Clinical Assessment of Dry Eye Disease in Patients with Keratoconus in Saudi Arabia
Abstract
1. Introduction
2. Methodology
3. Results
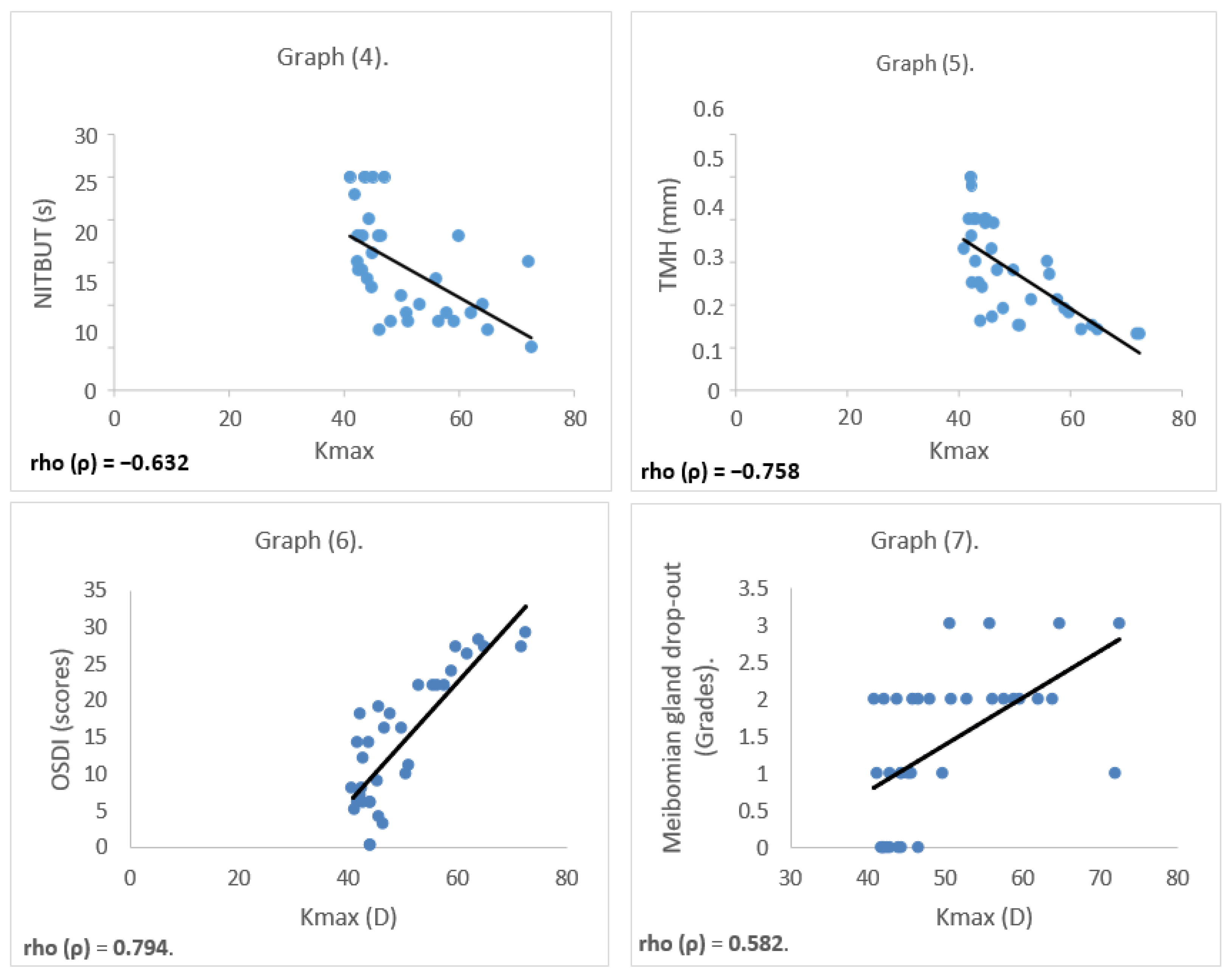
Relationship Between Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krachmer, J.H.; Feder, R.S.; Belin, M.W. Keratoconus and related noninflammatory corneal thinning disorders. Surv. Ophthalmol. 1984, 28, 293–322. [Google Scholar] [CrossRef] [PubMed]
- Mirza, E.; Oltulu, R.; Oltulu, P.; Mirza, G.D.; Okka, M. Dry eye disease and ocular surface characteristics in patients with keratoconus. Saudi J. Ophthalmol. 2022, 36, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, G.; Recchioni, A.; Alejandre-Alba, N.; Martin-Gil, A.; Crooke, A.; Morote, I.J.; Pintor, J. Signs and symptoms of dry eye in keratoconus patients: A pilot study. Curr. Eye Res. 2015, 40, 1088–1094. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Benítez-Del-Castillo, J.; Loya-Garcia, D.; Inomata, T.; Iyar, G.; Liang, L.; Pult, H.; Sabater, A.L.; Starr, C.E.; Vehof, J.; et al. TFOS DEWS III Diagnostic Methodology. Am. J. Ophthalmol. 2025, 279, 387–450. [Google Scholar] [CrossRef]
- Althomali, T.A.; Al-Qurashi, I.M.; Al-Thagafi, S.M.; Mohammed, A.; Almalki, M. Prevalence of keratoconus among patients seeking laser vision correction in Taif area of Saudi Arabia. Saudi J. Ophthalmol. 2018, 32, 114–118. [Google Scholar] [CrossRef]
- Assiri, A.A.; Yousuf, B.I.; Quantock, A.J.; Murphy, P.J. Incidence and severity of keratoconus in Asir province, Saudi Arabia. Br. J. Ophthalmol. 2005, 89, 1403–1406. [Google Scholar] [CrossRef]
- Netto, E.A.; Al-Otaibi, W.M.; Hafezi, N.L.; Kling, S.; Al-Farhan, H.M.; Randleman, J.B.; Hafezi, F. Prevalence of keratoconus in paediatric patients in Riyadh, Saudi Arabia. Br. J. Ophthalmol. 2018, 102, 1436–1441. [Google Scholar] [CrossRef]
- Alkhaldi, S.A.; Allam, K.H.; Radwan, M.A.; Sweeney, L.E.; Alshammeri, S. Estimates of dry eye disease in Saudi Arabia based on a short questionnaire of prevalence, symptoms, and risk factors: The Twaiq Mountain Eye Study I. Contact Lens Anterior Eye 2023, 46, 101770. [Google Scholar] [CrossRef]
- Shetty, R.; Sharma, A.; Pahuja, N.; Chevour, P.; Padmajan, N.; Dhamodaran, K.; Jayadev, C.; Nuijts, R.M.M.A.; Ghosh, A.; Nallathambi, J. Oxidative stress induces dysregulated autophagy in corneal epithelium of keratoconus patients. PLoS ONE 2017, 12, e0184628. [Google Scholar] [CrossRef]
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef] [PubMed]
- Bakkar, M.M.; El-Sharif, A.K.; Al Qadire, M. Validation of the Arabic version of the ocular surface disease index questionnaire. Int. J. Ophthalmol. 2021, 14, 1595. [Google Scholar] [CrossRef] [PubMed]
- Lema, I.; Durán, J.A. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology 2005, 112, 654–659. [Google Scholar] [CrossRef] [PubMed]
- West-Mays, J.A.; Sadow, P.M.; Tobin, T.W.; Strissel, K.J.; Cintron, C.; Fini, M.E. Repair phenotype in corneal fibroblasts is controlled by an interleukin-1 alpha autocrine feedback loop. Investig. Ophthalmol. Vis. Sci. 1997, 38, 1367–1379. [Google Scholar]
- Wilson, S.E.; He, Y.G.; Weng, J.; Li, Q.; McDowall, A.W.; Vital, M.; Chwang, E.L. Epithelial injury induces keratocyte apoptosis: Hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp. Eye Res. 1996, 62, 325–338. [Google Scholar] [CrossRef]
- Becker, J.; Salla, S.; Dohmen, U.; Redbrake, C.; Reim, M. Explorative study of interleukin levels in the human cornea. Graefe’s Arch. Clin. Exp. Ophthalmol. 1995, 233, 766–771. [Google Scholar] [CrossRef]
- Bosnar, D.; Dekaris, I.; Gabrić, N.; Markotić, A.; Lazić, R.; Špoljarić, N. Influence of interleukin-1α and tumor necrosis factor-α production on corneal graft survival. Croat. Med. J. 2006, 47, 59–66. [Google Scholar]
- Maertzdorf, J.; Osterhaus, A.D.; Verjans, G.M. IL-17 expression in human herpetic stromal keratitis: Modulatory effects on chemokine production by corneal fibroblasts. J. Immunol. 2002, 169, 5897–5903. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp. Eye Res. 2012, 96, 132–137. [Google Scholar] [CrossRef]
- Santos, A.; José Filho, A.P.; Cenedeze, M.A.; Hiyane, M.I.; Amano, M.T.; Cruz, M.C.; Hirai, F.E.; Camara, N.O.; de Sousa, L.B.; de Oliveira, L.A. Increased inflammatory mediators in the ocular surface tissue in keratoconus. Mol. Vis. 2024, 30, 279. [Google Scholar]
- Stern, M.E.; Pflugfelder, S.C. Inflammation in dry eye. Ocul. Surf. 2004, 2, 124–130. [Google Scholar] [CrossRef]
- Nichani, P.A.; Solomon, B.; Trinh, T.; Mimouni, M.; Rootman, D.; Singal, N.; Chan, C.C. Investigating the role of inflammation in keratoconus: A retrospective analysis of 551 eyes. Eur. J. Ophthalmol. 2023, 33, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dogru, M.; Karakaya, H.; Özçetin, H.; Ertürk, H.; Yücel, A.; Özmen, A.; Baykara, M.; Tsubota, K. Tear function and ocular surface changes in keratoconus. Ophthalmology 2003, 110, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II diagnostic methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. Tfos dews ii pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Hong, J.; Sun, X.; Wei, A.; Cui, X.; Li, Y.; Qian, T.; Wang, W.; Xu, J. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea 2013, 32, 716–721. [Google Scholar] [CrossRef]
- Mostafa, E.M.; Abdellah, M.M.; Elhawary, A.M.; Mounir, A. Research Article Noncontact Meibography in Patients with Keratoconus. J. Ophthalmol. 2019, 2019, 2965872. [Google Scholar]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Willcox, M.D.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Roll, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II tear film report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Shorter, E.; Harthan, J.; Nau, A.; Fogt, J.; Cao, D.; Schornack, M.; Nau, C. Dry eye symptoms in individuals with keratoconus wearing contact lenses. Eye Contact Lens 2021, 47, 515–519. [Google Scholar] [CrossRef]


| Mean ± SD | p | ||
|---|---|---|---|
| Keratoconus Group | Control Group | ||
| Age (years) | 25.9 ± 6.8 | 22.6 ± 8.4 | 0.890 |
| Kmax (dioptry) | 56.85 ± 8.41 D | 43.59 ± 1.81 D | <0.0001 |
| Thinnest Corneal thickness (µm) | 419.52 ± 58.61 µm | 535.47 ± 38.34 µm | <0.0001 |
| NITBUT (s) | 9.88 ± 3.25 s | 18.94 ± 4.26 s | <0.0001 |
| TMH (mm) | 0.185 ± 0.053 mm | 0.358 ± 0.076 mm | <0.0001 |
| OSDI score | 21.41 ± 5.93 | 7.52 ± 5.05 | <0.0001 |
| Mean ± SD | p | ||
|---|---|---|---|
| Keratoconus Group | Control Group | p | |
| Grade 0 (n) | 0 | 8 | <0.001 |
| Grade 1 (n) | 2 | 6 | <0.001 |
| Grade 2 (n) | 11 | 3 | <0.001 |
| Grade 3 (n) | 4 | 0 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammeri, S.; Alharbi, A. Clinical Assessment of Dry Eye Disease in Patients with Keratoconus in Saudi Arabia. Healthcare 2025, 13, 2890. https://doi.org/10.3390/healthcare13222890
Alshammeri S, Alharbi A. Clinical Assessment of Dry Eye Disease in Patients with Keratoconus in Saudi Arabia. Healthcare. 2025; 13(22):2890. https://doi.org/10.3390/healthcare13222890
Chicago/Turabian StyleAlshammeri, Saleh, and Azzam Alharbi. 2025. "Clinical Assessment of Dry Eye Disease in Patients with Keratoconus in Saudi Arabia" Healthcare 13, no. 22: 2890. https://doi.org/10.3390/healthcare13222890
APA StyleAlshammeri, S., & Alharbi, A. (2025). Clinical Assessment of Dry Eye Disease in Patients with Keratoconus in Saudi Arabia. Healthcare, 13(22), 2890. https://doi.org/10.3390/healthcare13222890





