Preliminary Cost-Effectiveness of Re-Purposing β-Blockers as an Adjunct Treatment for Women with Triple-Negative Breast Cancer
Highlights
- Observational studies suggest that β-blockers, a low-cost generic medication, improve survival in triple-negative breast cancer.
- This economic evaluation estimates that adjunct use of β-blockers alongside standard care reduces healthcare costs and increases quality-adjusted life years when compared to standard care alone.
- While further monitoring of long-term mortality outcomes and adverse events is warranted, further tightly controlled randomised clinical trials will be difficult to justify economically given the extremely low cost and robust safety profile of generic β-blocker medications.
Abstract
1. Introduction
2. Materials and Methods
2.1. Population of Interest
2.2. β-Blocker Intervention
2.3. Mortality Risk Under Usual Care
2.4. Intervention Efficacy
2.5. Health Utilities
2.6. Health Costs
2.7. Sub-Group Analysis
2.8. Scenario Analysis
2.9. Sensitivity Analysis
2.10. Value of Information Analysis Methods
3. Results
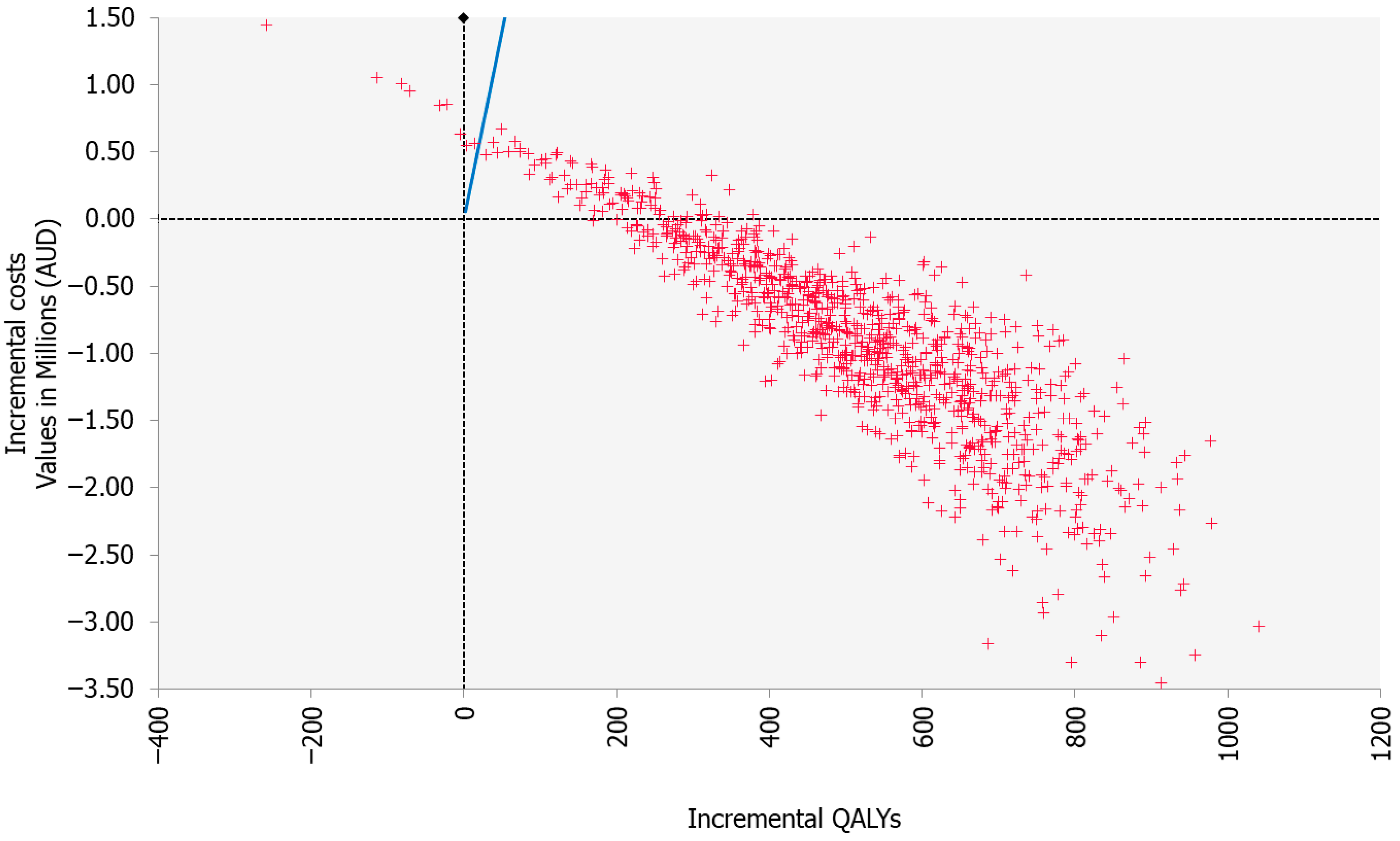
3.1. Base Case
3.2. Sub-Group Analysis
3.3. Scenario and Sensitivity Analysis
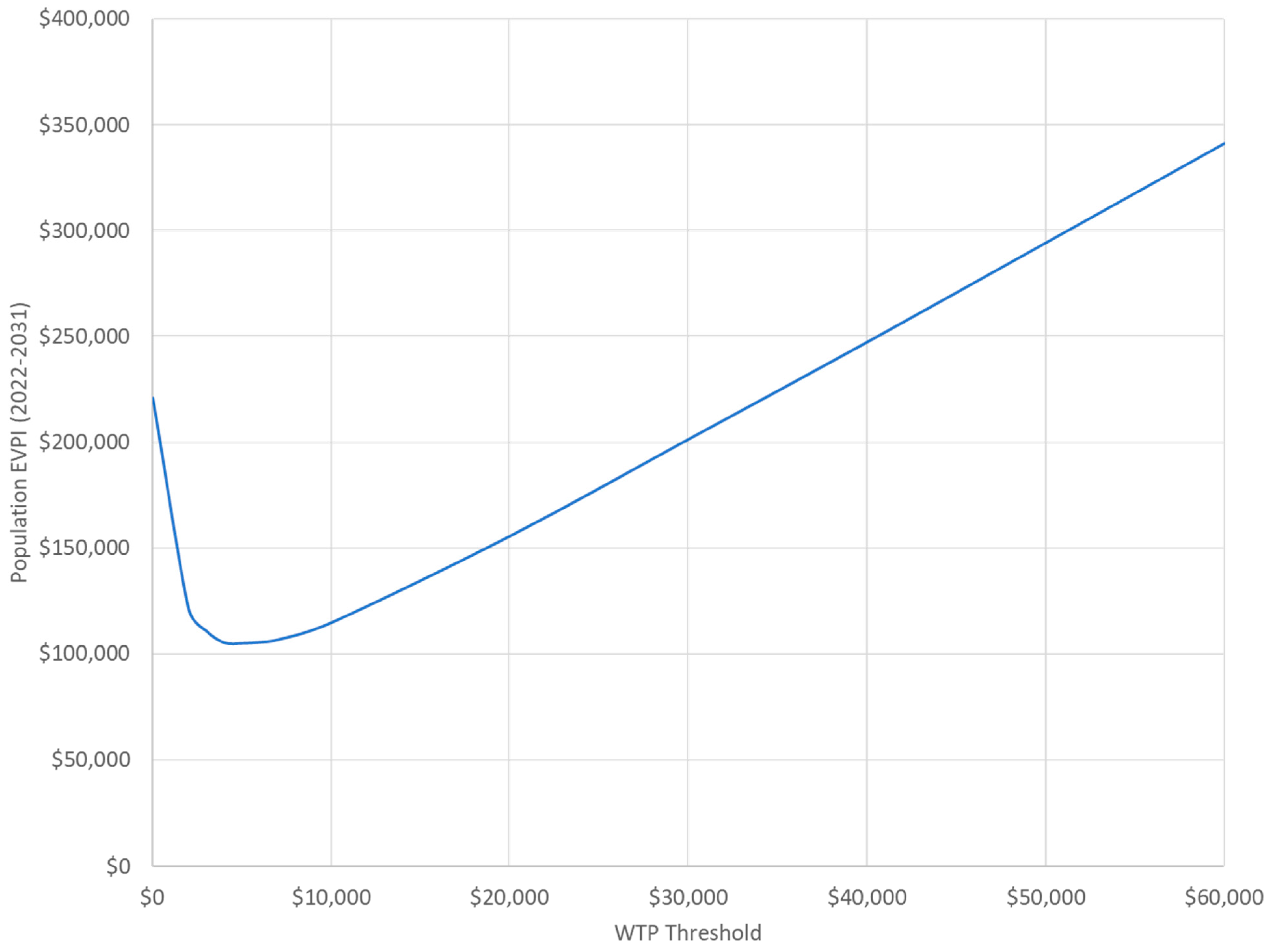
3.4. Value of Information Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AUD | Australian dollar |
| BC | Breast cancer |
| evLY | Equal-value life year |
| HTA | Health technology assessment |
| ICER | Incremental cost-effectiveness ratio |
| QALY | Quality-adjusted life year |
| TNBC | Triple-negative breast cancer |
| YLL | Years of life lived |
Appendix A
| Category | Item | Checked |
| Logical tests | Set all utility values equal to 1. QALYs should equal LYs. | Yes |
| Set all utility values to zero. There should be zero QALYs accrued for all included treatments. | Yes | |
| Set all mortality rates (including background mortality) to 1. All patients should be dead in cycle 1, but still produce (some) expected costs and QALYs (due to half cycle, and one-off costs/disutilities). | Yes | |
| Set mortality rate (including background mortality) at age X to 1. All patients should survive until age X, and still produce expected costs and QALYs. | Yes | |
| If included, set all AE probabilities to zero. Make sure that no AEs occur, and that AE-related costs and disutilities are also estimated to be zero. | Yes | |
| Set unit costs for all included treatments to zero. Estimated treatment costs should be zero. | Yes | |
| Halve and double treatment unit costs for each treatment. Estimated undiscounted treatment costs should also halve and double in response. | Yes | |
| For other included cost categories: halve, double, and set to zero. Ensure that all undiscounted model results respond as expected. | Yes | |
| If included as an input: increase and decrease treatment durations. Do treatment costs increase and decrease appropriately in response? | N/A | |
| Explore higher and lower time horizons. LYs, QALYs and total costs should increase/decrease with longer/shorter time horizons. Set time horizon to zero—all costs and outcomes should be zero/undefined. | N/A | |
| Set the discount rate of benefits to 100%. Total QALYs should dramatically decrease. Repeat for cost discount rate. | Yes | |
| Set the discount rate of benefits to 0%. Total discounted QALYs should increase and match undiscounted results exactly. Repeat for cost discount rate. | Yes | |
| Technical implementation | Check that half cycle correction has been appropriately applied. | N/A |
| Check that background mortality is correctly applied (for the correct age, adjusted for cycle length, reactive to changes in age and gender distribution). Pay special attention to the last model cycle, and any assumptions made for ages that fall outside of the life table (e.g., 100+). | Yes | |
| Has discounting been appropriately applied using the correct formula? ((1 + p)^(−t)). And is it implemented separately for costs and benefits? (Check cell references). | Yes | |
| Check that the sum of all health state membership in the model sums to 1 for all cycles. Check that this is not simply the result of one state’s membership being equal to 1 minus all others (this can hide errors). | Yes | |
| Check that probabilities and rates have been handled correctly (i.e., rates are converted to probabilities before being used as transitions). | Yes | |
| Check that the starting distribution of health states is correct, and consistent across the included treatments. Check that it makes sense given the decision problem (e.g., patients who have the disease, vs. patients who have been diagnosed with disease). | Yes | |
| Ensure that the cumulative probability to die in any given cycle is equal to or greater than that of the age-matched general population mortality. Use the background mortality tables to check. | Yes | |
| Confirm that the relationship with the cycle length and time horizon is correct. | Yes | |
| Following on from the above, ensure that treatment durations are correct by confirming that the model is incurring treatment costs for the correct number of cycles. Ensure any stopping rules are appropriately timed. | Yes | |
| Confirm that disutilities are correctly subtracted from QALYs, by ensuring that duration is taken into account in the calculations. | Yes |
References
- Australian Institute of Health and Welfare. Deaths in Australia. 2021. Available online: https://www.aihw.gov.au/reports/life-expectancy-deaths/deaths-in-australia/contents/leading-causes-of-death (accessed on 1 December 2023).
- Almansour, N.M. Triple-negative breast cancer: A brief review about epidemiology, risk factors, signaling pathways, treatment and role of artificial intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Cortes, J.; Rugo, H.S.; Cescon, D.W.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Perez-Garcia, J.; Iwata, H.; et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med. 2022, 387, 217–226. [Google Scholar] [CrossRef]
- Emens, L.A.; Loi, S. Immunotherapy approaches for breast cancer patients in 2023. Cold Spring Harb. Perspect. Med. 2023, 13, a041332. [Google Scholar] [CrossRef]
- Hiller, J.G.; Perry, N.J.; Poulogiannis, G.; Riedel, B.; Sloan, E.K. Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 2018, 15, 205–218. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Creed, S.J.; Le, C.P.; Hassan, M.; Pon, C.K.; Albold, S.; Chan, K.T.; Berginski, M.E.; Huang, Z.; Bear, J.E.; Lane, J.R.; et al. β2-adrenoceptor ignalling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Devi, S.; Alexandre, Y.O.; Loi, J.K.; Gillis, R.; Ghazanfari, N.; Creed, S.J.; Holz, L.E.; Shackleford, D.; Mackay, L.K.; Heath, W.R.; et al. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity 2021, 54, 1219–1230.E7. [Google Scholar] [CrossRef] [PubMed]
- Le, C.P.; Nowell, C.J.; Kim-Fuchs, C.; Botteri, E.; Hiller, J.G.; Ismail, H.; Pimentel, M.A.; Chai, M.G.; Karnezis, T.; Rotmensz, N.; et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat. Commun. 2016, 7, 10634. [Google Scholar] [CrossRef] [PubMed]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.G.; Morizono, K.; Karanikolas, B.D.W.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef]
- Chang, A.; Botteri, E.; Gillis, R.D.; Löfling, L.; Le, C.P.; Ziegler, A.I.; Chung, N.-C.; Rowe, M.C.; Fabb, S.A.; Hartley, B.J.; et al. Beta-blockade enhances anthracycline control of metastasis in triple-negative breast cancer. Sci. Transl. Med. 2023, 15, eadf1147. [Google Scholar] [CrossRef]
- Kokolus, K.M.; Zhang, Y.; Sivik, J.M.; Schmeck, C.; Zhu, J.; Repasky, E.A.; Drabick, J.J.; Schell, T.D. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 2018, 7, e1405205. [Google Scholar] [CrossRef] [PubMed]
- Løfling, L.L.; Støer, N.C.; Sloan, E.K.; Chang, A.; Gandini, S.; Ursin, G.; Botteri, E. β-blockers and breast cancer survival by molecular subtypes: A population-based cohort study and meta-analysis. Br. J. Cancer 2022, 127, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Hiller, J.G.; Cole, S.W.; Crone, E.M.; Byrne, D.J.; Shackleford, D.M.; Pang, J.-M.B.; Henderson, M.A.; Nightingale, S.S.; Ho, K.M.; Myles, P.S.; et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: A phase II randomized trial. Clin. Cancer Res. 2020, 26, 1803–1811. [Google Scholar] [CrossRef]
- Shaashua, L.; Shabat-Simon, M.; Haldar, R.; Matzner, P.; Zmora, O.; Shabtai, M.; Sharon, E.; Allweis, T.; Barshack, I.; Hayman, L.; et al. Perioperative COX-2 and β-adrenergic blockade improves metastatic biomarkers in breast cancer patients in a phase-II randomized trial. Clin. Cancer Res. 2017, 23, 4651–4661. [Google Scholar] [CrossRef]
- Hopson, M.B.; Lee, S.; Accordino, M.; Trivedi, M.; Maurer, M.; Crew, K.D.; Hershman, D.L.; Kalinsky, K. Phase II study of propranolol feasibility with neoadjuvant chemotherapy in patients with newly diagnosed breast cancer. Breast Cancer Res. Treat. 2021, 188, 427–432. [Google Scholar] [CrossRef]
- Government of Australia—Department of Health and Aged Care. Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (PBAC)—Version 5.0. 2016. Available online: https://pbac.pbs.gov.au/ (accessed on 1 December 2023).
- Grutters, J.P.; Bouttell, J.; Abrishami, P.; Ahmed, S.Y.; Cole, A.; Dawoud, D.; Fernández-Barceló, C.; Frederix, G.W.; Hawkins, N.; Karnon, J.; et al. Defining early health technology assessment: Building consensus using Delphi technique. Int. J. Technol. Assess. Health Care 2025, 41, e34. [Google Scholar] [CrossRef]
- Vreman, R.A.; Geenen, J.W.; Hövels, A.M.; Goettsch, W.G.; Leufkens, H.G.; Al, M.J. Phase I/II clinical trial-based early economic evaluation of acalabrutinib for relapsed chronic lymphocytic leukaemia. Appl. Health Econ. Health Policy 2019, 17, 883–893. [Google Scholar] [CrossRef]
- Pearson, I.; Rothwell, B.; Olaye, A.; Knight, C. Economic modeling considerations for rare diseases. Value Health 2018, 21, 515–524. [Google Scholar] [CrossRef]
- Edney, L.C.; Haji Ali Afzali, H.; Cheng, T.C.; Karnon, J. Estimating the reference incremental cost-effectiveness ratio for the Australian health system. Pharmacoeconomics 2018, 36, 239–252. [Google Scholar] [CrossRef]
- Barratt, A.L.; Irwig, L.M.; Salkeld, G.P.; Glasziou, P.P.; Houssami, N. Benefits, harms and costs of screening mammography in women 70 years and over: A systematic review. Med. J. Aust. 2002, 176, 266–271. [Google Scholar] [CrossRef]
- LaPointe, N.M.A.; Chen, A.Y.; Roe, M.T.; Cohen, D.J.; Diercks, D.B.; Hoekstra, J.W.; Fesmire, F.M.; Gibler, W.B.; Ohman, E.M.; Peterson, E.D. Relation of patient age and mortality to reported contraindications to early beta-blocker use for non-ST elevation acute coronary syndrome. Am. J. Cardiol. 2009, 104, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. Cancer Data in Australia 2021—Web Report. Available online: https://www.aihw.gov.au/reports/cancer/cancer-in-australia-2021/data (accessed on 1 December 2023).
- Farshid, G.; Walters, D. Molecular subtypes of screen-detected breast cancer. Breast Cancer Res. Treat. 2018, 172, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Australian Bureau of Statistics. Australian Population Clock and Pyramid. 2023. Available online: https://www.abs.gov.au/statistics/people/population/population-clock-pyramid (accessed on 1 December 2023).
- Knight, J.M.; Kerswill, S.A.; Hari, P.; Cole, S.W.; Logan, B.R.; D’Souza, A.; Shah, N.N.; Horowitz, M.M.; Stolley, M.R.; Sloan, E.K.; et al. Repurposing existing medications as cancer therapy: Design and feasibility of a randomized pilot investigating propranolol administration in patients receiving hematopoietic cell transplantation. BMC Cancer 2018, 18, 593. [Google Scholar] [CrossRef] [PubMed]
- Australian Institute of Health and Welfare. General Record of Incidence of Mortality (GRIM) Data. Available online: https://www.aihw.gov.au/reports/life-expectancy-deaths/grim-books/contents/general-record-of-incidence-of-mortality-grim-data (accessed on 11 July 2023).
- McCaffrey, N.; Kaambwa, B.; Currow, D.C.; Ratcliffe, J. Health-related quality of life measured using the EQ-5D–5L: South Australian population norms. Health Qual. Life Outcomes 2016, 14, 1–12. [Google Scholar] [CrossRef]
- Brown, D.S.; Trogdon, J.G.; Ekwueme, D.U.; Chamiec-Case, L.; Guy Jr, G.P.; Tangka, F.K.; Li, C.; Trivers, K.F.; Rodriguez, J.L. Health state utility impact of breast cancer in US women aged 18–44 years. Am. J. Prev. Med. 2016, 50, 255–261. [Google Scholar] [CrossRef]
- Kaur, M.N.; Yan, J.; Klassen, A.F.; David, J.P.; Pieris, D.; Sharma, M.; Bordeleau, L.; Xie, F. A systematic literature review of health utility values in breast cancer. Med. Decis. Mak. 2022, 42, 704–719. [Google Scholar] [CrossRef]
- Government of Australia. Pharmaceutical Benefits Scheme. Available online: https://www.pbs.gov.au/pbs/home (accessed on 11 July 2023).
- Goldsbury, D.E.; Yap, S.; Weber, M.F.; Veerman, L.; Rankin, N.; Banks, E.; Canfell, K.; O’Connell, D.L. Health services costs for cancer care in Australia: Estimates from the 45 and Up Study. PLoS ONE 2018, 13, e0201552. [Google Scholar] [CrossRef]
- Marquina, C.; Lacaze, P.; Tiller, J.; Riaz, M.; Sturm, A.C.; Nelson, M.R.; A Ference, B.; Pang, J.; Watts, G.F.; Nicholls, S.J.; et al. Population genomic screening of young adults for familial hypercholesterolaemia: A cost-effectiveness analysis. Eur. Heart J. 2022, 43, 3243–3254. [Google Scholar] [CrossRef]
- Campbell, J.D.; Whittington, M.D.; Pearson, S.D. An alternative measure of health for value assessment: The equal value life-year. Pharmacoeconomics 2023, 41, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Independent Hospital Pricing Authority Australia. National Hospital Cost Data Collection—Round 24 (2019–2020); IHPA: Sydney, Australian, 2022. [Google Scholar]
- Guglin, M.; Krischer, J.; Tamura, R.; Fink, A.; Bello-Matricaria, L.; McCaskill-Stevens, W.; Munster, P.N. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J. Am. Coll. Cardiol. 2019, 73, 2859–2868. [Google Scholar] [CrossRef]
- Avila, M.S.; Ayub-Ferreira, S.M.; de Barros Wanderley, M.R.; das Dores Cruz, F.; Goncalves Brandao, S.M.; Rigaud, V.O.C.; Higuchi-Dos-Santos, M.H.; Hajjar, L.A.; Filho, R.K.; Hoff, P.M.; et al. Carvedilol for prevention of chemotherapy-related cardiotoxicity: The CECCY trial. J. Am. Coll. Cardiol. 2018, 71, 2281–2290. [Google Scholar] [CrossRef]
- Briggs, A. Decision Modelling for Health Economic Evaluation; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Huang, M.; Fasching, P.; Haiderali, A.; Pan, W.; Gray, E.; Zhou, Z.-Y.; Hu, P.; Chaudhuri, M.; Tilleghem, C.L.B.D.; Cappoen, N.; et al. Cost–effectiveness of pembrolizumab plus chemotherapy as first-line treatment in PD-L1-positive metastatic triple-negative breast cancer. Immunotherapy 2022, 14, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Ma, F. Cost-effectiveness of adding atezolizumab to first-line chemotherapy in patients with advanced triple-negative breast cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920916000. [Google Scholar] [CrossRef] [PubMed]
- Phua, L.C.; Lee, S.C.; Ng, K.; Abdul Aziz, M.I. Cost-effectiveness analysis of atezolizumab in advanced triple-negative breast cancer. BMC Health Serv. Res. 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Boscolo Bielo, L.; Trapani, D.; Curigliano, G. Pharmacoeconomics of novel pharmacotherapies in triple-negative breast cancer. Expert Opin. Pharmacother. 2023, 24, 789–801. [Google Scholar] [CrossRef]
- Ijzerman, M.J.; Koffijberg, H.; Fenwick, E.; Krahn, M. Emerging use of early health technology assessment in medical product development: A scoping review of the literature. Pharmacoeconomics 2017, 35, 727–740. [Google Scholar] [CrossRef]
- Hernán, M.A.; Wang, W.; Leaf, D.E. Target trial emulation: A framework for causal inference from observational data. JAMA 2022, 328, 2446–2447. [Google Scholar] [CrossRef]
- Le, T.T.; Payne, S.L.; Buckwald, M.N.; Hayes, L.A.; Parker, S.R.; Burge, C.B.; Oudin, M.J. Sensory nerves enhance triple-negative breast cancer invasion and metastasis via the axon guidance molecule PlexinB3. NPJ Breast Cancer 2022, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Moshides, L.; Minderman, H.; Mohammadpour, H.; Rosario, S.; Kalinsky, K.; Repasky, E.A. A phase Il trial to assess the impact of β2 adrenergic receptor (β2-AR) blockade in metastatic triple negative breast cancer (mTNBC). Am. Soc. Clin. Oncol. 2025, 43, 16. [Google Scholar] [CrossRef]
- Vögele, A.; Johansson, T.; Renom-Guiteras, A.; Reeves, D.; Rieckert, A.; Schlender, L.; Teichmann, A.-L.; Sönnichsen, A.; Martinez, Y.V. Effectiveness and safety of beta blockers in the management of hypertension in older adults: A systematic review to help reduce inappropriate prescribing. BMC Geriatr. 2017, 17 (Suppl. 1), 224. [Google Scholar] [CrossRef] [PubMed]
| Variable | Fixed or Age-Specific | Distribution | Mean | Range | Source |
|---|---|---|---|---|---|
| 1-year mortality probability | |||||
| All-cause mortality | Age-specific | See Supplementary Table S2. | |||
| TNBC-specific mortality | |||||
| All | Fixed | Beta | 0.039 | 0.034–0.041 | Cancer Registry of Norway |
| Localized | Fixed | Beta | 0.017 | 0.012–0.019 | |
| Regional | Fixed | Beta | 0.066 | 0.058–0.077 | |
| Distal | Fixed | Beta | 0.290 | 0.204–0.369 | |
| Effect of treatment—TNBC-specific mortality risk reduction | |||||
| All | Fixed | Lognormal | 0.66 | 0.47–0.91 | Løfling et al. [16] |
| Localized | Fixed | Lognormal | 0.86 | 0.48–1.56 | |
| Regional | Fixed | Lognormal | 0.54 | 0.34–0.86 | |
| Distal | Fixed | Lognormal | 0.69 | 0.24–2.04 | |
| Utilities | |||||
| Healthy Australian population | Age-specific | See Supplementary Table S3. | |||
| Healthy Australian population average | Fixed | Beta | 0.90 | 0.89, 0.91 | McCaffrey et al. [32] |
| TNBC health state penalty | Fixed | Beta | −0.024 | −0.03, −0.02 | Brown et al. [33] |
| Terminal TNBC cycle penalty | Fixed | Beta | −0.110 | −0.27, 0.05 | Kaur et al. [34] |
| Treatment costs (AUD, 2022 prices)—β-blocker intervention | |||||
| Propranolol 40 mg b.d. | Fixed | Gamma | 113.95/year | 9.,62, 136.29 | PBS [35] |
| Carvedilol 12.5 mg b.d. | Fixed | Gamma | 258.18/year | 207.57, 308.78 | |
| Breast cancer management costs (AUD, 2022 prices) | |||||
| Time since diagnosis: | |||||
| 0–1 years | Fixed | Gamma | 42,409 | 34,097, 50,721 | Goldsbury et al. [36] (inflated to 2022 prices) |
| 1–2 years | Fixed | Gamma | 8745 | 7031, 10,459 | |
| 2–3 years | Fixed | Gamma | 3487 | 2804, 4170 | |
| 3–4 years | Fixed | Gamma | 3595 | 2890, 4300 | |
| 4–5 years | Fixed | Gamma | 2839 | 2282, 3395 | |
| Terminal phase | Fixed | Gamma | 44,327 | 35,639, 53,015 | |
| Non-TNBC death | Fixed | Gamma | 5844 | 4698, 6989 | Marquina et al. [37] (inflated to 2022 prices) |
| Standard Care | Standard Care Plus β-Blockers | Difference | 95% CI | |
|---|---|---|---|---|
| Total years of life lived (discounted) | 6233 | 6870 | 628 | 139–1035 |
| QALYs (discounted) | 5166 | 5698 | 526 | 116–865 |
| evLYs (discounted) | 5166 | 5738 | 566 | 125–932 |
| Total β-blocker treatment cost (AUD) | 0 | 1,136,662 | 1,142,294 | 893,676–1,377,890 |
| Total health costs (AUD—discounted) | 53,029,462 | 52,084,447 | −935,116 | −2,365,417–405,350 |
| Total population treated (n) | 767 | |||
| Results per patient treated: | ||||
| Years of life lived gained | 0.82 | |||
| QALYs gained | 0.69 | |||
| evLYs gained | 0.74 | |||
| Mean β-blocker treatment cost (AUD) | 1489 | |||
| Health costs saved (AUD) | −1219 | |||
| ICER—Cost (AUD)/QALY | −1778 (Dominant) | −3284–1951 | ||
| ICER—Cost (AUD)/evLY | −1653 (Dominant) | −2757–783 |
| Incremental YLL (Discounted) | Incremental QALYs (Discounted) | Total β-Blocker Cost (AUD) | Incremental Total Health Costs (AUD, Discounted) | ICER (AUD/QALY) | |
|---|---|---|---|---|---|
| Age group: | |||||
| 50–54 (n = 137) | 152 | 129 | 243,184 | −218,141 | Dominant |
| 55–59 (n = 162) | 157 | 132 | 255,397 | −194,415 | Dominant |
| 60–64 (n = 158) | 125 | 104 | 213,306 | −182,693 | Dominant |
| 65–69 (n = 170) | 127 | 105 | 188,022 | −179,220 | Dominant |
| 70–74 (n = 189) | 92 | 75 | 158,525 | −159,948 | Dominant |
| 75–79 (n = 143) | 33 | 28 | 78,229 | −63,932 | Dominant |
| Tumour stage at diagnosis: | |||||
| Local (60.5%) | 82 | 68 | 773,279 | 246,207 | 3602 |
| Regional (35.6%) | 427 | 357 | 365,685 | −604,284 | Dominant |
| Distal (3.9%) | 29 | 24 | 13,740 | 190,226 | 7775 |
| Scenarios: | |||||
| 1179 | 978 | 1,136,662 | −1,338,484 | Dominant |
| 754 | 629 | 1,136,662 | −1,049,370 | Dominant |
| 478 | 400 | 1,102,710 | −503,655 | Dominant |
| 636 | 533 | 2,575,269 | 45749 | 86 |
| 636 | 533 | 2,273,324 | −162,199 | Dominant |
| 636 | 533 | 1,136,662 | 654,216 | 1229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lloyd, M.; Sloan, E.K.; Marquina, C.; Bouttell, J.; Hassanien, O.; Botteri, E.; Ademi, Z. Preliminary Cost-Effectiveness of Re-Purposing β-Blockers as an Adjunct Treatment for Women with Triple-Negative Breast Cancer. Healthcare 2025, 13, 2929. https://doi.org/10.3390/healthcare13222929
Lloyd M, Sloan EK, Marquina C, Bouttell J, Hassanien O, Botteri E, Ademi Z. Preliminary Cost-Effectiveness of Re-Purposing β-Blockers as an Adjunct Treatment for Women with Triple-Negative Breast Cancer. Healthcare. 2025; 13(22):2929. https://doi.org/10.3390/healthcare13222929
Chicago/Turabian StyleLloyd, Melanie, Erica K. Sloan, Clara Marquina, Janet Bouttell, Omar Hassanien, Edoardo Botteri, and Zanfina Ademi. 2025. "Preliminary Cost-Effectiveness of Re-Purposing β-Blockers as an Adjunct Treatment for Women with Triple-Negative Breast Cancer" Healthcare 13, no. 22: 2929. https://doi.org/10.3390/healthcare13222929
APA StyleLloyd, M., Sloan, E. K., Marquina, C., Bouttell, J., Hassanien, O., Botteri, E., & Ademi, Z. (2025). Preliminary Cost-Effectiveness of Re-Purposing β-Blockers as an Adjunct Treatment for Women with Triple-Negative Breast Cancer. Healthcare, 13(22), 2929. https://doi.org/10.3390/healthcare13222929






