.A.; Zaleski, C.; Brown, T.; Keiles, S.; Powers, A.; Ghate, S.; et al. Molecular testing for cystic fibrosis carrier status practice guidelines: Recommendations of the National Society of Genetic Counselors. J. Genet. Couns. 2014, 23, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Foil, K.E.; Powers, A.; Raraigh, K.S.; Wallis, K.; Southern, K.W.; Salinas, D. The increasing challenge of genetic counseling for cystic fibrosis. J. Cyst. Fibros. 2019, 18, 167–174. [Google Scholar] [CrossRef]
- Stalker, H.J.; Jonasson, A.R.; Hopfer, S.M.; Collins, M.S. Improvement in cystic fibrosis newborn screening program outcomes with genetic counseling via telemedicine. Pediatr. Pulmonol. 2023, 58, 3478–3486. [Google Scholar] [CrossRef]
- Moreland, A.; Herlihy, C.; Tynan, M.A.; Sunshine, G.; McCord, R.F.; Hilton, C.; Poovey, J.; Werner, A.K.; Jones, C.D.; Fulmer, E.B.; et al. Timing of State and Territorial COVID-19 Stay-at-Home Orders and Changes in Population Movement—United States, March 1-May 31, 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1198–1203. [Google Scholar] [CrossRef]
- Pasnick, S.; Carlos, W.G.; Cruz, C.S.D.; Gross, J.E.; Garrison, G.; Jamil, S. SARS-CoV-2 Transmission and the Risk of Aerosol Generating Procedures. Am. J. Respir. Crit. Care Med. 2020. [Google Scholar] [CrossRef]
- Wat, D.; Doull, I. Respiratory virus infections in cystic fibrosis. Paediatr. Respir. Rev. 2003, 4, 172–177. [Google Scholar] [CrossRef]
- Flight, W.G.; Bright-Thomas, R.J.; Tilston, P.; Mutton, K.J.; Guiver, M.; Morris, J.; Webb, A.K.; Jones, A.M. Incidence and clinical impact of respiratory viruses in adults with cystic fibrosis. Thorax 2014, 69, 247–253. [Google Scholar] [CrossRef]
- Tuckson, R.V.; Edmunds, M.; Hodgkins, M.L. Telehealth. N. Engl. J. Med. 2017, 377, 1585–1592. [Google Scholar] [CrossRef]
- Association, A.T. Telehealth: Defining 21st Century Care. Available online: https://www.americantelemed.org/resource/why-telemedicine/ (accessed on 3 February 2023).
- Services, C.f.M.a.M. Centers for Medicare and Medicaid Services COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers. 2020. Available online: https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf (accessed on 19 August 2025).
- Cox, N.S.; Alison, J.A.; Button, B.M.; Wilson, J.W.; Holland, A.E. Feasibility and acceptability of an internet-based program to promote physical activity in adults with cystic fibrosis. Respir. Care 2015, 60, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Cooper, D.M.; Haddad, F.; Sladkey, A.; Nussbaum, E.; Radom-Aizik, S. Tele-Exercise as a Promising Tool to Promote Exercise in Children with Cystic Fibrosis. Front. Public Health 2018, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- O’HAyer, C.V.; O’LOughlin, C.M.; Nurse, C.N.; Smith, P.J.; Stephen, M.J. ACT with CF: A telehealth and in-person feasibility study to address anxiety and depressive symptoms among people with cystic fibrosis. J. Cyst. Fibros. 2021, 20, 133–139. [Google Scholar] [CrossRef]
- Enochs, C.; Filbrun, A.G.; Iwanicki, C.; Moraniec, H.; Lehrmann, J.; Stiffler, J.; Dagher, S.; Tapley, C.; Phan, H.; Raines, R.; et al. Development of an Interdisciplinary Telehealth Care Model in a Pediatric Cystic Fibrosis Center. Telemed. Rep. 2021, 2, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Rad, E.J.; Mirza, A.A.; Chhatwani, L.; Purington, N.; Mohabir, P.K. Cystic fibrosis telemedicine in the era of COVID-19. JAMIA Open 2022, 5, ooac005. [Google Scholar] [CrossRef] [PubMed]
- List, R.; Compton, M.; Soper, M.; Bruschwein, H.; Gettle, L.; Bailey, M.; Starheim, E.; Kalmanek, J.; Somerville, L.; Albon, D. Preserving Multidisciplinary Care Model and Patient Safety During Reopening of Ambulatory Cystic Fibrosis Clinic for Nonurgent Care: A Hybrid Telehealth Model. Telemed. J. E Health 2021, 27, 193–199. [Google Scholar] [CrossRef]
- Womack, C.; Farsin, R.; Farsad, M.; Chaudary, N. Emerging Alternatives to Conventional Clinic Visits in the Era of COVID-19: Adoption of Telehealth at VCU Adult Cystic Fibrosis Center. Int. J. Gen. Med. 2020, 13, 1175–1186. [Google Scholar] [CrossRef]
- Desimone, M.E.; Sherwood, J.; Soltman, S.C.; Moran, A. Telemedicine in cystic fibrosis. J. Clin. Transl. Endocrinol. 2021, 26, 100270. [Google Scholar] [CrossRef]
- Hendra, K.; Neemuchwala, F.; Chan, M.; Ly, N.P.; Gibb, E.R. Patient and Provider Experience with Cystic Fibrosis Telemedicine Clinic. Front. Pediatr. 2021, 9, 784692. [Google Scholar] [CrossRef]
- Jaclyn, D.; Andrew, N.; Ryan, P.; Julianna, B.; Christopher, S.; Nauman, C.; Powers, M.; Sawicki, G.S.; George, M.S. Patient and family perceptions of telehealth as part of the cystic fibrosis care model during COVID-19. J. Cyst. Fibros. 2021, 20, e23–e28. [Google Scholar] [CrossRef]
- Rimbaldo, K.; Frayman, K.B.; Shanthikumar, S. The impact of telehealth based care on paediatric cystic fibrosis outcomes. J. Cyst. Fibros. 2023, 22, 706–709. [Google Scholar] [CrossRef]
- Thee, S.; Busack, L.M.; Mall, M.A.; Stahl, M. Impact of lockdown during the COVID-19 pandemic on health status in patients with cystic fibrosis: A mono-centre observational study. ERJ Open Res. 2022, 8, 00588–02021. [Google Scholar] [CrossRef] [PubMed]
- Servidio, A.G.; Capata, G.; Levantino, L.; Riccio, G.; Contorno, S.; Barbi, E.; Maschio, M. COVID-19 lockdown beneficial effects on lung function in a cohort of cystic fibrosis patients. Ital. J. Pediatr. 2021, 47, 12. [Google Scholar] [CrossRef] [PubMed]
- Loukou, I.; Moustaki, M.; Petrocheilou, A.; Zarkada, I.; Douros, K. Impact of COVID-19 Lockdown on Pulmonary and Nutritional Status in Children and Young Adults with Cystic Fibrosis, in Greece. J. Patient Exp. 2021, 8, 23743735211008295. [Google Scholar] [CrossRef]
- Hatziagorou, E.; Toulia, I.; Kalaitzidou, E.; Chrysochoou, E.-A.; Galogavrou, M.; Tsanakas, J. Change in CF care during COVID-19 pandemic: Single-center experience in a middle-income setting. Pediatr. Pulmonol. 2021, 56, 3065–3067. [Google Scholar] [CrossRef]
- Albon, D.; Ong, T.; Horton, B.; Brighton, D.; Shen, S.; List, R.; Antos, N.; Asfour, F.; Balasa, E.; Beachler, D.; et al. Cystic Fibrosis Learning Network Telehealth Innovation Lab During the COVID-19 Pandemic: Impact on Access to Care, Outcomes, and a New CF Care Model. Pediatr. Pulmonol. 2025, 60, e71102. [Google Scholar] [CrossRef]
- Compton, M.; List, R.; Starheim, E.; Somerville, L.; Williamson, L.; Murray, R.; Jennings, D.; Bruschwein, H.; Albon, D. Home spirometry utilisation in telemedicine clinic for cystic fibrosis care during COVID-19 pandemic: A quality improvement process. BMJ Open Qual. 2021, 10, e001529. [Google Scholar] [CrossRef]
- Paynter, A.; Khan, U.; Heltshe, S.L.; Goss, C.H.; Lechtzin, N.; Hamblett, N.M. A comparison of clinic and home spirometry as longtudinal outcomes in cystic fibrosis. J. Cyst. Fibros. 2022, 21, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, C.; Westrupp, N.; Short, C.; Seddon, P.; Olden, C.; Wallis, C.; Brodlie, M.; Baxter, F.; McCormick, J.; MacFarlane, S.; et al. Unsupervised home spirometry is not equivalent to supervised clinic spirometry in children and young people with cystic fibrosis: Results from the CLIMB-CF study. Pediatr. Pulmonol. 2023, 58, 2871–2880. [Google Scholar] [CrossRef]
- Oppelaar, M.C.; van Helvoort, H.A.; Bannier, M.A.; Reijers, M.H.; van der Vaart, H.; van der Meer, R.; Altenburg, J.; Conemans, L.; Rottier, B.L.; Nuijsink, M.; et al. Accuracy, Reproducibility, and Responsiveness to Treatment of Home Spirometry in Cystic Fibrosis: Multicenter, Retrospective, Observational Study. J. Med. Internet Res. 2024, 26, e60892. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation. Patient Registry: 2022 Annual Data Report; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2023. [Google Scholar]
- Cystic Fibrosis Foundation. 2024 Patient Registry Highlights; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2025. [Google Scholar]
- Faiman, B.; Tariman, J.D. Shared Decision Making: Improving Patient Outcomes by Understanding the Benefits of and Barriers to Effective Communication. Clin. J. Oncol. Nurs. 2019, 23, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.; Frosch, D.; Thomson, R.; Joseph-Williams, N.; Lloyd, A.; Kinnersley, P.; Cording, E.; Tomson, D.; Dodd, C.; Rollnick, S.; et al. Shared decision making: A model for clinical practice. J. Gen. Intern. Med. 2012, 27, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Settipane, R.A.; Bukstein, D.A. Allergen immunotherapy and shared decision-making. Allergy Asthma Proc. 2022, 43, 350–355. [Google Scholar] [CrossRef]
- Van Citters, A.D.; Holthoff, M.M.; Kennedy, A.M.; Melmed, G.Y.; Oberai, R.; Siegel, C.A.; Weaver, A.; Nelson, E.C. Point-of-care dashboards promote coproduction of healthcare services for patients with inflammatory bowel disease. Int. J. Qual. Health Care 2021, 33 (Suppl. S2), ii40–ii47. [Google Scholar] [CrossRef]
- Forner, D.; Noel, C.W.; Shuman, A.G.; Hong, P.; Corsten, M.; Rac, V.E.; Pieterse, A.H.; Goldstein, D. Shared Decision-making in Head and Neck Surgery: A Review. JAMA Otolaryngol. Head Neck. Surg. 2020, 146, 839–844. [Google Scholar] [CrossRef]
- Aoki, Y. Shared decision making for adults with severe mental illness: A concept analysis. Jpn. J. Nurs. Sci. 2020, 17, e12365. [Google Scholar] [CrossRef]
- Rostoft, S.; van den Bos, F.; Pedersen, R.; Hamaker, M.E. Shared decision-making in older patients with cancer—What does the patient want? J. Geriatr. Oncol. 2021, 12, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, K.D.; List, B.; Brinkman, W.B.; Lopez, G.P.; Asi, N.; Erwin, P.; Wang, Z.; Garces, J.P.D.; Montori, V.M.; LeBlanc, A. Shared Decision Making in Pediatrics: A Systematic Review and Meta-analysis. Acad. Pediatr. 2015, 15, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Homa, K.; Stevens, G.; Forcino, R.; Scalia, P.; Mertz, P.; Elwyn, G. Assessing Shared Decision-Making in Cystic Fibrosis Care Using collaboRATE: A Cross-Sectional Study of 159 Programs. J. Patient Exp. 2021, 8, 23743735211034032. [Google Scholar] [CrossRef]
- Eckman, M.H.; Kopras, E.J.; Montag-Leifling, K.; Kirby, L.P.; Burns, L.; Indihar, V.M.; Joseph, P.M. Shared Decision-Making Tool for Self-Management of Home Therapies for Patients with Cystic Fibrosis. MDM Policy Pract. 2017, 2, 2381468317715621. [Google Scholar] [CrossRef]
- Vandemheen, K.L.; O’COnnor, A.; Bell, S.C.; Freitag, A.; Bye, P.; Jeanneret, A.; Berthiaume, Y.; Brown, N.; Wilcox, P.; Ryan, G.; et al. Randomized trial of a decision aid for patients with cystic fibrosis considering lung transplantation. Am. J. Respir. Crit. Care Med. 2009, 180, 761–768. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation. Partnerships for Sustaining Daily Care; Cystic Fibrosis Foundation: Bethesda, MD, USA, 2016. [Google Scholar]
- Hood-Medland, E.A.; White, A.E.C.; Kravitz, R.L.; Henry, S.G. Agenda setting and visit openings in primary care visits involving patients taking opioids for chronic pain. BMC Fam. Pract. 2021, 22, 4. [Google Scholar] [CrossRef]
- Cooley, L.; Hudson, J.; Potter, E.; Raymond, K.F.; George, C.; Georgiopoulos, A.M. Clinical communication preferences in cystic fibrosis and strategies to optimize care. Pediatr. Pulmonol. 2020, 55, 948–958. [Google Scholar] [CrossRef]
- Hempstead, S.E.; Fredkin, K.; Hovater, C.; Naureckas, E.T. Patient and Family Participation in Clinical Guidelines Development: The Cystic Fibrosis Foundation Experience. J. Particip. Med. 2020, 12, e17875. [Google Scholar] [CrossRef] [PubMed]
- Sabadosa, K.A.; Batalden, P.B. The interdependent roles of patients, families and professionals in cystic fibrosis: A system for the coproduction of healthcare and its improvement. BMJ Qual. Saf. 2014, 23 (Suppl. S1), i90–i94. [Google Scholar] [CrossRef] [PubMed]
- Van Citters, A.D.; Gifford, A.H.; Brady, C.; Dunitz, J.M.; Elmhirst, M.; Flath, J.; Laguna, T.A.; Moore, B.; Prickett, M.L.; Riordan, M.; et al. Formative evaluation of a dashboard to support coproduction of healthcare services in cystic fibrosis. J. Cyst. Fibros. 2020, 19, 768–776. [Google Scholar] [CrossRef] [PubMed]
| Modulator Name | Year of First US-FDA Approval | Initial CFTR Variants Approved | CFTR Mutations for Which It Is Approved | Initial Age Approval | Current Age Approval |
|---|---|---|---|---|---|
| Ivacaftor | 2012 | G551D | 97 variants a | ≥6 years | ≥1 month |
| Lumacaftor/ivacaftor | 2015 | Homozygous Phe508del | Homozygous Phe508del | ≥12 years | ≥1 years |
| Tezacaftor/ivacaftor | 2018 | Homozygous Phe508del or at least 1 of 26 additional TEZ/IVA responsive mutations | Additional 127 TEZ/IVA responsive variants b | ≥12 years | ≥6 years |
| Elexacaftor/tezacaftor/ivacaftor | 2019 | At least 1 copy of Phe508del | Additional 272 ETI-responsive variants c | ≥12 years | ≥2 years |
| Vanzacaftor/tezacaftor/deutivacaftor | 2024 | At least 1 copy of phe508del, OR ETI responsive variants OR 31 additional variants d | ≥6 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diener, B.L.; Berdella, M.; DeCelie-Germana, J.; Stables-Carney, T.; Kier, C. Transforming Care Models in Cystic Fibrosis: A Review. Healthcare 2025, 13, 3022. https://doi.org/10.3390/healthcare13233022
Diener BL, Berdella M, DeCelie-Germana J, Stables-Carney T, Kier C. Transforming Care Models in Cystic Fibrosis: A Review. Healthcare. 2025; 13(23):3022. https://doi.org/10.3390/healthcare13233022
Chicago/Turabian StyleDiener, Barry Lawrence, Maria Berdella, Joan DeCelie-Germana, Teresa Stables-Carney, and Catherine Kier. 2025. "Transforming Care Models in Cystic Fibrosis: A Review" Healthcare 13, no. 23: 3022. https://doi.org/10.3390/healthcare13233022
APA StyleDiener, B. L., Berdella, M., DeCelie-Germana, J., Stables-Carney, T., & Kier, C. (2025). Transforming Care Models in Cystic Fibrosis: A Review. Healthcare, 13(23), 3022. https://doi.org/10.3390/healthcare13233022






