Abstract
Background/Objectives: A successful implementation of VBHC requires significant transformations, spanning from structural adjustments to a fundamental shift in organizational culture. However, organizations often adopt VBHC principles piecemeal, while managers overlook diverse staff reactions and their leadership role. To address these challenges, we developed and validated a multi-source assessment tool for use by organizations during their transition to VBHC to determine whether they are progressing effectively in both the dimensions. Method: To develop the first version of the tool, a multidisciplinary group translated strategies identified in a previous scoping review into questionnaire items through structured sessions. To validate it, a two round Delphi analysis was conducted. Experts in VBHC, management, health economy and clinical/researcher were contacted to ask for their participation. Consensus was established based on the following criteria: a median (Mdn) score of ≥4, an interquartile range (IQR) of ≤1.5 or ≤2, and a frequency of ratings in the range [4–5] ≥ 70%. Results: A total of 54 experts were invited to participate in the Delphi survey. The first round received 25 responses, while the second round had 23 responses. The final version of the assessment tool consists of 30 questions divided into two macro-areas: the Value Agenda Section and the Change Management Section. All items achieved a minimum frequency of ratings > 80% during both the rounds. Conclusion: By comparing the perspectives of managers and clinicians, the resulting tool enables organizations to assess the adoption of the Value Agenda component, as well as the change management strategies supporting its implementation.
1. Introduction
In recent decades, healthcare systems worldwide have faced growing pressure to enhance efficiency, improve patient outcomes, and ensure financial sustainability. Rising healthcare expenditures, variations in care quality, medical errors, and persistent barriers to access underscore the urgency need for transformation.
In response, Value-Based Healthcare (VBHC) has emerged as a paradigm shift that reorients care delivery from a volume to value [1,2,3]. Introduced by M.E. Porter and E.O. Teisberg in 2006 [2], VBHC defines value as the “health outcomes that matter to patients divided by the costs needed to achieve them” [2]. This framework challenges traditional, specialty-based organization models and promotes patient-centered systems that emphasize integration, multidisciplinary collaboration, and continuity of care [4,5,6,7]. Despite its conceptual appeal, VBHC implementation remain complex, requiring both structural and culture transformation [8,9].
To guide this transformation M.E. Porter and T.H. Lee introduced the “Value Agenda” [10], which comprises six interdependent elements. Its cornerstone is the reorganization of care around patients’ medical conditions through Integrated Practice Units (IPUs). The agenda also highlights systematic measurement of outcomes and costs, the development of care networks to deliver the right services in the right settings, and the use of integrated Information technology (IT) platforms to coordinate all components [2]. In terms of reimbursement, the agenda advocates shifting from the DRG payment system to bundled payments for care cycles, aligned with value creation [2].
Although many healthcare providers worldwide have begun adopting VBHC principles [11,12,13], several studies indicate that few have implemented VBHC as an integrated management strategy [13,14]. Most have selectively focused on elements that fit with existing organizational structure. This selective approach inhibits the development of a truly value-oriented care model, particularly when considering the van der Nat’s extended strategic agenda [15]. According to him, for VBHC to become fully operational, the Value Agenda needs to be extended with four additional elements: setting up value-based quality improvement processes, integrating value into patient communication, investing in a culture focused on value delivery, and creating learning platforms for healthcare professionals [15].
The implementation of these elements profoundly affects established practices and processes. Consequently, if such structural and transformative interventions are not accompanied by a strategy on cultural change, their successful implementation will be compromised [9]. Organizational culture, shaped by shared values and behavioral norms, often represents the greatest barrier to VBHC adoption [16]. Therefore, leaders must actively promote a VBHC-oriented culture through targeted change management strategies that engage staff and promote understanding of the benefits of transformation [17]. Coetsee et al. [17] identified five strategies to achieve what Weiner et al. [18] defined as “organizational readiness” (OR), namely “the degree to which organizational members are psychologically and behaviorally prepared to implement change”. These strategies include fostering a learning environment, ensuring transparent communication, empowering staff participation, recognizing and rewarding performance, and promoting a shared vision that aligns individual and organizational goals. Similarly, J. P. Kotter [19] outlined eight essential steps for successful organizational transformations, from creating a sense of urgency and forming a powerful coalition to anchoring new approaches in organizational culture.
However, many managers tend to underestimate the diversity of employee responses to organizational change and their role of leadership in shaping them [16]. This lacuna, combined with the “piece-meal adoption” of the VBHC tenets [20], remains a major barrier to comprehensive implementation.
The present study aims to develop and validate an assessment tool that organizations can use during their transition to VBHC with a dual aim:
- (1)
- To assess the extent to which the Value Agenda elements have been implemented within an organization, and
- (2)
- To evaluate the change management strategies employed by organizational leaders to drive and sustain the transition to VBHC.
2. Materials and Methods
The study was conducted between 2023 and early 2025 and consisted of 2 phases. a preparatory literature search and experts’ involvement (phase 1: August 2023–November 2024) followed by a survey using the Delphi method (phase 2: November 2024–January 2025). This article presents phase 2, while the methodology and findings from of phase 1 were published elsewhere [9].
2.1. Phase 1: Tool Development
The present assessment tool builds upon the findings of our previous scoping review [9], which aimed to identify the operational and management strategies employed at various levels—healthcare policymakers, hospital management, and healthcare providers—to implement VBHC principles in real-world settings. For this assessment tool, the focus is narrowed to strategies within hospital boundaries, specifically addressing change management and implementing the Value Agenda.
To translate operational strategies into practical questionnaire items, a series of structured sessions were organized between September 2024 and November 2024. This process involved a multidisciplinary working group composed of 12 experts in VBHC, change management, hospital operations, clinical care, and statistics.
2.2. Phase 2: Tool Validation
To validate the initial version of the assessment tool developed in Phase 1, a two-round Delphi survey was conducted. The Delphi survey involved the following steps: (i) Development of an online survey; (ii) Recruitment and consenting of participants to the Delphi panel; (iii) Two rounds of consultation on the proposed topics in the survey.
2.2.1. The Panel Members
For the Delphi panel, potential members, who spoke English, were selected among international experts based on their Curriculum Vitae, scientific publications, and demonstrated expertise in the following domains: (i) VBHC, (ii) healthcare management, (iii) health economics, and (iv) clinical practice and research. Experts were identified through literature searches, professional networks, and members of learning communities.
Experts were invited to complete the Delphi survey via email, using a Google Forms questionnaire. A cover letter outlined the survey’s purpose, relevance, and significance. Responses were collected in real-time and anonymously. Two reminder emails were sent at one-week intervals to increase the response rate. Expecting a response rate in the range of 30–40 percent, a total of 54 experts were invited.
2.2.2. Data Analysis and Definition of Consensus
The experts panel assessed the adequacy of the proposed items using a 5-point scale, ranging from 1 (not adequate) to 5 (completely adequate). Data collected during the initial consultation round were used to calculate the Kendall’s W coefficient and establish consensus criteria among panelists. Consensus was establishing based on the following criteria: a median (Mdn) score of ≥4; an interquartile range (IQR) was used as a non-parametric consensus metric; lower IQR values indicate tighter agreement among experts. A priori, consensus was defined as IQR ≤ 1.5 (primary threshold), with IQR ≤ 2 adopted as a lenient criterion in sensitivity checks; and a frequency of ratings in the range [4–5] ≥ 70% [21].
Items meeting inclusion criteria in the first round were carried forward to the second round, where their stability within expert consensus was re-evaluated. The purpose of round 2 was to confirm the robustness of the agreement obtained in round 1. The final set of items included those that met the consensus criteria in both rounds.
To further validate the findings, several complementary statistical analyses were performed:
- Central tendency and dispersion analysis;
- Consensus evaluation between rounds: To gauge agreement, we relied on the IQR, a non-parametric measure in which smaller values reflect tighter convergence of ratings and thus greater consensus. We complemented this with the Relative Interquartile Range (RIR), which expresses how dispersed the responses are in relation to the central tendency; it is computed as (IQR/Median) × 100, so lower RIR values indicate that judgments cluster more closely around the median. To assess how stable the panel’s views remained across rounds, we examined the Variation in the Coefficient of Variation (VCV), defined as the percentage change in the coefficient of variation (CV) from Round 1 to Round 2. The CV is calculated as (Standard Deviation/Mean) × 100, and VCV as ((CV_Round2 − CV_Round1)/CV_Round1) × 100. In this framework, smaller—and especially negative—VCV values signal that variability decreased over time, consistent with increasing stability of expert judgments across successive Delphi rounds.
- Reliability assessment using Cronbach’s alpha;
- Stability analysis of group responses through the median test and U test.
This methodology replicated one already applied in an another study [21].
3. Results
3.1. Tool Development
The initial pool of items was derived from the operational strategies identified in the scoping review. After three rounds of expert review and refinement meetings, redundant or unclear items were removed, resulting in a final 30-item questionnaire structured into two main sections: the Value Agenda (13 items) and Change Management (17 items). Each section is subdivided into thematic sub-areas (4 and 5, respectively), with mirrored items designed for both healthcare managers and frontline professionals.
3.2. Tool Validation
A two-round modified Delphi study was carried out for this study.
- Panel Participation
A total of 54 experts were invited to participate in the Delphi survey. The first round received 25 responses (46%), while the second round had 23 responses (43%) (Table 1). This slight decrease in participation is consistent with the Delphi methodology, where attrition between rounds is expected as participants refine their evaluations.

Table 1.
Panel composition and expertise.
- Value Agenda Section
In the first round (R1), most items in this section received ratings concentrated in the 4–5 range, indicating strong agreement among panel members. However, some priorities had a wider distribution of responses, prompting further refinement. The second round (R2) showed a shift toward greater consensus, with a reduction in response variability. Items that initially had more dispersed ratings (including scores of 2 and 3) saw an increase in higher agreement (4–5 scores) (Figure 1). These findings indicate that after initial feedback, experts refined their evaluations, leading to a stronger collective endorsement of the priorities. The level of agreement among the 13 items, measured using Kendall’s coefficient of concordance (W), increased slightly between Delphi rounds—from W = 0.196 (χ2(24) = 61.1, p < 0.001) in Round 1 to W = 0.222 (χ2(22) = 63.6, p < 0.001) in Round 2. The statistically significant results indicate a progressive trend toward consensus among panelists.
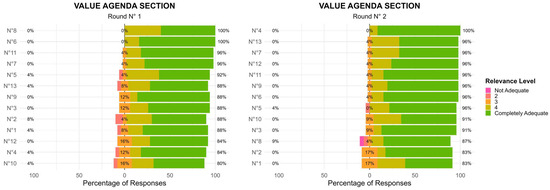
Figure 1.
Value Agenda Section: Two-Round Delphi Results.
- Change Management Section
Compared to the Value Agenda section, the Change Management section exhibited slightly more variability in responses during the R1 with some items receiving scores across the full 1–5 scale. This suggests that panel members had differing perspectives on the importance or feasibility of certain priorities within this domain. In the R2, consensus improved for several key priorities, with a notable decrease in lower ratings (1–2 scores) (Figure 2). This indicates that after reviewing collective input, panel members aligned more closely on the critical importance of change management strategies. However, a few priorities continued to display some variability, suggesting the need for further discussion or clarification in future iterations. In the Change Management domain, the level of agreement among the 17 items, assessed using Kendall’s coefficient of concordance, increased from W = 0.208 (χ2(24) = 84.7, p < 0.001) in Round 1 to W = 0.280 (χ2(22) = 105, p < 0.001) in Round 2. Although overall concordance remained moderate, the significant increase in W indicates a progressive alignment of expert opinions across rounds.
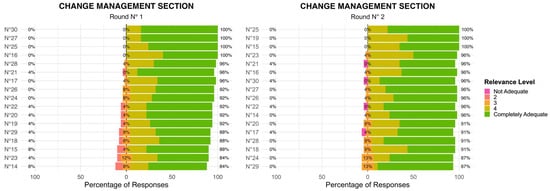
Figure 2.
Change Management Section: Two-Round Delphi Results.
- Data Analysis
The analysis of responses collected during the R1 revealed broad agreement among panelists regarding the two proposed dimensions. As a result, all items were included in the R2 for further assessment. After R2, the selection criteria for the final items were specified through an interpretation of the contingency table with consensus results. Highly significant items demonstrated unanimous consensus across both rounds, while non-significant items were excluded at each stage of evaluation.
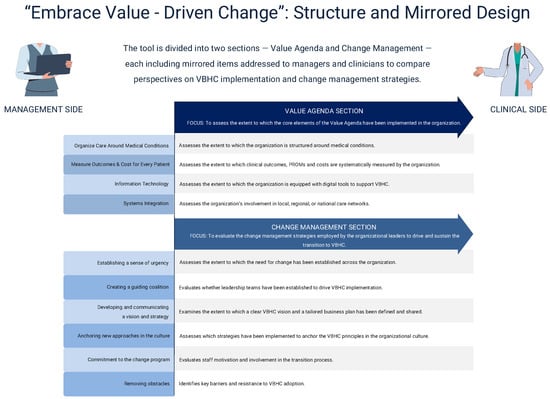
Figure 3.
”Embrace Value-Driven Change” assessment tool.

Table 2.
“Embrace Value-Driven Change” assessment tool.
- ○
- Analysis of Consensus Level and Group Stability in Answers
To assess the proximity and stability of responses between rounds, the IQR and VCV were calculated. The analysis indicated an acceptable degree of proximity and stability among panellists’ opinions (IQR < 0.5).
Group stability was confirmed when the RIR between rounds was < 0.20. Additionally, consensus was established when the CV remained below 22% for most items.
Table S1 shows that the VRIR values for all items remained below the 0.30 threshold, confirming stability in responses. Consensus among panelists is further substantiated by the VCV data.
The Delphi process was deemed complete once consensus and stability levels were reached, as additional rounds would not have produced significant variations in the results.
- ○
- Questionnaire Validity and Reliability Analysis
The reliability of the questionnaire was assessed using Cronbach’s alpha, which yielded a high overall score of 0.962, confirming strong internal consistency. Individual reliability scores for each dimension exceeded 0.8, confirming internal consistency [R1: Value Agenda section = 0.822; Change Management section = 0.884; R2: Value Agenda section = 0.876; Change Management section = 0.932].
An item-total correlation analysis was performed, revealing an optimal inter-item correlation range. The removal of any individual items did not enhance reliability, indicating a robust instrument. Inspection of “alpha if item deleted” indicated no item would increase α; differences were trivial, confirming that item removal would not yield a meaningful gain in reliability. Accordingly, all items were retained.
- ○
- Independent Samples t-Test
To compare responses between rounds, an independent samples Mann–Whitney U test was performed. The results, presented in Table S1, indicate that no statistically significant differences were found between the rounds (p > 0.05), further reinforcing the stability of panelist responses over time.
4. Discussion
In this paper, we presented the development and validation of an assessment tool designed to support healthcare organization in their transition towards VBHC. We called it “Embrace Value-Driven Change”. This tool serves a dual aim: (i) to assess the extent to which the Value Agenda elements have been implemented within an organization, and (ii) to evaluate the change management strategies employed by organizational leaders to drive and sustain this transition.
As structured, the tool incorporates innovative features.
The first distinctive feature lies in its design. The final version consists of 30 items divided into two sections: 13 items in the Value Agenda Section and 17 in the Change Management Section. Each area includes “main questions” (e.g., “To what extent does the hospital systematically measure clinical and patient reported outcomes?”), followed by “secondary questions” (e.g., “If so, which of the following measures are used?”), which explore specific aspects of each topic. To safeguard objectivity and reproducibility, multiple choice questions were chosen.
To capture potential misalignment between hospital top management and healthcare personnel, the tool was designed as mirror survey. For instance, hospital managers are asked: “To what extent are members of the organization regularly updated on the progress of the VBHC plan over time?”, while healthcare personnel are asked the mirror question: “To what extent are you informed about the progress of such VBHC plan over time?”. This design, which has been already employed in other settings [53,54,55,56], enables a comprehensive understanding of how VBHC is being implemented and helps identity whether further alignment ore corrective actions are needed.
The second feature lies in the scope of investigation. Existing instruments typically focus on either the implementation of value agenda elements or the organizational strategies for change management; none integrate both dimensions within a single framework. For example, the questionnaire developed by H.J. Westerink et al. [57] captures the perspective of care teams and focuses solely on the structural adoption of Value Agenda components. In contrast, our tool collects data from both managers and clinicians and also explores the change management strategies underpinning VBHC implementation.
Although the content of the first section, albeit expressed differently (e.g., ”To what extent are outcomes measures and casemix variables structurally being measured for the medical condition?” versus “To what extent does the hospital systematically measure clinical and patient reported outcomes?”), partly overlaps with Westerink’s instrument (for instance, in evaluating the organization of care around medical conditions or the measurement of outcomes and costs), our tool further examines whether these measures are shared internally to inform quality improvement processes and communicated to patients to promote engagement and shared decision-making. In line with van der Nat’s extended strategic agenda [15], these aspects reflect the integration of value into patient communication and the creation of a value-oriented culture. However, our tool does not yet include two key pillars of van der Nat’s extended strategic agenda: build learning platforms for healthcare professionals and aligning reimbursement with value. These elements may be included in future iterations of the tool.
On the change management side, numerous frameworks have been developed to help managers evaluate their change initiatives. One example is the DICE framework. This framework, developed by H.L. Sirkin et al. [58], evaluates change initiatives based on duration, integrity, commitment, and effort. Unlike such models, our tool measures, from both top management and healthcare personnel perspectives, the implementation of key actions and strategies identified in our previous scoping review [9] as critical for successful change management. These strategies are consistent with those proposed by Coetsee [17] and Kotter [19], spanning from creating a sense of urgency (“To what extent did your organization establish a sense of urgency to implement the principles of VBHC?”), forming a guiding coalition (“To what extent was a formal group of people—key leaders and representatives from clinical and managerial teams—brought together to drive the implementation of VBHC?”) to developing and communicating a vision for the project (“a future-oriented perspective that emphasizes the reasons why you should strive to create that future”). The tool also investigates how organizations anchor VBHC principles in their culture through education, training and career progression opportunities. Finally, it explores barriers to implementation, asking respondents to identify the main obstacles encountered during the transition to VBHC (“Which were the main obstacles you encountered during the implementation of VBHC?”).
Limitations
The development of this assessment tool could have been affected by some limitations. The first is related to the methodology chosen to validate it. We used the Delphi methodology. It ensured methodological rigor and expert consensus on the instrument’s key elements [59], but it has its own inherent limitations. Although this provides an evidence-based basis, we may have omitted elements that were not included in the scoping review but were worthy of inclusion in our tool, such as the role of the hospital in contracting for value-based payment mechanisms and the presence of platform-based learning systems. Future versions could explore how these missing pillars might be integrated or further examined to expand the instrument’s scope.
Second, the Delphi methodology is susceptible to high dropout rates due to the length of commitment and distractions between rounds. In our case, the first round received 25 responses, while the second round had 23 responses.
Finally, although the panel of experts included were of international standing, the majority of respondents (72%) were based in Italy. This limited geographical representation may have influenced the results. Future validations should aim for a more balanced international composition and include a greater proportion of non-clinical stakeholders such as managers, health economists, and representatives of patient organizations.
5. Conclusions
The resulting evidence-based assessment tool was designed to enable organizations to monitor the progress of VBHC adoption and evaluate the effectiveness of the change management strategies employed.
Its dual-perspective structure facilitates the assessment of the alignment between leadership and clinical teams, ensuring that the transition is both strategically driven and operationally embraced. This approach empowers managers to proactively steer change, address resistance, and enhance organizational readiness, ultimately fostering more effective and sustainable VBHC adoption.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/healthcare13222849/s1, Table S1: Contingency table corresponding to the analysis of the results obtained during the Delphi rounds.
Author Contributions
Conceptualization, E.d.M., D.v.S., P.B.v.d.N. and A.G.d.B.; methodology, E.d.M., D.v.S., I.V. and A.G.d.B.; validation, P.B.v.d.N. and A.G.d.B.; statistical analysis: I.V.; writing—original draft preparation, E.d.M.; writing—review and editing, D.v.S.; visualization, D.v.S.; supervision, P.B.v.d.N. and A.G.d.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Policlinico Universitario A. Gemelli (ID 7823- approval date: 25 September 2025).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Porter, M.E. What Is Value in Health Care? N. Engl. J. Med. 2010, 363, 2477–2481. [Google Scholar] [CrossRef]
- Porter, M.E.; Teisberg, E.O. Redefining Health Care: Creating Value-Based Competition on Results; Harvard Business School Press: Boston, MA, USA, 2006. [Google Scholar]
- Porter, M.E. Value-Based Health Care Delivery. Ann. Surg. 2008, 248, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Plsek, P.E. Systematic Design of Healthcare Processes. Qual. Health Care 1997, 6, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gabutti, I.; Mascia, D.; Cicchetti, A. Exploring “Patient-Centered” Hospitals: A Systematic Review to Understand Change. BMC Health Serv. Res. 2017, 17, 364. [Google Scholar] [CrossRef]
- Coulson-Thomas, C. Re-Engineering Hospital and Healthcare Processes. Health Estate J. 1997, 51, 14–15. [Google Scholar] [PubMed]
- Porter, M.E.; Lee, T.H. Integrated Practice Units: A Playbook for Health Care Leaders. NEJM Catal. Innov. Care Deliv. 2021, 2. [Google Scholar] [CrossRef]
- Conrad, D.; Grembowski, D.; Gibbons, C.; Marcus-Smith, M.; Hernandez, S.; Chang, J.; Renz, A.; Lau, B.; dela Cruz, E. A Report On Eight Early-Stage State And Regional Projects Testing Value-Based Payment. Health Aff. 2013, 32, 998–1006. [Google Scholar] [CrossRef]
- De Mattia, E.; Angioletti, C.; D’Agostino, M.; Paoletti, F.; de Belvis, A.G. Moving from Principles to Practice: A Scoping Review of Value-Based Healthcare (VBHC) Implementation Strategies. Healthcare 2024, 12, 2457. [Google Scholar] [CrossRef]
- Porter, M.; Lee, T. The Strategy That Will Fix Health Care. Harv. Bus. Rev. 2013, 91, 50–70. [Google Scholar]
- Gallani, S.; Witkowski, M.; Corsi, E.; Jonsson, N. Transforming Healthcare Delivery at Karolinska University Hospital. Available online: https://www.hbs.edu/faculty/Pages/item.aspx?num=65111 (accessed on 5 November 2025).
- EIT Health. Implementing Value-Based Health Care in Europe: Handbook for Pioneers; EIT Health: Munich, Germany, 2020. [Google Scholar]
- Ramos, P.; Savage, C.; Thor, J.; Atun, R.; Carlsson, K.S.; Makdisse, M.; Neto, M.C.; Klajner, S.; Parini, P.; Mazzocato, P. It Takes Two to Dance the VBHC Tango: A Multiple Case Study of the Adoption of Value-Based Strategies in Sweden and Brazil. Soc. Sci. Med. 2021, 282, 114145. [Google Scholar] [CrossRef]
- Van Staalduinen, D.J.; Van Den Bekerom, P.; Groeneveld, S.; Kidanemariam, M.; Stiggelbout, A.M.; Van Den Akker-van Marle, M.E. The Implementation of Value-Based Healthcare: A Scoping Review. BMC Health Serv. Res. 2022, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Van der Nat, P.B. The New Strategic Agenda for Value Transformation. Health Serv. Manag. Res. 2022, 35, 189–193. [Google Scholar] [CrossRef]
- Kotter, J.P. Leading Change; Harvard Business Review Press: Brighton, MA, USA, 2012. [Google Scholar]
- Coetsee, L. From Resistance to Commitment. Public. Adm. Q. 1999, 23, 204–222. [Google Scholar]
- Weiner, B.J.; Amick, H.; Lee, S.-Y.D. Conceptualization and Measurement of Organizational Readiness for Change: A Review of the Literature in Health Services Research and Other Fields. Med. Care Res. Rev. 2008, 65, 379–436. [Google Scholar] [CrossRef] [PubMed]
- Kotter, J.P. Leading Change; Harvard Business Press: Boston, MA, USA, 1996. [Google Scholar]
- Ramos, P. The Adoption, Adaptation, and Abandonment of Value-Based Health Care. Ph.D. Thesis, Karolinska Institutet, Stockholm, Sweden, 2023. [Google Scholar]
- Mengual-Andrés, S.; Roig-Vila, R.; Mira, J.B. Delphi Study for the Design and Validation of a Questionnaire about Digital Competences in Higher Education. Int. J. Educ. Technol. High. Educ. 2016, 13, 12. [Google Scholar] [CrossRef]
- Keswani, A.; Koenig, K.; Bozic, K. Value-Based Healthcare: Part 1-Designing and Implementing Integrated Practice Units for the Management of Musculoskeletal Disease. Clin. Orthop. Relat. Res. 2016, 474, 2100–2103. [Google Scholar] [CrossRef]
- Conrad, D.; Vaughn, M.; Grembowski, D.; Marcus-Smith, M. Implementing Value-Based Payment Reform: A Conceptual Framework and Case Examples. Med. Care Res. Rev. 2016, 73, 437–457. [Google Scholar] [CrossRef]
- Steinmann, G.; Daniels, K.; Mieris, F.; Delnoij, D.; van de Bovenkamp, H.; van Der Nat, P. Redesigning Value-Based Hospital Structures: A Qualitative Study on Value-Based Health Care in the Netherlands. BMC Health Serv. Res. 2022, 22, 1193. [Google Scholar] [CrossRef]
- Van Veghel, D.; Soliman-Hamad, M.; Schulz, D.; Cost, B.; Simmers, T.; Dekker, L. Improving Clinical Outcomes and Patient Satisfaction among Patients with Coronary Artery Disease: An Example of Enhancing Regional Integration between a Cardiac Centre and a Referring Hospital. BMC Health Serv. Res. 2020, 20, 494. [Google Scholar] [CrossRef]
- Goretti, G.; Marinari, G.; Vanni, E.; Ferrari, C. Value-Based Healthcare and Enhanced Recovery After Surgery Implementation in a High-Volume Bariatric Center in Italy. Obes. Surg. 2020, 30, 2519–2527. [Google Scholar] [CrossRef]
- Phillips, R.; Cyr, J.; Keaney, J.; Messina, L.; Meyer, T.; Tam, S.; Korenda, K.; Darrigo, M.; Kumar, P.; Challapalli, S. Creating and Maintaining a Successful Service Line in an Academic Medical Center at the Dawn of Value-Based Care: Lessons Learned From the Heart and Vascular Service Line at UMass Memorial Health Care. Acad. Med. 2015, 90, 1340–1346. [Google Scholar] [CrossRef]
- Zipfel, N.; Van Der Nat, P.B.; Rensing, B.J.W.M.; Daeter, E.J.; Westert, G.P.; Groenewoud, A.S. The Implementation of Change Model Adds Value to Value-Based Healthcare: A Qualitative Study. BMC Health Serv. Res. 2019, 19, 643. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.; Baathe, F.; Andersson, A.; Sandoff, M. Value-Based Healthcare as a Trigger for Improvement Initiatives. Leadersh. Health Serv. 2017, 30, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Angioletti, C.; de Mattia, E.; Carloni, L.M.; Morsella, A.; Fabi, A.; Orlandi, A.; Tortora, G.; de Belvis, A.G. Definition of a Tool to Assess Shared Decision-Making (SDM) on Women with Breast Cancer: A Value-Based Approach. Health Sci. Rep. 2022, 5, e817. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, G.; Brown, Z. Transitioning from Volume to Value: A Strategic Approach to Design and Implementation. Nurs. Adm. Q. 2016, 40, 130–136. [Google Scholar] [CrossRef]
- Nilsson, K.; Baathe, F.; Andersson, A.; Sandoff, M. The Need to Succeed—Learning Experiences Resulting from the Implementation of Value-Based Healthcare. Leadersh. Health Serv. 2018, 31, 2–16. [Google Scholar] [CrossRef]
- Daniels, K.; van der Voort, M.; Biesma, D.; van der Nat, P. Five Years’ Experience with Value-Based Quality Improvement Teams: The Key Factors to a Successful Implementation in Hospital Care. BMC Health Serv. Res. 2022, 22, 1271. [Google Scholar] [CrossRef]
- Gray, C.F.; Parvataneni, H.K.; Bozic, K.J. Value-Based Healthcare: “Physician Activation”: Healthcare Transformation Requires Physician Engagement and Leadership. Clin. Orthop. Relat. Res. 2020, 478, 954–957. [Google Scholar] [CrossRef]
- Nilsson, K.; Baathe, F.; Andersson, A.; Wikstrom, E.; Sandoff, M. Experiences from Implementing Value-Based Healthcare at a Swedish University Hospital—A Longitudinal Interview Study. BMC Health Serv. Res. 2017, 17, 169. [Google Scholar] [CrossRef]
- Van der Nat, P.; van Veghel, D.; Daeter, E.; Crijns, H.; Koolen, J.; Houterman, S.; Soliman, M.; de Mol, B. Meetbaar Beter Study Grp Insights on Value-Based Healthcare Implementation from Dutch Heart Care. Int. J. Healthc. Manag. 2020, 13, 189–192. [Google Scholar] [CrossRef]
- Van Veghel, H.; Dekker, L.; Theunissen, L.; Janssen, J.; Burg, M.; Huijbers, P.; Voermans, P.; van der Wees, P.; Cremers, H. Introducing a Method for Implementing Value Based Health Care Principles in the Full Cycle of Care: Using Atrial Fibrillation as a Proof of Concept. Int. J. Healthc. Manag. 2022, 15, e009054. [Google Scholar] [CrossRef]
- Lansdaal, D.; van Nassau, F.; van der Steen, M.; de Bruijne, M.; Smeulers, M. Lessons Learned on the Experienced Facilitators and Barriers of Implementing a Tailored VBHC Model in a Dutch University Hospital from a Perspective of Physicians and Nurses. BMJ Open 2022, 12, e051764. [Google Scholar] [CrossRef]
- Van Veghel, D.; Daeter, E.; Bax, M.; Amoroso, G.; Blaauw, Y.; Camaro, C.; Cummins, P.; Halfwerk, F.; Hamer, I.; de Jong, J.; et al. Organization of Outcome-Based Quality Improvement in Dutch Heart Centres. Eur. Heart J. Qual. CARE Clin. Outcomes 2020, 6, 49–54. [Google Scholar] [CrossRef]
- Chatfield, J.; Longenecker, C.; Fink, L.; Gold, J. Ten CEO Imperatives for Healthcare Transformation: Lessons from Top-Performing Academic Medical Centers. J. Healthc. Manag. 2017, 62, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Collden, C.; Hellstrom, A. Value-Based Healthcare Translated: A Complementary View of Implementation. BMC Health Serv. Res. 2018, 18, 681. [Google Scholar] [CrossRef]
- Kaplan, R.S. Improving Value with TDABC. Healthc. Financ. Manag. 2014, 68, 76–83. [Google Scholar]
- Hurh, J.; Ko, Y.; Lee, S. Value-Based Healthcare: Prerequisites and Suggestions for Full-Fledged Implementation in the Republic of Korea. J. Korean Med. Assoc. 2017, 60, 826–840. [Google Scholar] [CrossRef]
- De Vries, E.; Drewes, H.; Struijs, J.; Heijink, R.; Baan, C. Barriers to Payment Reform: Experiences from Nine Dutch Population Health Management Sites. Health Policy 2019, 123, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, E.; Odelade, O.; Gostkorzewicz, J.; Cordero, L. Demonstrating Proof of Concept for Value-Based Agreements in Europe: Two Real-World Cases. Int. J. Technol. Assess. Health Care 2023, 39, e30. [Google Scholar] [CrossRef]
- Conrad, D.; Grembowski, D.; Hernandez, S.; Lau, B.; Marcus-Smith, M. Emerging Lessons from Regional and State Innovation in Value-Based Payment Reform: Balancing Collaboration and Disruptive Innovation. Milbank Q. 2014, 92, 568–623. [Google Scholar] [CrossRef]
- Kissam, S.; Beil, H.; Cousart, C.; Greenwald, L.; Lloyd, J. States Encouraging Value-Based Payment: Lessons from CMS’s State Innovation Models Initiative. Milbank Q. 2019, 97, 506–542. [Google Scholar] [CrossRef]
- Leao, D.L.L.; Cremers, H.-P.; Van Veghel, D.; Pavlova, M.; Hafkamp, F.J.; Groot, W.N.J. Facilitating and Inhibiting Factors in the Design, Implementation, and Applicability of Value-Based Payment Models: A Systematic Literature Review. Med. Care Res. Rev. 2023, 80, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Ng, S. A Qualitative Study on Relationships and Perceptions between Managers and Clinicians and Its Effect on Value-Based Healthcare within the National Health Service in the UK. Health Serv. Manag. Res. 2022, 35, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Theunissen, L.; Cremers, H.-P.; Dekker, L.; Janssen, H.; Burg, M.; Huijbers, E.; Voermans, P.; Kemps, H.; Van Veghel, D. Implementing Value-Based Health Care Principles in the Full Cycle of Care: The Pragmatic Evolution of the Netherlands Heart Network. Circ. Cardiovasc. Qual. Outcomes 2023, 16, e009054. [Google Scholar] [CrossRef]
- Stefánsdóttir, N.T.; Nilsen, P.; Lindstroem, M.B.; Andersen, O.; Powell, B.J.; Tjørnhøj-Thomsen, T.; Kirk, J.W. Implementing a New Emergency Department: A Qualitative Study of Health Professionals’ Change Responses and Perceptions. BMC Health Serv. Res. 2022, 22, 447. [Google Scholar] [CrossRef]
- Shea, C.M.; Jacobs, S.R.; Esserman, D.A.; Bruce, K.; Weiner, B.J. Organizational Readiness for Implementing Change: A Psychometric Assessment of a New Measure. Implement. Sci. 2014, 9, 7. [Google Scholar] [CrossRef]
- Chaganty, S.S.; Walter, H.; Smith, F.; Sharma, H. Evaluating Patient and Neurosurgeon Perspectives on Virtualisation of Neurosurgery Clinics in the COVID-19 Era: A Prospective Mirror Survey Study. Br. J. Neurosurg. 2023, 37, 142–147. [Google Scholar] [CrossRef]
- De Mattia, E.; Angioletti, C.; Perilli, A.; Guajardo Rios, L.S.; Garganese, G.; Tagliaferri, L.; Scambia, G.; Fragomeni, S.M.; de Belvis, A.G. Gov➔Value: How to Combine Reported Quality Experiences and Patient-Reported Outcome Measures. First Results on Vulvar Cancer Patients in an Italian Research Hospital. Front. Public Health 2022, 10, 1014651. [Google Scholar] [CrossRef]
- Gimenez, L.; Kelly-Irving, M.; Delpierre, C.; Rougé-Bugat, M.-E.; Lepage, B.; Lang, T. Interaction between Patient and General Practitioner According to the Patient Body Weight: A Cross-Sectional Survey. Fam. Pract. 2023, 40, 218–225. [Google Scholar] [CrossRef]
- Kirren, Q.; Daste, C.; Foissac, F.; Abdoul, H.; Alami, S.; Carrier, M.-E.; Kwakkenbos, L.; Lefèvre-Colau, M.-M.; Rannou, F.; Papelard, A.; et al. Differences in Disability Perception in Systemic Sclerosis: A Mirror Survey of Patients and Health Care Providers. J. Clin. Med. 2023, 12, 1359. [Google Scholar] [CrossRef] [PubMed]
- Westerink, H.J.; Steinmann, G.; Koomans, M.; van der Kemp, M.H.; van der Nat, P.B. Value-Based Healthcare Implementation in the Netherlands: A Quantitative Analysis of Multidisciplinary Team Performance. BMC Health Serv. Res. 2024, 24, 224. [Google Scholar] [CrossRef] [PubMed]
- Sirkin, H.; Keenan, P.; Jackson, A. The Hard Side of Change Management. Harv. Bus. Rev. 2005, 83, 108–118, 158. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, H.M.; Needham, R.D. Moving Best Practice Forward: Delphi Characteristics, Advantages, Potential Problems, and Solutions. Int. J. Tour. Res. 2009, 11, 415–437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).