2050: An Arthroplasty Odyssey †
Highlights
- Advocates the inclusion of critical raw material management and supply chain resilience in health policy planning to address imminent scarcities and ensure the sustainability of essential medical services, such as arthroplasty, potentially impacted by Net Zero by 2050.
- Interdisciplinary health policy frameworks that incorporate insights from healthcare, environmental science, and supply chain management are essential to effectively address the complex challenges of achieving sustainable, equitable, and ethical arthroplasty practices during the escalating osteoarthritis epidemic.
- Highlights the need for preventive health policies that promote early detection and treatment of osteoarthritis, aiming to decrease long-term reliance on joint replacement surgeries, thereby reducing future demand pressures and enhancing patient outcomes.
Abstract
1. The Dawn of the Arthroplasty Era
2. The Golden Age of Arthroplasty
3. Net Zero by 2050: Implications for Routine Arthroplasty Procedures
4. Beyond 2050: The Future of Arthroplasty—Continuity or Transcendence?
5. Concluding Remarks and Recommendations
| Key Policy Takeaways |
|
6. Limitations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACL | Anterior cruciate ligament |
| ADLs | Activities of daily living |
| AI | Artificial intelligence |
| BAU | Business-as-usual |
| BMI | Body Mass Index |
| CMS | Centers for Medicare & Medicaid Services |
| CPRD | Clinical Practice Research Datalink |
| COVID-19 | Coronavirus disease 2019 |
| CRMs | Critical raw materials |
| CT | Computed Tomography |
| HDPE | High-density polyethylene |
| IPCC | Intergovernmental Panel on Climate Change |
| JSN | Joint space narrowing |
| KL | Kellgren-Lawrence |
| LLDPE | Linear low-density polyethylene |
| LLMs | Large language models |
| LMICs | Low- and middle-income countries |
| MDPE | Medium-density polyethylene |
| MEP | Movement-evoked pain |
| MRI | Magnetic Resonance Imaging |
| NZE | Net Zero Emissions |
| OA | Osteoarthritis |
| OARSI | Osteoarthritis Research Society International |
| PREVENT | Patient-centered, Risk reducing, Equity, inclusiveness, accessibility, Vigilant, Evidence-based, Nurturing health promotion, Transparency |
| PEEK | Polyetheretherketone |
| PEKK | Polyetherketoneketone |
| PROMs | Patient-reported outcome measures |
| PRP | Platelet-rich plasma |
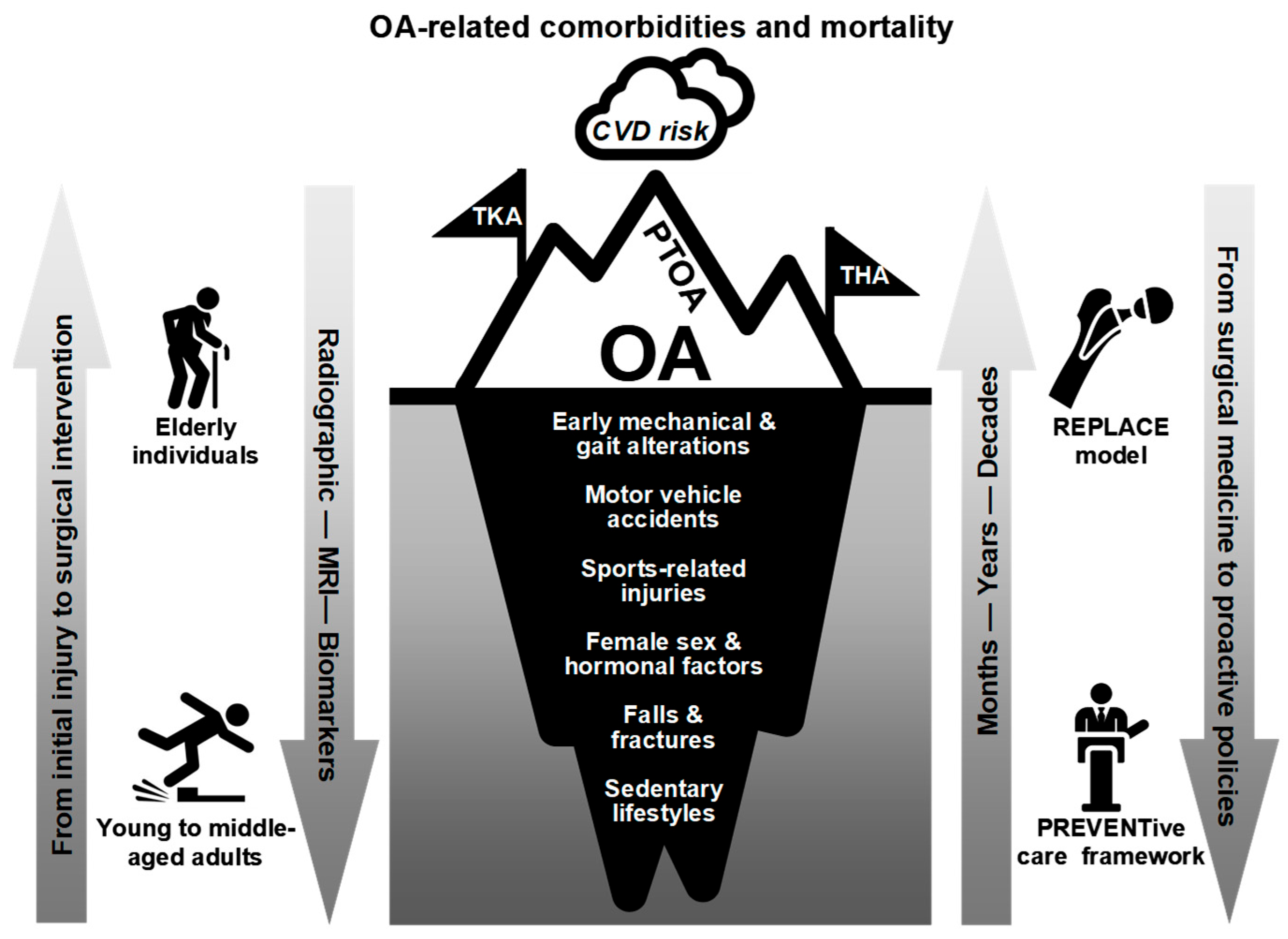
| PTOA | Post-traumatic OA |
| QoL | Quality of life |
| REPLACE | Reactive End-stage Prosthesis for Load-bearing Arthritic Cartilage Erosion |
| R&D | Research and development |
| THA | Total hip arthroplasty |
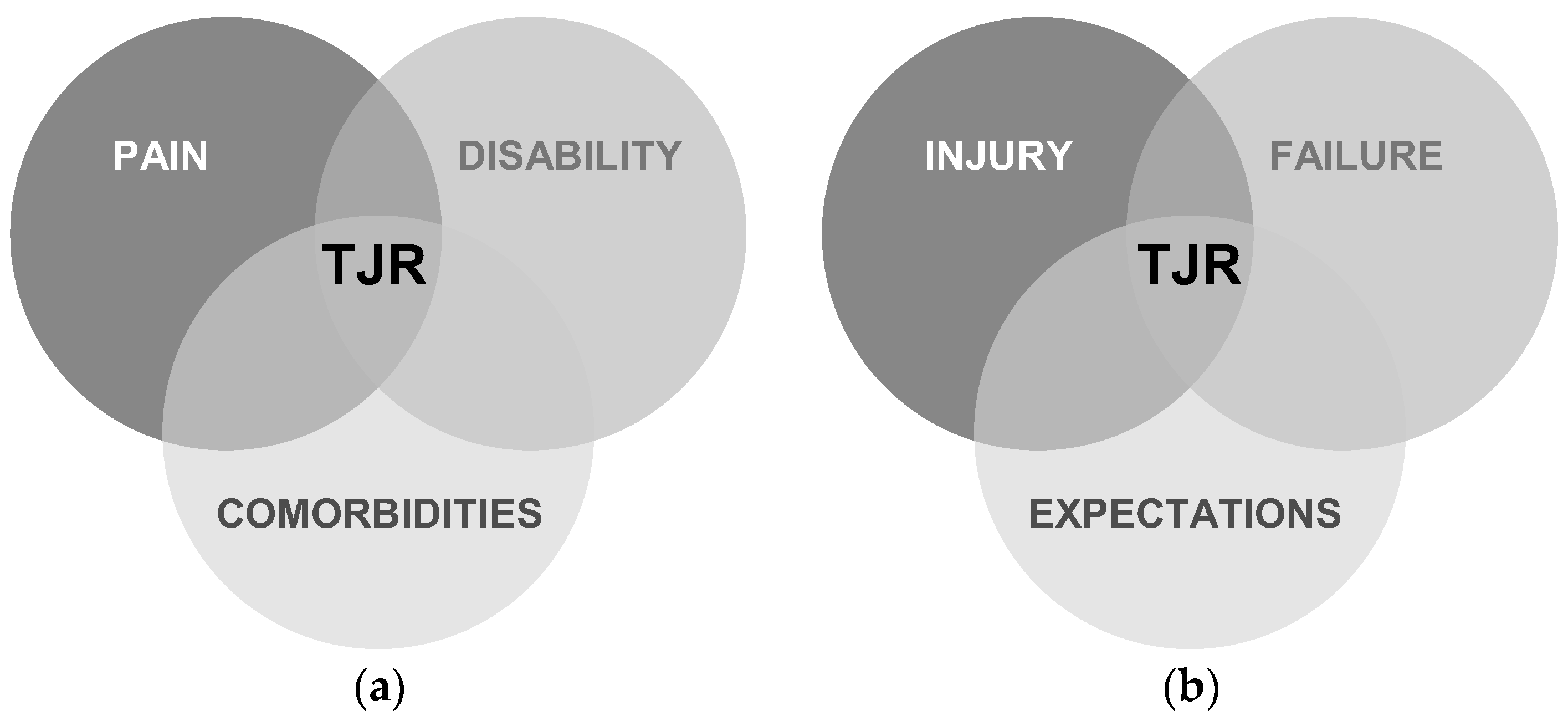
| TJR | Total joint replacement |
| TKA | Total knee arthroplasty |
| UHMWPE | Ultra-high-molecular-weight polyethylene |
| U.S. | United States |
| WHO | World Health Organization |
| XPE | Cross-linked polyethylene |
Appendix A
References
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sánchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal health conditions represent a global threat to healthy aging: A report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 2016, 56 (Suppl. S2), S243–S255. [Google Scholar] [CrossRef]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar]
- Jennifer, M.H.; Charles, G.H.; Teresa, J.B. A public health approach to addressing arthritis in older adults: The most common cause of disability. Am. J. Public Health 2012, 102, 426–433. [Google Scholar] [CrossRef]
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of total hip and knee replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Deshpande, B.R.; Katz, J.N.; Solomon, D.H.; Yelin, E.H.; Hunter, D.J.; Messier, S.P.; Suter, L.G.; Losina, E. Number of persons with symptomatic knee osteoarthritis in the US: Impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. 2016, 68, 1743–1750. [Google Scholar] [CrossRef]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef]
- Steinmetz, J.D.; Culbreth, G.T.; Haile, L.M.; Rafferty, Q.; Lo, J.; Fukutaki, K.G.; Cruz, J.A.; Smith, A.E.; Vollset, S.E.; Brooks, P.M.; et al. Global, regional, and national burden of osteoarthritis, 1990-2020 and projections to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Rheumatol. 2023, 5, e508–e522. [Google Scholar] [CrossRef]
- Centers for Medicare and Medicaid Services. Comprehensive Care for Joint Replacement Model. Available online: https://www.cms.gov/priorities/innovation/innovation-models/cjr (accessed on 31 May 2021).
- Learmonth, I.D.; Young, C.; Rorabeck, C. The operation of the century: Total hip replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Ong, K.L.; Schmier, J.; Mowat, F.; Saleh, K.; Dybvik, E.; Karrholm, J.; Garellick, G.; Havelin, L.I.; Furnes, O.; et al. Future clinical and economic impact of revision total hip and knee arthroplasty. J. Bone Jt. Surg. Am. 2007, 89 (Suppl. S3), 144–151. [Google Scholar]
- Wilson, N.A.; Schneller, E.S.; Montgomery, K.; Bozic, K.J. Hip and knee implants: Current trends and policy considerations. Health Aff. 2008, 27, 1587–1598. [Google Scholar] [CrossRef]
- Bitton, R. The economic burden of osteoarthritis. Am. J. Manag. Care 2009, 15, S230–S235. [Google Scholar]
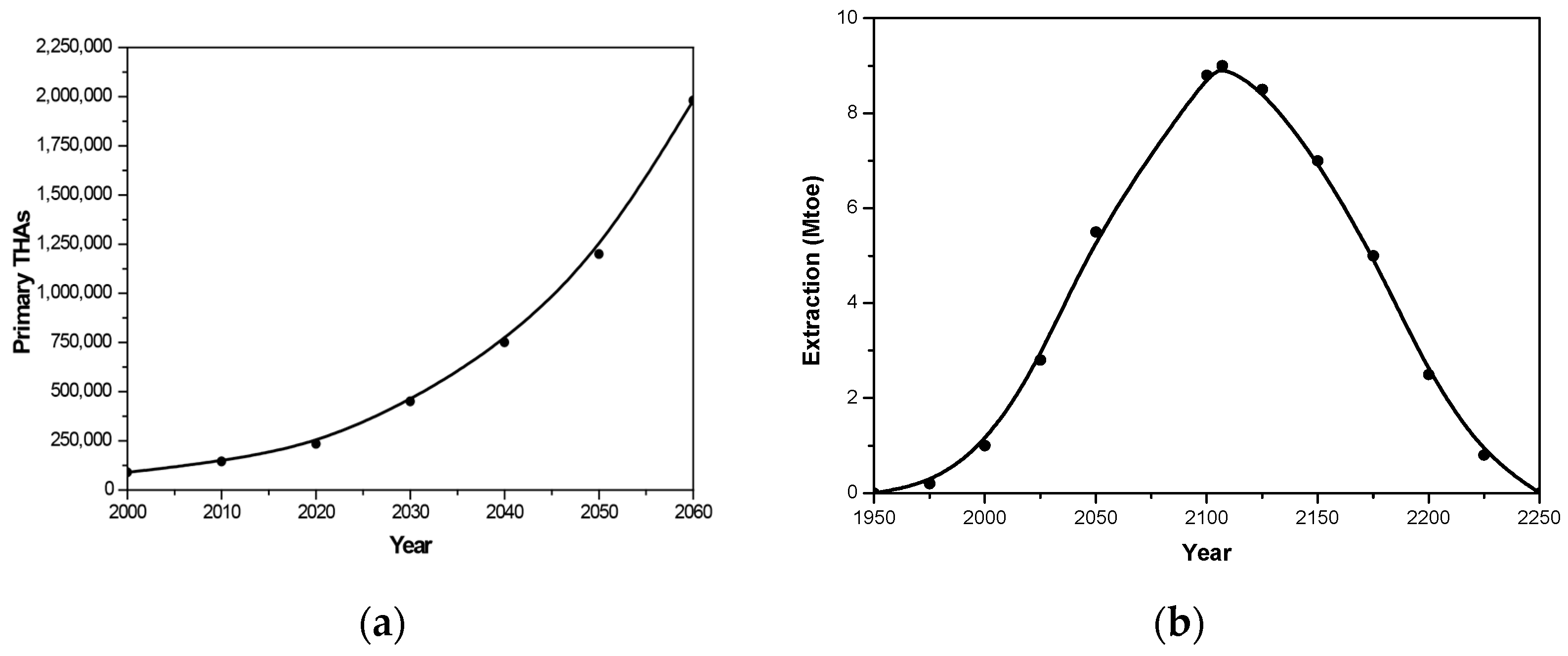
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Culliford, D.; Maskell, J.; Judge, A.; Cooper, C.; Prieto-Alhambra, D.; Arden, N.K. Future projections of total hip and knee arthroplasty in the UK: Results from the UK Clinical Practice Research Datalink. Osteoarthr. Cartil. 2015, 23, 594–600. [Google Scholar] [CrossRef]
- Shichman, I.; Roof, M.; Askew, N.; Nherera, L.; Rozell, J.C.; Seyler, T.M.; Schwarzkopf, R. Projections and epidemiology of primary hip and knee arthroplasty in medicare patients to 2040-2060. JB JS Open Access 2023, 8, e22. [Google Scholar] [CrossRef]
- Singh, J.A.; Yu, S.; Chen, L.; Cleveland, J.D. Rates of total joint replacement in the United States: Future projections to 2020–2040 using the national inpatient sample. J. Rheumatol. 2019, 46, 1134–1140. [Google Scholar] [CrossRef]
- Ackerman, I.N.; Bohensky, M.A.; Zomer, E.; Tacey, M.; Gorelik, A.; Brand, C.A.; de Steiger, R. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet. Disord. 2019, 20, 90. [Google Scholar] [CrossRef]
- Clement, N.D.; Scott, C.E.H.; Murray, J.R.D.; Howie, C.R.; Deehan, D.J. The number of patients “worse than death” while waiting for a hip or knee arthroplasty has nearly doubled during the COVID-19 pandemic. Bone Jt. J. 2021, 103-B, 672–680. [Google Scholar] [CrossRef]
- Farrow, L.; Gardner, W.T.; Tang, C.C.; Low, R.; Forget, P.; Ashcroft, G.P. Impact of COVID-19 on opioid use in those awaiting hip and knee arthroplasty: A retrospective cohort study. BMJ Qual. Saf. 2023, 32, 479–484. [Google Scholar] [CrossRef]
- Jabbal, M.; Burt, J.; Clarke, J.; Moran, M.; Walmsley, P.; Jenkins, P.J. Trends in incidence and average waiting time for arthroplasty from 1998-2021: An observational study of 282,367 patients from the Scottish arthroplasty project. Ann. R. Coll. Surg. Engl. 2023, 106, 249–255. [Google Scholar] [CrossRef]
- Sniderman, J.; Abdeen, A. The impact of the COVID-19 pandemic on the practice of hip and knee arthroplasty. JBJS Rev. 2023, 11, e23.00095. [Google Scholar] [CrossRef]
- French, J.M.R.; Deere, K.; Jones, T.; Pegg, D.J.; Reed, M.R.; Whitehouse, M.R.; Sayers, A. An analysis of the effect of the COVID-19-induced joint replacement deficit in England, Wales, and Northern Ireland suggests recovery will be protracted. Bone Jt. J. 2024, 106-B, 834–841. [Google Scholar] [CrossRef]
- Huynh, C.; Puyraimond-Zemmour, D.; Maillefert, J.F.; Conaghan, P.G.; Davis, A.M.; Gunther, K.P.; Hawker, G.; Hochberg, M.C.; Kloppenburg, M.; Lim, K.; et al. Factors associated with the orthopaedic surgeon’s decision to recommend total joint replacement in hip and knee osteoarthritis: An international cross-sectional study of 1905 patients. Osteoarthr. Cartil. 2018, 26, 1311–1318. [Google Scholar] [CrossRef]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in brief: Kellgren-Lawrence classification of osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef]
- Orchard, J.W.; Tutt, L.E.; Hines, A.; Orchard, J.J. Associations between common hip and knee osteoarthritis treatments and all-cause mortality. Healthcare 2025, 13, 2229. [Google Scholar] [CrossRef]
- Lee, K.; Goodman, S.B. Current state and future of joint replacements in the hip and knee. Expert Rev. Med. Devices 2008, 5, 383–393. [Google Scholar] [CrossRef]
- Lo, Y.C.; Chen, Y.P.; Lin, H.E.; Chang, W.C.; Ho, W.P.; Jang, J.P.; Kuo, Y.J. Factors associated with decisional regret after shared decision making for patients undergoing total knee arthroplasty. Healthcare 2025, 13, 1597. [Google Scholar] [CrossRef]
- Gunaratne, R.; Pratt, D.N.; Banda, J.; Fick, D.P.; Khan, R.J.K.; Robertson, B.W. Patient dissatisfaction following total knee arthroplasty: A systematic review of the literature. J. Arthroplast. 2017, 32, 3854–3860. [Google Scholar] [CrossRef]
- Bourne, R.B.; Chesworth, B.M.; Davis, A.M.; Mahomed, N.N.; Charron, K.D. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin. Orthop. Relat. Res. 2010, 468, 57–63. [Google Scholar] [CrossRef]
- Cronström, A.; Dahlberg, L.E.; Nero, H.; Hammarlund, C.S. “I was considering surgery because I believed that was how it was treated”: A qualitative study on willingness for joint surgery after completion of a digital management program for osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1026–1032. [Google Scholar] [CrossRef]
- United Nations. The Sustainable Development Goals Report 2023, Special ed.; Towards a Rescue Plan for People and Planet; United Nations Publications: New York, NY, USA, 2023; Available online: https://www.un.org/sustainabledevelopment/health/ (accessed on 1 June 2024).
- IEA. Net Zero by 2050; IEA: Paris, France, 2021; Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 1 June 2024).
- Delaie, C.; Cerlier, A.; Argenson, J.N.; Escudier, J.C.; Khakha, R.; Flecher, X.; Jacquet, C.; Ollivier, M. Ecological burden of modern surgery: An analysis of total knee replacement’s life cycle. Arthroplast. Today 2023, 23, 101187. [Google Scholar] [CrossRef]
- IPCC, 2018: Summary for Policymakers; Cambridge University Press: Cambridge, UK; New York, NY, USA; pp. 3–24. [CrossRef]
- Eggert, R.G. Minerals go critical. Nat. Chem. 2011, 3, 688–691. [Google Scholar] [CrossRef]
- Raabe, D. The materials science behind sustainable metals and alloys. Chem. Rev. 2023, 123, 2436–2608. [Google Scholar] [CrossRef]
- USGS. Mineral Commodity Summaries 2025; U.S. Department of the Interior; U.S. Geological Survey: Reston, VA, USA, 2025.
- Moradlou, H.; Reefke, H.; Skipworth, H.; Roscoe, S. Geopolitical disruptions and the manufacturing location decision in multinational company supply chains: A Delphi study on Brexit. Int. J. Oper. Prod. Manag. 2021, 41, 102–130. [Google Scholar] [CrossRef]
- Gulley, A.L. China, the Democratic Republic of the Congo, and artisanal cobalt mining from 2000 through 2020. Proc. Natl. Acad. Sci. USA 2023, 120, e2212037120. [Google Scholar] [CrossRef]
- Nze-Ekpebie, R.A. Global supply chain effects on medical devices. J. Healthc. Commun. 2023, 8, 170–182. [Google Scholar] [CrossRef]
- Watari, T.; Nansai, K.; Nakajima, K. Review of critical metal dynamics to 2050 for 48 elements. Resour. Conserv. Recycl. 2020, 155, 104669. [Google Scholar] [CrossRef]
- Bhaskar, S.; Tan, J.; Bogers, M.; Minssen, T.; Badaruddin, H.; Israeli-Korn, S.; Chesbrough, H. At the epicenter of COVID-19—the tragic failure of the global supply chain for medical supplies. Front. Public Health 2020, 8, 562882. [Google Scholar] [CrossRef]
- National Strategy for a Resilient Public Health Supply Chain; U.S. Department of Health and Human Services: Washington, DC, USA, 2021. Available online: https://www.aeaweb.org/forum/2616/national-strategy-resilient-implementation-measurement (accessed on 21 July 2025).
- International Energy Agency. The Role of Critical Minerals in Clean Energy Transitions; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Energy Transitions Commission. Material and Resource Requirements for the Energy Transition; Energy Transitions Commission: London, UK, 2023. [Google Scholar]
- Hudak, P.L.; Clark, S.J.; Raymond, G. The omni-relevance of surgery: How medical specialization shapes orthopedic surgeons’ treatment recommendations. Health Commun. 2013, 28, 533–545. [Google Scholar] [CrossRef]
- Quintana, J.M.; Escobar, A.; Arostegui, I.; Bilbao, A.; Azkarate, J.; Goenaga, J.I.; Arenaza, J.C. Health-related quality of life and appropriateness of knee or hip joint replacement. JAMA Intern. Med. 2006, 166, 220–226. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Milosev, I.; Trebse, R.; Rantanen, P.; Linden, R.; Tiainen, V.M.; Virtanen, S.; Revell, P.A. 6—Metals for joint replacement. In Joint Replacement Technology; Woodhead Publishing: Southstone, UK, 2008; pp. 115–162. [Google Scholar]
- Merola, M.; Affatato, S. Materials for hip prostheses: A review of wear and loading considerations. Materials 2019, 12, 495. [Google Scholar] [CrossRef]
- Szczęsny, G.; Kopec, M.; Politis, D.J.; Kowalewski, Z.L.; Łazarski, A.; Szolc, T. A review on biomaterials for orthopaedic surgery and traumatology: From past to present. Materials 2022, 15, 3622. [Google Scholar] [CrossRef]
- Ratti, M.; Ceriotti, D.; Rescinito, R.; Bibi, R.; Panella, M. Does robotic assisted technique improve patient utility in total knee arthroplasty? A comparative retrospective cohort study. Healthcare 2024, 12, 1650. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Calvo, G.; Valero, A.; Valero, A. Assessing maximum production peak and resource availability of non-fuel mineral resources: Analyzing the influence of extractable global resources. Resour. Conserv. Recycl. 2017, 125, 208–217. [Google Scholar] [CrossRef]
- OECD. Geographic Variations in Health Care: What Do We Know and What Can Be Done to Improve Health System Performance? OECD Health Policy Studies; OECD Publishing: Paris, France, 2014. [Google Scholar]
- Jennison, T.; MacGregor, A.; Goldberg, A. Hip arthroplasty practice across the Organisation for Economic Co-operation and Development (OECD) over the last decade. Ann. R. Coll. Surg. Engl. 2023, 105, 645–652. [Google Scholar] [CrossRef]
- Lübbeke, A.; Silman, A.J.; Barea, C.; Prieto-Alhambra, D.; Carr, A.J. Mapping existing hip and knee replacement registries in Europe. Health Policy 2018, 122, 548–557. [Google Scholar] [CrossRef]
- Rupp, M.; Lau, E.; Kurtz, S.M.; Alt, V. Projections of primary TKA and THA in Germany from 2016 through 2040. Clin. Orthop. Relat. Res. 2020, 478, 1622–1633. [Google Scholar] [CrossRef]
- Matharu, G.S.; Culliford, D.J.; Blom, A.W.; Judge, A. Projections for primary hip and knee replacement surgery up to the year 2060: An analysis based on data from The National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. Ann. R. Coll. Surg. Engl. 2022, 104, 443–448. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, R.; Liu, D.; Cheng, Y.; Sun, Y.; Huang, Z. Epidemiological trends in burden of osteoarthritis in China: An analysis from 1990 to 2021 with forecasts for 2022-2050. Front. Public Health 2025, 13, 1612596. [Google Scholar] [CrossRef]
- Feng, B.; Zhu, W.; Bian, Y.Y.; Chang, X.; Cheng, K.Y.; Weng, X.S. China artificial joint annual data report. Chin. Med. J. 2020, 134, 752–753. [Google Scholar] [CrossRef]
- Vaidya, S.V.; Jogani, A.D.; Pachore, J.A.; Armstrong, R.; Vaidya, C.S. India joining the world of hip and knee registries: Present status—a leap forward. Indian J. Orthop. 2020, 55, 46–55. [Google Scholar] [CrossRef]
- Davies, P.S.; Graham, S.M.; Maqungo, S.; Harrison, W.J. Total joint replacement in sub-Saharan Africa: A systematic review. Trop. Doct. 2019, 49, 120–128. [Google Scholar] [PubMed]
- Laubscher, K.; Dey, R.; Nortje, M.; Held, M.; Kauta, N. Primary hip and knee arthroplasty at district level is safe and may reduce the burden on tertiary care in a low-income setting. BMC Musculoskelet. Disord. 2022, 23, 1014. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Y.; Wong, M.S.; Humbyrd, C.J. Eligibility criteria for lower extremity joint replacement may worsen racial and socioeconomic disparities. Clin. Orthop. Relat. Res. 2018, 476, 2301–2308. [Google Scholar] [CrossRef]
- Thirukumaran, C.P.; Cai, X.; Glance, L.G.; Kim, Y.; Ricciardi, B.F.; Fiscella, K.A.; Li, Y. Geographic variation and disparities in total joint replacement Use for Medicare beneficiaries: 2009 to 2017. J. Bone Jt. Surg. Am. 2020, 102, 2120–2128. [Google Scholar] [CrossRef]
- Thirukumaran, C.P.; Kim, Y.; Cai, X.; Ricciardi, B.F.; Li, Y.; Fiscella, K.A.; Mesfin, A.; Glance, L.G. Association of the comprehensive care for joint replacement model with disparities in the use of total hip and total knee replacement. JAMA Netw. Open 2021, 4, e2111858. [Google Scholar] [CrossRef]
- Goodman, S.M.; Mannstadt, I.; Gibbons, J.A.B.; Rajan, M.; Bass, A.; Russell, L.; Mehta, B.; Figgie, M.; Parks, M.L.; Venkatachalam, S.; et al. Healthcare disparities: Patients’ perspectives on barriers to joint replacement. BMC Musculoskelet Disord. 2023, 24, 976. [Google Scholar] [CrossRef]
- Amen, T.B.; Liimakka, A.P.; Jain, B.; Rudisill, S.S.; Bedair, H.S.; Chen, A.F. Total joint arthroplasty utilization after orthopaedic surgery referral: Identifying disparities along the care pathway. J. Arthroplast. 2023, 38, 424–430. [Google Scholar] [CrossRef]
- Malchau, H.; Bragdon, C.R.; Muratoglu, O.K. The stepwise introduction of innovation into orthopedic surgery: The next level of dilemmas. J. Arthroplast. 2011, 26, 825–831. [Google Scholar] [CrossRef]
- Inabathula, A.; Semerdzhiev, D.I.; Srinivasan, A.; Amirouche, F.; Puri, L.; Piponov, H. Robots on the stage: A snapshot of the American robotic total knee arthroplasty market. JB JS Open Access 2024, 9, e24. [Google Scholar] [CrossRef]
- Wu, H.; Yao, S.; Bao, H.; Guo, Y.; Xu, C.; Ma, J. ChatGPT-4.0 and DeepSeek-R1 does not yet provide clinically supported answers for knee osteoarthritis. Knee 2025, 56, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Barakat, N.; Ramamurti, P.; Duensing, I.M.; Browne, J.A. Financial conflicts of interest and industry funding are associated with conclusions favorable to new technologies: A review of published economic analyses in hip and knee arthroplasty. J. Arthroplast. 2024, 39, S299–S305.e299. [Google Scholar] [CrossRef]
- Peters, R.M.; Ten Have, B.; Rykov, K.; Van Steenbergen, L.; Putter, H.; Rutgers, M.; Vos, S.; Van Steijnen, B.; Poolman, R.W.; Vehmeijer, S.B.W.; et al. The learning curve of the direct anterior approach is 100 cases: An analysis based on 15,875 total hip arthroplasties in the Dutch Arthroplasty Register. Acta Orthop. 2022, 93, 775–782. [Google Scholar] [CrossRef]
- Sarpong, N.O.; Herndon, C.L.; Held, M.B.; Neuwirth, A.L.; Hickernell, T.R.; Geller, J.A.; Cooper, H.J.; Shah, R.P. What is the learning curve for new technologies in total joint arthroplasty? A review. Curr. Rev. Musculoskelet. Med. 2020, 13, 675–679. [Google Scholar] [CrossRef]
- Ravi, B.; Jenkinson, R.; Austin, P.C.; Croxford, R.; Wasserstein, D.; Escott, B.; Paterson, J.M.; Kreder, H.; Hawker, G.A. Relation between surgeon volume and risk of complications after total hip arthroplasty: Propensity score matched cohort study. BMJ 2014, 348, g3284. [Google Scholar] [CrossRef]
- Patel, R.V.; Gonzalez, M.R.; Attaar, N.; Patel, M.V.; Lozano-Calderon, S.A. Analyzing orthopaedic workforce trends in an ever-changing landscape. J. Am. Acad. Orthop. Surg. 2025, 33, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Rullán, P.J.; Deren, M.E.; Zhou, G.; Emara, A.K.; Klika, A.K.; Schiltz, N.K.; Barsoum, W.K.; Koroukian, S.; Piuzzi, N.S. The arthroplasty surgeon growth indicator: A tool for monitoring supply and demand trends in the orthopaedic surgeon workforce from 2020 to 2050. J. Bone Jt. Surg. Am. 2023, 105, 1038–1045. [Google Scholar] [CrossRef]
- Cheng, H.Y.; Beswick, A.D.; Bertram, W.; Siddiqui, M.A.; Gooberman-Hill, R.; Whitehouse, M.R.; Wylde, V. What proportion of people have long-term pain after total hip or knee replacement? An update of a systematic review and meta-analysis. BMJ Open 2025, 15, e088975. [Google Scholar] [CrossRef]
- Ayers, D.C.; Yousef, M.; Zheng, H.; Yang, W.; Franklin, P.D. The prevalence and predictors of patient dissatisfaction 5-years following primary total knee arthroplasty. J. Arthroplast. 2022, 37, S121–S128. [Google Scholar] [CrossRef]
- Ames, S.E.; Cowan, J.B.; Kenter, K.; Emery, S.; Halsey, D. Burnout in orthopaedic surgeons: A challenge for leaders, learners, and colleagues: AOA critical issues. J. Bone Jt. Surg. Am. 2017, 99, e78. [Google Scholar] [CrossRef] [PubMed]
- Mihcin, S.; Sahin, A.M.; Yilmaz, M.; Alpkaya, A.T.; Tuna, M.; Akdeniz, S.; Korkmaz, N.C.; Tosun, A.; Sahin, S. Database covering the prayer movements which were not available previously. Sci. Data 2023, 10, 276. [Google Scholar] [CrossRef]
- Sorenson, C.; Drummond, M. Improving medical device regulation: The United States and Europe in perspective. Milbank. Q. 2014, 92, 114–150. [Google Scholar] [CrossRef] [PubMed]
- Graedel, T.E.; Harper, E.M.; Nassar, N.T.; Reck, B.K. On the materials basis of modern society. Proc. Natl. Acad. Sci. USA 2015, 112, 6295–6300. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Valero, A.; Calvo, G.; Ortego, A. Material bottlenecks in the future development of green technologies. Renew. Sustain. Energy Rev. 2018, 93, 178–200. [Google Scholar] [CrossRef]
- Rhodes, C.J. Endangered elements, critical raw materials and conflict minerals. Sci. Prog. 2019, 102, 304–350. [Google Scholar] [CrossRef]
- Girtan, M.; Wittenberg, A.; Grilli, M.L.; de Oliveira, D.P.S.; Giosue, C.; Ruello, M.L. The critical raw materials issue between scarcity, supply risk, and unique properties. Materials 2021, 14, 1826. [Google Scholar] [CrossRef] [PubMed]
- Murphy, L.B.; Cisternas, M.G.; Theis, K.A.; Brady, T.J.; Bohm, M.K.; Guglielmo, D.; Hootman, J.M.; Barbour, K.E.; Boring, M.A.; Helmick, C.G. All-cause opioid prescriptions dispensed: The outsized role of adults with arthritis. Am. J. Prev. Med. 2020, 59, 355–366. [Google Scholar] [CrossRef]
- Dowell, D.; Ragan, K.R.; Jones, C.M.; Baldwin, G.T.; Chou, R. CDC clinical practice guideline for prescribing opioids for pain — United States. MMWR Recomm. Rep. 2022, 71, 1–95. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, P.; Jha, R. COVID-19: Impact on health supply chain and lessons to be learnt. J. Health Man. 2020, 22, 248–261. [Google Scholar] [CrossRef]
- Emanuel, E.J.; Persad, G. The shared ethical framework to allocate scarce medical resources: A lesson from COVID-19. Lancet 2023, 401, 1892–1902. [Google Scholar] [CrossRef]
- OECD. Ready for the Next Crisis? Investing in Health System Resilience; OECD Health Policy Studies; OECD Publishing: Paris, France, 2023. [Google Scholar]
- OECD. In Securing Medical Supply Chains in a Post-Pandemic World; OECD (2024) Health Policy Studies; OECD Publishing: Paris, France, 2024. [CrossRef]
- OECD. The Supply of Critical Raw Materials Endangered by Russia’s War on Ukraine; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Hurst, J.; Siciliani, L. Tackling Excessive Waiting Times for Elective Surgery: A Comparison of Policies in Twelve OECD Countries; OECD Health Working Papers, No. 6; OECD Publishing: Paris, France, 2003. [Google Scholar]
- Porter, G.M.; Balian, J.; Ng, A.P.; Mannings, H.; Jeffcoat, D.M.; Benharash, P. Cost-volume analysis of primary total knee and hip arthroplasty in the United States. J. Arthroplast. 2025, 40, 2259–2267.e1. [Google Scholar] [CrossRef]
- Hannon, C.P.; Goodman, S.M.; Austin, M.S.; Yates, A., Jr.; Guyatt, G.; Aggarwal, V.K.; Baker, J.F.; Bass, P.; Bekele, D.I.; Dass, D.; et al. 2023 American College of Rheumatology and American Association of Hip and Knee Surgeons Clinical Practice Guideline for the optimal timing of elective hip or knee arthroplasty for patients with symptomatic moderate-to-severe osteoarthritis or advanced symptomatic osteonecrosis with secondary arthritis for whom nonoperative therapy is ineffective. Arthritis Care Res. 2023, 75, 2227–2238. [Google Scholar]
- Valero, A.; Valero, A. Thermodynamic rarity and recyclability of raw materials in the energy transition: The need for an in-spiral economy. Entropy 2019, 21, 873. [Google Scholar] [CrossRef]
- Sarfraz, S.; Mäntynen, P.H.; Laurila, M.; Rossi, S.; Leikola, J.; Kaakinen, M.; Suojanen, J.; Reunanen, J. Comparison of titanium and PEEK medical plastic implant materials for their bacterial biofilm formation properties. Polymers 2022, 14, 3862. [Google Scholar] [CrossRef] [PubMed]
- Al-Shalawi, F.D.; Mohamed Ariff, A.H.; Jung, D.W.; Mohd Ariffin, M.K.A.; Seng Kim, C.L.; Brabazon, D.; Al-Osaimi, M.O. Biomaterials as implants in the orthopedic field for regenerative medicine: Metal versus synthetic polymers. Polymers 2023, 15, 2601. [Google Scholar] [CrossRef] [PubMed]
- Said, A.I.; Patricia, D.F. Race and elective joint replacement: Where a disparity meets patient preference. Am. J. Public Health 2013, 103, 583–584. [Google Scholar] [CrossRef]
- Arcaya, M.C.; Figueroa, J.F. Emerging trends could exacerbate health inequities in the United States. Health Aff. 2017, 36, 992–998. [Google Scholar] [CrossRef]
- Cogburn, C.D. Culture, race, and health: Implications for racial inequities and population health. Milbank. Q. 2019, 97, 736–761. [Google Scholar] [CrossRef]
- Faison, W.E.; Harrell, P.G.; Semel, D. Disparities across diverse populations in the health and treatment of patients with osteoarthritis. Healthcare 2021, 9, 1421. [Google Scholar] [CrossRef]
- Cullen, M.R.; Lemeshow, A.R.; Russo, L.J.; Barnes, D.M.; Ababio, Y.; Habtezion, A. Disease-specific health disparities: A targeted review focusing on race and ethnicity. Healthcare 2022, 10, 603. [Google Scholar] [CrossRef]
- Humphreys, P.; Spratt, B.; Tariverdi, M.; Burdett, R.L.; Cook, D.; Yarlagadda, P.; Corry, P. An overview of hospital capacity planning and optimisation. Healthcare 2022, 10, 826. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.F.; Ambrose, K.R.; Albright, A.L.; Altpeter, M.; Golightly, Y.M.; Huffman, K.F.; Nelson, A.E.; Weisner, S.E. Public Health Interventions for Osteoarthritis - updates on the Osteoarthritis Action Alliance’s efforts to address the 2010 OA Public Health Agenda Recommendations. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 31–39. [Google Scholar] [PubMed]
- Jinks, C.; Botto-van Bemden, A.; Bunzli, S.; Bowden, J.; Egerton, T.; Eyles, J.; Foster, N.; Healey, E.L.; Maddison, J.; O’Brien, D.; et al. Changing the narrative on osteoarthritis: A call for global action. Osteoarthr. Cartil. 2024, 32, 414–420. [Google Scholar] [CrossRef]
- Nguyen, A.; Lee, P.; Rodriguez, E.K.; Chahal, K.; Freedman, B.R.; Nazarian, A. Addressing the growing burden of musculoskeletal diseases in the ageing US population: Challenges and innovations. Lancet Healthy Longev. 2025, 6, 100707. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.D.; Dieppe, P.; Radin, E.L. Etiopathogenesis of osteoarthritis. Rheum. Dis. Clin. N. Am. 2008, 34, 531–559. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Goldring, S.R. The role of synovitis in osteoarthritis pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef]
- Houard, X.; Goldring, M.B.; Berenbaum, F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 2013, 15, 375. [Google Scholar] [CrossRef]
- Chu, C.R.; Andriacchi, T.P. Dance between biology, mechanics, and structure: A systems-based approach to developing osteoarthritis prevention strategies. J. Orthop. Res. 2015, 33, 939–947. [Google Scholar] [CrossRef]
- Whittaker, J.L.; Runhaar, J.; Bierma-Zeinstra, S.; Roos, E.M. A lifespan approach to osteoarthritis prevention. Osteoarthr. Cartil. 2021, 29, 1638–1653. [Google Scholar] [CrossRef]
- Woolf, A.D. The bone and joint decade 2000-2010. Ann. Rheum. Dis. 2000, 59, 81–82. [Google Scholar] [CrossRef]
- Lidgren, L. The bone and joint decade 2000-2010. Bull. World Health Organ. 2003, 81, 629. [Google Scholar]
- Osteoarthritis Research Society International (OARSI). Osteoarthritis: A Serious Disease; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2016. [Google Scholar]
- Young, A.; Flower, L. Patients as partners, patients as problem-solvers. Health Commun. 2002, 14, 69–97. [Google Scholar] [CrossRef]
- Foote, S.B.; Blewett, L.A. Politics of prevention: Expanding prevention benefits in the Medicare program. J. Public Health Pol. 2003, 24, 26–40. [Google Scholar] [CrossRef]
- Dumay, A.C.M.; Blank, J.L.T. Healthcare prosumerism. In Future Visions on Biomedicine and Bioinformatics 1: A Liber Amicorum in Memory of Swamy Laxminarayan; Bos, L., Carroll, D., Kun, L., Marsh, A., Roa, L.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 43–52. [Google Scholar]
- de Melo, L.R.S.; Hunter, D.; Fortington, L.; Peeters, A.; Seward, H.; Vertullo, C.; Hills, A.P.; Brown, W.; Feng, Y.; Lloyd, D.G. National Osteoarthritis Strategy brief report: Prevention of osteoarthritis. Aust. J. Gen. Pract. 2020, 49, 272–275. [Google Scholar] [CrossRef]
- Ambrose, K.R.; Huffman, K.F.; Odom, E.L.; Foster, A.L.; Turkas, N.; Callahan, L.F. A public health approach to osteoarthritis in the United States. Osteoarthr. Cartil. 2024, 32, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F.; Wallace, I.J.; Lieberman, D.E.; Felson, D.T. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2018, 14, 674–681. [Google Scholar] [CrossRef]
- Noto, S. Perspectives on aging and quality of life. Healthcare 2023, 11, 2131. [Google Scholar] [CrossRef] [PubMed]
- del Río, E. Thick or thin? Implications of cartilage architecture for osteoarthritis risk in sedentary lifestyles. Biomedicines 2025, 13, 1650. [Google Scholar] [CrossRef]
- Bircher, J.; Kuruvilla, S. Defining health by addressing individual, social, and environmental determinants: New opportunities for health care and public health. J. Public Health Pol. 2014, 35, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Katz, D.L. Disease prevention and health promotion: How integrative medicine fits. Am. J. Prev. Med. 2015, 49, S230–S240. [Google Scholar] [CrossRef]
- Batalden, M.; Batalden, P.; Margolis, P.; Seid, M.; Armstrong, G.; Opipari-Arrigan, L.; Hartung, H. Coproduction of healthcare service. BMJ Qual. Saf. 2016, 25, 509–517. [Google Scholar] [CrossRef]
- Chauvin, J.; Perera, Y.; Clarke, M. Digital technologies for population health and health equity gains: The perspective of public health associations. J. Public Health Pol. 2016, 37, 232–248. [Google Scholar] [CrossRef][Green Version]
- Russo, G.; Moretta Tartaglione, A.; Cavacece, Y. Empowering patients to co-create a sustainable healthcare value. Sustainability 2018, 11, 1315. [Google Scholar] [CrossRef]
- Andriacchi, T.P.; Griffin, T.M.; Loeser, R.F.; Chu, C.R.; Roos, E.M.; Hawker, G.A.; Erhart-Hledik, J.C.; Fischer, A.G. Bridging Disciplines as a pathway to Finding New Solutions for Osteoarthritis a collaborative program presented at the 2019 Orthopaedic Research Society and the Osteoarthritis Research Society International. Osteoarthr. Cartil. Open 2020, 2, 100026. [Google Scholar] [CrossRef]
- Goulbourne, T.; Yanovitzky, I. The communication infrastructure as a social determinant of health: Implications for health policymaking and practice. Milbank. Q. 2021, 99, 24–40. [Google Scholar] [CrossRef]
- González-Cacheda, B.; Outeda, C.C. Understanding attitudes, knowledge, and use of e-health services in the health system in Spain. J. Public Health Pol. 2025, 46, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, A.J.; Gray, B.; Wallis, J.A.; Taylor, N.F.; Kemp, J.L.; Hunter, D.J.; Barton, C.J. Recommendations for the management of hip and knee osteoarthritis: A systematic review of clinical practice guidelines. Osteoarthr. Cartil. 2023, 31, 1280–1292. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Kramer, W.C.; Hendricks, K.J.; Wang, J. Pathogenetic mechanisms of posttraumatic osteoarthritis: Opportunities for early intervention. Int. J. Clin. Exp. Med. 2011, 4, 285–298. [Google Scholar]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, O.; Cooper, C.; Pelletier, J.P.; Maheu, E.; Rannou, F.; Branco, J.; Luisa Brandi, M.; Kanis, J.A.; Altman, R.D.; Hochberg, M.C.; et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-From evidence-based medicine to the real-life setting. Semin. Arthritis Rheum. 2016, 45, S3–S11. [Google Scholar] [CrossRef]
- Watt, F.E.; Corp, N.; Kingsbury, S.R.; Frobell, R.; Englund, M.; Felson, D.T.; Levesque, M.; Majumdar, S.; Wilson, C.; Beard, D.J.; et al. Towards prevention of post-traumatic osteoarthritis: Report from an international expert working group on considerations for the design and conduct of interventional studies following acute knee injury. Osteoarthr. Cartil. 2019, 27, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Petrigna, L.; Roggio, F.; Trovato, B.; Zanghi, M.; Guglielmino, C.; Musumeci, G. How physical activity affects knee cartilage and a standard intervention procedure for an exercise program: A systematic review. Healthcare 2022, 10, 1821. [Google Scholar] [CrossRef] [PubMed]
- del Río, E.; Vergés, J. Exploring the influence of physical activity on the efficacy of chondroprotective agents for osteoarthritis: The role of diffusion conditions. Med. Hypotheses 2024, 182, 111244. [Google Scholar] [CrossRef]
- del Río, E. Rethinking osteoarthritis management: Synergistic effects of chronoexercise, circadian rhythm, and chondroprotective agents. Biomedicines 2025, 13, 598. [Google Scholar] [CrossRef]
- Editorial. A national health program for the United States: The need for a citizens coalition. J. Public Health Pol. 1984, 5, 10–17. [Google Scholar] [CrossRef]
- Editorial. The role of medical schools in the second epidemiologic revolution. J. Public Health Pol. 1987, 8, 148–150. [Google Scholar] [CrossRef]
- Baker, E.L.; Melius, J.M.; Millar, J.D. Surveillance of occupational illness and injury in the United States: Current perspectives and future directions. J. Public Health Pol. 1988, 9, 198–221. [Google Scholar] [CrossRef]
- Griffin, L.Y.; Albohm, M.J.; Arendt, E.A.; Bahr, R.; Beynnon, B.D.; Demaio, M.; Dick, R.W.; Engebretsen, L.; Garrett, W.E., Jr.; Hannafin, J.A.; et al. Understanding and preventing noncontact anterior cruciate ligament injuries: A review of the Hunt Valley II meeting, January 2005. Am. J. Sports Med. 2006, 34, 1512–1532. [Google Scholar] [CrossRef]
- Bart, K.; Wilma, J.N.; Caspar, W.L.; Johan, P.M. Educational disparities in the burden of disability: Contributions of disease prevalence and disabling impact. Am. J. Public Health 2014, 104, e141–e148. [Google Scholar] [CrossRef]
- Chauvin, J.; Pauls, J.; Strobl, L. Building codes: An often overlooked determinant of health. J. Public Health Pol. 2016, 37, 136–148. [Google Scholar] [CrossRef]
- Cylus, J.; Papanicolas, I.; Smith, P.C. Health System Efficiency: How to Make Measurement Matter for Policy and Management; European Observatory on Health Systems and Policies: Copenhagen, Denmark, 2016. [Google Scholar]
- Sakellariou, G.; Conaghan, P.G.; Zhang, W.; Bijlsma, J.W.J.; Boyesen, P.; D’Agostino, M.A.; Doherty, M.; Fodor, D.; Kloppenburg, M.; Miese, F.; et al. EULAR recommendations for the use of imaging in the clinical management of peripheral joint osteoarthritis. Ann. Rheum. Dis. 2017, 76, 1484–1494. [Google Scholar] [CrossRef]
- Fries, J.F. The compression of morbidity. Milbank. Q. 2005, 83, 801–823. [Google Scholar] [CrossRef]
- Iolascon, G.; Migliore, A.; Beretta, G.; Bernetti, A.; Bortolotti, R.; Celano, A.; Giarratano, A.; Marinangeli, F.; Momoli, A.; Sebastiani, G.D.; et al. Pain management in knee osteoarthritis: Insights from an exploratory online survey of Italian patients and physicians. Healthcare 2024, 12, 2077. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.N.; Arant, K.R.; Loeser, R.F. Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA 2021, 325, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Alsobhi, M.; Gmmash, A.; Aldhabi, R.; Almaddah, M.R.; Ameen, A.; Almotairi, F.; Basuodan, R.; Khan, F. Physical therapists’ attitudes, beliefs, and barriers regarding fall screening and prevention among patients with knee osteoarthritis: A cross-sectional study. Healthcare 2024, 12, 718. [Google Scholar] [CrossRef]
- Pijls, B.G. Technology assistance in primary total knee replacement: Hype or hope? Expert Rev. Med. Devices 2024, 21, 11–14. [Google Scholar] [CrossRef]
- Larson, H.J. The biggest pandemic risk? Viral misinformation. Nature 2018, 562, 309. [Google Scholar] [CrossRef] [PubMed]
- Mheidly, N.; Fares, J. Leveraging media and health communication strategies to overcome the COVID-19 infodemic. J. Public Health Pol. 2020, 41, 410–420. [Google Scholar] [CrossRef]
- Wainwright, T.W.; Burgess, L.C.; Immins, T.; Middleton, R.G. Self-management of hip osteoarthritis five years after a cycling and education treatment pathway. Healthcare 2020, 8, 37. [Google Scholar] [CrossRef]
- O’Connor, M.I. Osteoarthritis of the hip and knee: Sex and gender differences. Orthop. Clin. N. Am. 2006, 37, 559–568. [Google Scholar] [CrossRef]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef]
- del Río, E. A novel etiological approach for the development of knee osteoarthritis in sedentary adults. Med. Hypotheses 2024, 185, 111291. [Google Scholar] [CrossRef]
- Pineda-Escobar, S.; Matias-Soto, J.; García-Muñoz, C.; Martinez-Calderon, J. Protecting athletes: The clinical relevance of meta-analyses on injury prevention programs for sports and musculoskeletal body regions: An overview of systematic reviews with meta-analyses of randomized clinical trials. Healthcare 2025, 13, 1530. [Google Scholar] [CrossRef]
- Brown, T.D.; Johnston, R.C.; Saltzman, C.L.; Marsh, J.L.; Buckwalter, J.A. Posttraumatic osteoarthritis: A first estimate of incidence, prevalence, and burden of disease. J. Orthop. Trauma. 2006, 20, 739–744. [Google Scholar] [CrossRef]
- Thomas, A.C.; Hubbard-Turner, T.; Wikstrom, E.A.; Palmieri-Smith, R.M. Epidemiology of posttraumatic osteoarthritis. J. Athl. Train. 2017, 52, 491–496. [Google Scholar] [CrossRef]
- Robson, K.; Pope, R.; Orr, R. Incidence and risk factors for acute articular cartilage tears in military and other occupational settings: A systematic review. Healthcare 2024, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Shino, K.; Horibe, S.; Tanaka, Y.; Toritsuka, Y.; Nakamura, N.; Koyanagi, M.; Yoshikawa, H. Arthroscopic anterior cruciate ligament reconstruction using fresh-frozen bone plug-free allogeneic tendons: 10-year follow-up. Arthroscopy 2008, 24, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Sadoghi, P.; von Keudell, A.; Vavken, P. Effectiveness of anterior cruciate ligament injury prevention training programs. J. Bone Jt. Surg. Am. 2012, 94, 769–776. [Google Scholar] [CrossRef]
- Yubo, M.; Yanyan, L.; Li, L.; Tao, S.; Bo, L.; Lin, C. Clinical efficacy and safety of mesenchymal stem cell transplantation for osteoarthritis treatment: A meta-analysis. PLoS One 2017, 12, e0175449. [Google Scholar] [CrossRef]
- Bennell, K.L.; Hunter, D.J.; Paterson, K.L. Platelet-rich plasma for the management of hip and knee osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 24. [Google Scholar] [CrossRef]
- Bensa, A.; Bianco Prevot, L.; Moraca, G.; Sangiorgio, A.; Boffa, A.; Filardo, G. Corticosteroids, hyaluronic acid, platelet-rich plasma, and cell-based therapies for knee osteoarthritis - literature trends are shifting in the injectable treatments’ evidence: A systematic review and expert opinion. Expert Opin. Biol. Ther. 2025, 25, 309–318. [Google Scholar] [CrossRef]
- Moseng, T.; Vliet Vlieland, T.P.M.; Battista, S.; Beckwée, D.; Boyadzhieva, V.; Conaghan, P.G.; Costa, D.; Doherty, M.; Finney, A.G.; Georgiev, T.; et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis: 2023 update. Ann. Rheum. Dis. 2024, 83, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Grønne, D.T.; Roos, E.M.; Ibsen, R.; Kjellberg, J.; Skou, S.T. Cost-effectiveness of an 8-week supervised education and exercise therapy programme for knee and hip osteoarthritis: A pre-post analysis of 16 255 patients participating in Good Life with osteoArthritis in Denmark (GLA:D). BMJ Open 2021, 11, e049541. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Musculoskeletal Conditions: Fact Sheet. 2022. Geneva, Switzerland. Available online: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions (accessed on 20 July 2025).
- Walker, A.; Boaz, A.; Gibney, A.; Zambelli, Z.; Hurley, M.V. Scaling-up an evidence-based intervention for osteoarthritis in real-world settings: A pragmatic evaluation using the RE-AIM framework. Implement. Sci. Commun. 2020, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.; Ademi, Z.; Barton, C.; Wallis, J.A.; Harris, I.A.; de Steiger, R.; Buchbinder, R.; Brusco, N.; Young, K.; Pazzinatto, M.F.; et al. Lifetime cost-effectiveness of structured education and exercise therapy for knee osteoarthritis in Australia. JAMA Netw. Open 2024, 7, e2436715. [Google Scholar] [CrossRef]
- Hallab, N.; Merritt, K.; Jacobs, J.J. Metal sensitivity in patients with orthopaedic implants. J. Bone Jt. Surg. Am. 2001, 83, 428–436. [Google Scholar] [CrossRef]
- Gessner, B.D.; Steck, T.; Woelber, E.; Tower, S.S. A systematic review of systemic cobaltism after wear or corrosion of chrome-cobalt hip implants. J. Patient Saf. 2019, 15, 97–104. [Google Scholar] [CrossRef]
- Zhong, Q.; Pan, X.; Chen, Y.; Lian, Q.; Gao, J.; Xu, Y.; Wang, J.; Shi, Z.; Cheng, H. Prosthetic metals: Release, metabolism and toxicity. Int. J. Nanomed. 2024, 19, 5245–5267. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.T.; Mambu Vangu, A.; Balu Mabiala, H.; Bambi Mangungulu, H.; Tissingh, E.K. Toxicity in the supply chain: Cobalt, orthopaedics, and the Democratic Republic of the Congo. Lancet Planet Health 2021, 5, e327–e328. [Google Scholar] [CrossRef] [PubMed]
- Gorban, A.N.; Pokidysheva, L.I.; Smirnova, E.V.; Tyukina, T.A. Law of the minimum paradoxes. Bull. Math. Biol. 2011, 73, 2013–2044. [Google Scholar] [CrossRef]
- World Population Prospects 2022: Highlights (ST/ESA/SER.A/470); United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2022; Available online: https://population.un.org/wpp/ (accessed on 1 May 2024).
- Yahaya, I.; Wright, T.; Babatunde, O.O.; Corp, N.; Helliwell, T.; Dikomitis, L.; Mallen, C.D. Prevalence of osteoarthritis in lower middle- and low-income countries: A systematic review and meta-analysis. Rheumatol. Int. 2021, 41, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Howard, N.; Moffat, C.; Lubega, N.; Mkandawire, N.; Harrison, W.J. Total hip arthroplasty in a low-income country: Ten-year outcomes from the National Joint Registry of the Malawi Orthopaedic Association. JB JS Open Access 2019, 4, e0027. [Google Scholar] [CrossRef]
- Graham, S.M.; Moffat, C.; Lubega, N.; Mkandawire, N.; Burgess, D.; Harrison, W.J. Total knee arthroplasty in a low-income country: Short-term outcomes from a National Joint Registry. JB JS Open Access 2018, 3, e0029. [Google Scholar] [CrossRef]
- Watt, T.; Charlesworth, A.; Gershlick, B. Health and care spending and its value, past, present and future. Future Healthc. J. 2019, 6, 99–105. [Google Scholar] [CrossRef]
- Hartman, M.; Martin, A.B.; Whittle, L.; Catlin, A. National health care spending in 2022: Growth similar to prepandemic rates. Health Aff. 2024, 43, 6–17. [Google Scholar] [CrossRef]
| Grade | Description | Features |
|---|---|---|
| 0 | No OA | No osteophytes, normal joint space, no signs of sclerosis or bone deformity |
| 1 | Doubtful OA | Possible osteophytic lipping, joint space is normal or slightly decreased. No definite joint deformity or sclerosis |
| 2 | Mild OA | Definite osteophytes, appreciable joint space narrowing, no significant bone deformities |
| 3 | Moderate OA | Prominent osteophyte formation, marked joint-space narrowing, potential subchondral sclerosis (increased bone density), and subtle bony deformities |
| 4 | Severe OA | Large osteophytes, significant joint space narrowing, subchondral sclerosis, deformity of bones/joints, and cyst formation |
| Phase | Main Features | Indicative Timeframe and Uncertainty | Primary System-Level Risks |
|---|---|---|---|
| Early warning (critical stage) | Rapid exponential rise in primary TKA/THA volumes; rising peri-operative and rehabilitation demand; emerging short lead-time pressures on implant supply; localized capacity strain in operating rooms and recovery services | Short–medium term. Moderate uncertainty (driven by registry and administrative data trends) | Growing waitlists; regional access inequities; perioperative bottlenecks; localized procurement volatility; early workforce overload |
| System strain (financial non-viability) | Budget plateaus and diminishing marginal gains from efficiency; widening payer funding gaps; sustained supply-chain disruption; longer implant lead-times and routine backorders | Medium term. Higher uncertainty (sensitive to policy, reimbursement, and market dynamics) | Deferred or rationed care; increased out-of-pocket expenditure; cost shifting between payers; erosion of equity and quality of care; accelerated workforce attrition |
| Critical shortage (material constraints) | Severe CRM scarcity or major, sustained price shocks; geopolitical supply instability, logistical challenges and export restrictions; substantial reduction in the range of available implant models, leading to routine case delays and cancellations | Medium–long term. High uncertainty (contingent on global supply and markets, substitution/recycling success, and technological change) | Widespread case cancellations/delays; severe backlog accumulation; compromised clinical outcomes; amplified global inequities; disproportionate impact on LMICs and vulnerable populations; emergency procurement pressures and ethical allocation dilemmas |
| Policy Principles 1 | Description | Expected Outcomes |
|---|---|---|
| Patient-centered | Implement personalized care that prioritizes patient preferences and needs, ensuring active involvement in decision-making, and tailored rehabilitation programs involving a multidisciplinary team to restore function, strength, and mobility in OA patients | Enhanced patient satisfaction and treatment adherence, improved recovery rates, reduced disability, and better overall QoL |
| Risk reducing | Develop and enforce strategies for injury prevention in sports, workplaces, and road safety. Collaborate with urban planners to design supportive built environments aimed at minimizing the risk of falls and fractures | Reduced incidence of joint injuries and PTOA, improved joint health, and decreased need for surgical interventions such as TKA and THA |
| Equity, inclusiveness, accessibility | Ensure equitable access to OA prevention and treatment programs across all socioeconomic, racial/ethnic, and geographic groups | Reduced disparities in OA prevalence and care outcomes, leading to improved QoL for marginalized populations |
| Vigilant | Establish comprehensive surveillance systems for monitoring OA and PTOA, utilizing advanced data analytics for timely intervention | Early detection of OA trends and risk factors, enabling proactive management, better disease control, and reduced long-term healthcare costs |
| Evidence-based | Formulate policies and treatment guidelines based on robust scientific evidence. Regularly assess the economic impact and cost-effectiveness of interventions | Improved patient outcomes, enhanced policy effectiveness, optimized use of healthcare resources, and reduced costs associated with OA management |
| Nurturing health promotion | Launch public health initiatives that promote joint health through education, community programs, and lifestyle interventions. Focus on non-pharmacological strategies | Increased public awareness and adoption of healthy behaviors, leading to early detection and prevention of OA, improved joint function, and overall well-being within urban and rural communities |
| Transparency | Ensure clear communication of treatment options, risks, and benefits to individuals. Improve transparency in healthcare data sharing and clinician-patient interactions | Increased patient trust and satisfaction, informed decision-making, and enhanced effectiveness of public health policies and clinical practices in OA management |
| Level/Objective | Typical Timing/Target Population | Key Outcome Measures 1 | Linked Pillar(s) |
|---|---|---|---|
| Primary prevention/Prevent development of risk factors or first joint injury | Childhood, adolescence, early adulthood; general population and at-risk groups | Incidence of index injuries; prevalence of obesity; biomechanical measures; adherence/process metrics | Research (efficacy), policy (population programs), delivery (community/school implementation) |
| Secondary prevention/Modify risk factors or halt progression from early structural change to symptomatic OA | Immediately post-injury or when early structural changes detected; at-risk cohorts | Change in risk factor (BMI, strength); imaging surrogates (MRI compositional measures, cartilage T2); symptom scores; time-to-symptomatic OA | Research (surrogates), delivery (clinical pathways), policy (coverage) |
| Tertiary prevention/Reduce progression to advanced disease, disability, or surgery | Symptomatic OA with established disease; patients at risk of rapid progression | Time-to-joint replacement; pain/function PROMs; health-related QoL; healthcare utilization/costs | Delivery (care pathways), policy (access, financing), research (comparative effectiveness) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Río, E.d. 2050: An Arthroplasty Odyssey. Healthcare 2025, 13, 2730. https://doi.org/10.3390/healthcare13212730
Río Ed. 2050: An Arthroplasty Odyssey. Healthcare. 2025; 13(21):2730. https://doi.org/10.3390/healthcare13212730
Chicago/Turabian StyleRío, Eloy del. 2025. "2050: An Arthroplasty Odyssey" Healthcare 13, no. 21: 2730. https://doi.org/10.3390/healthcare13212730
APA StyleRío, E. d. (2025). 2050: An Arthroplasty Odyssey. Healthcare, 13(21), 2730. https://doi.org/10.3390/healthcare13212730






